Mobility of Nucleostemin in Live Cells Is Specifically Related to Transcription Inhibition by Actinomycin D and GTP-Binding Motif
Abstract
1. Introduction
2. Results
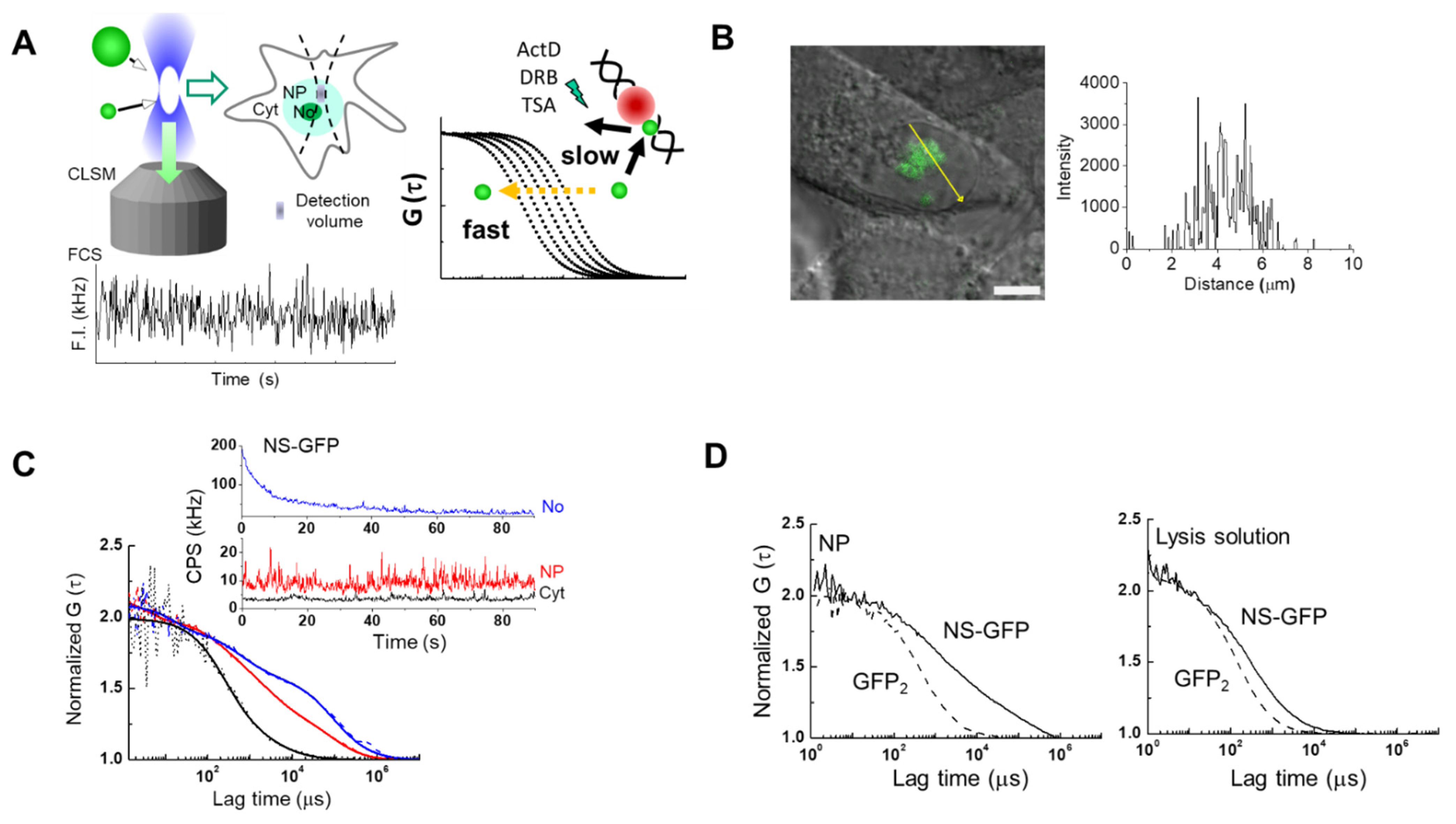
2.1. FCS Analysis of NS-GFP Diffusion in Aqueous Solution and in Live Cells
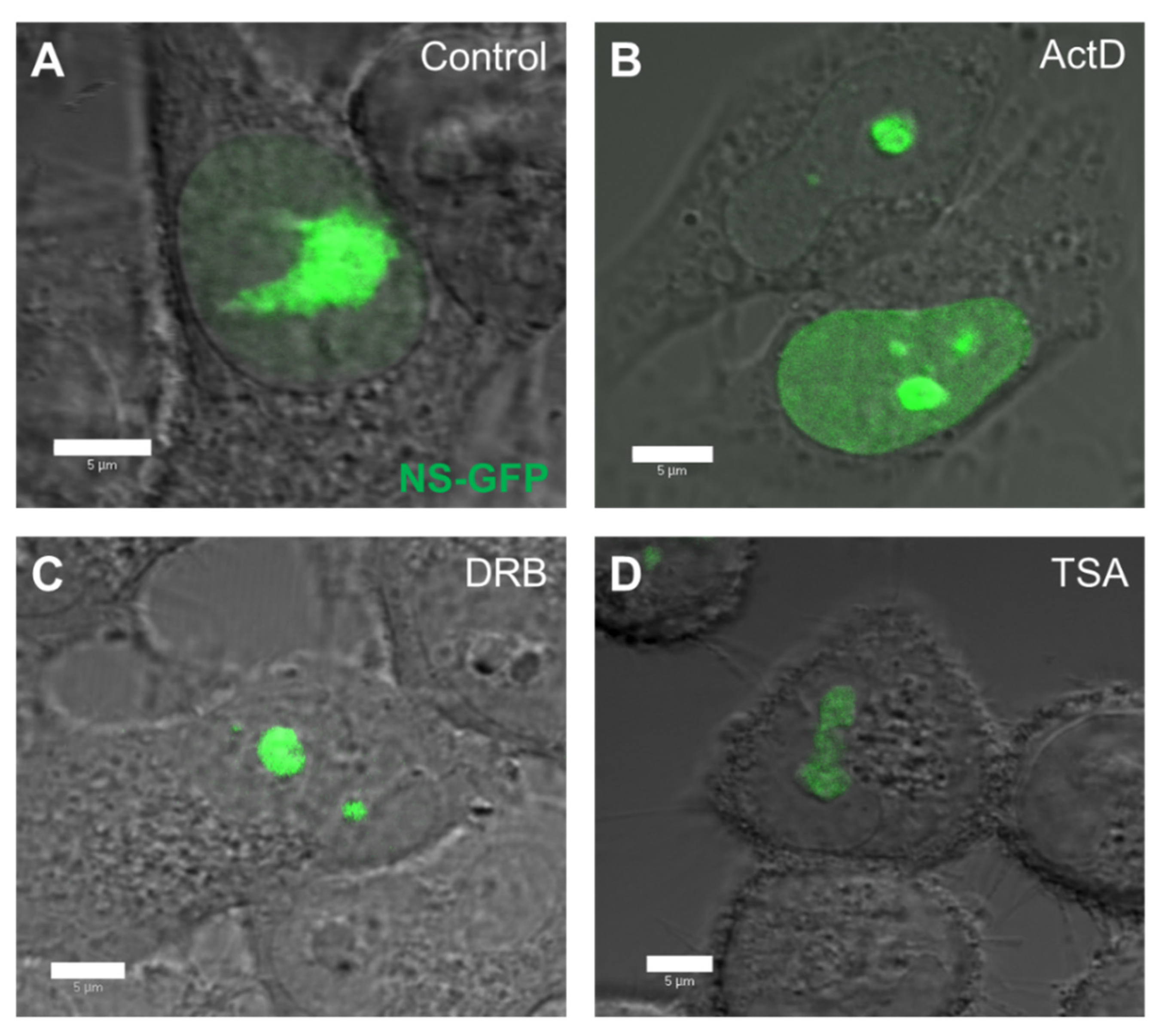
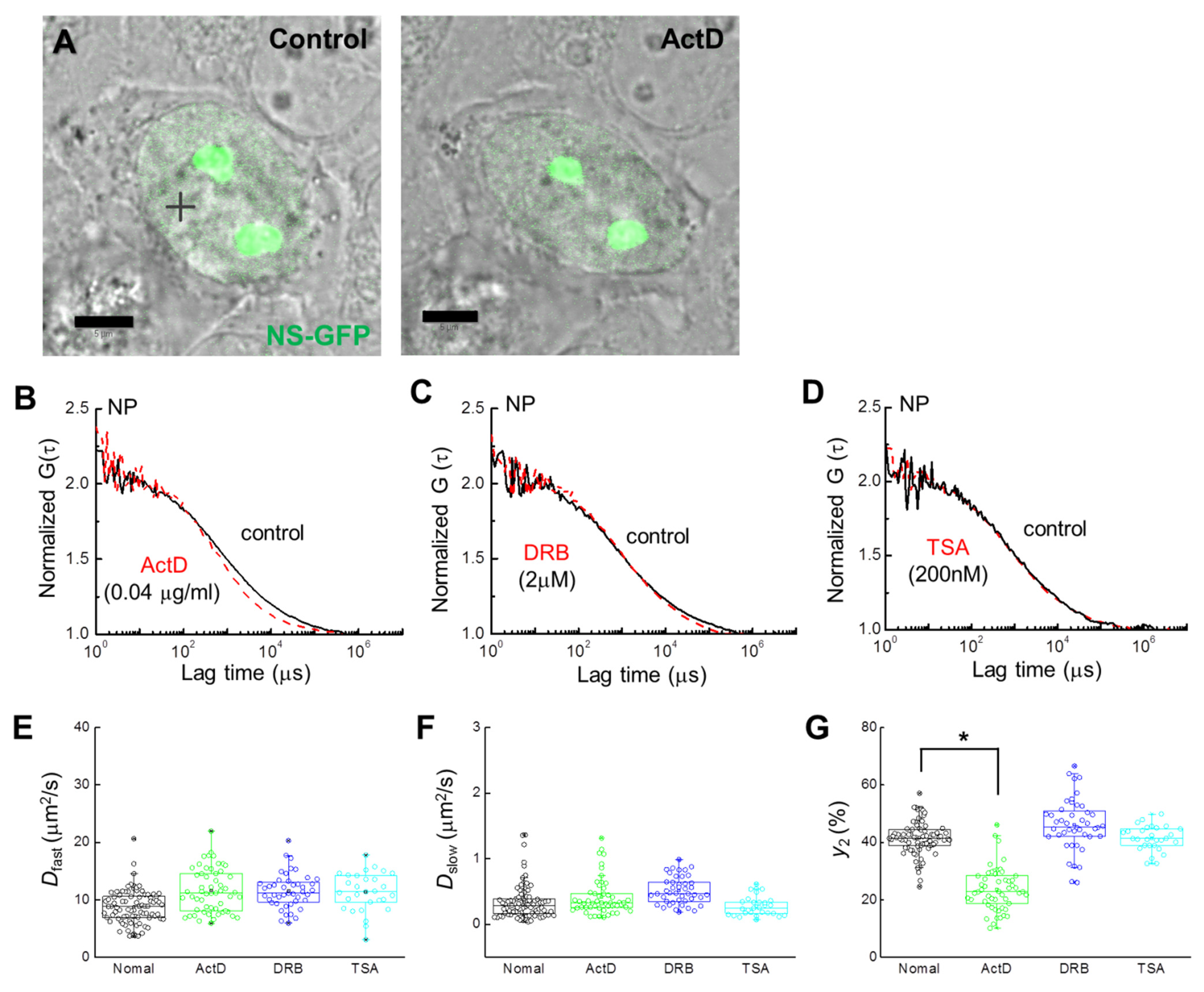
2.2. Localization and Diffusion of NS-GFP before and after Inhibitor Treatment
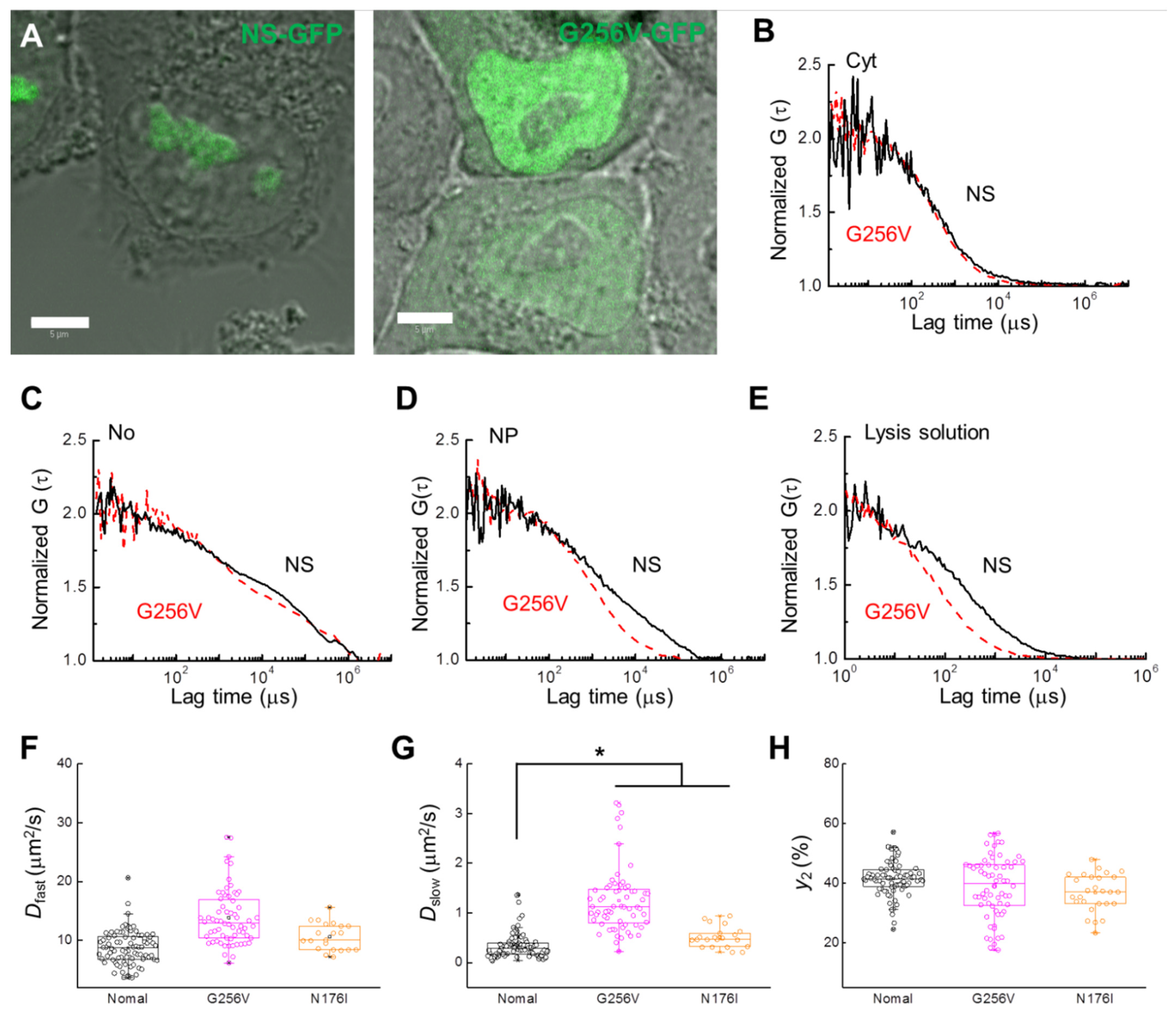
2.3. Inhibitor Dosage Dependence and Effect on Multimeric GFP and GFP-B23
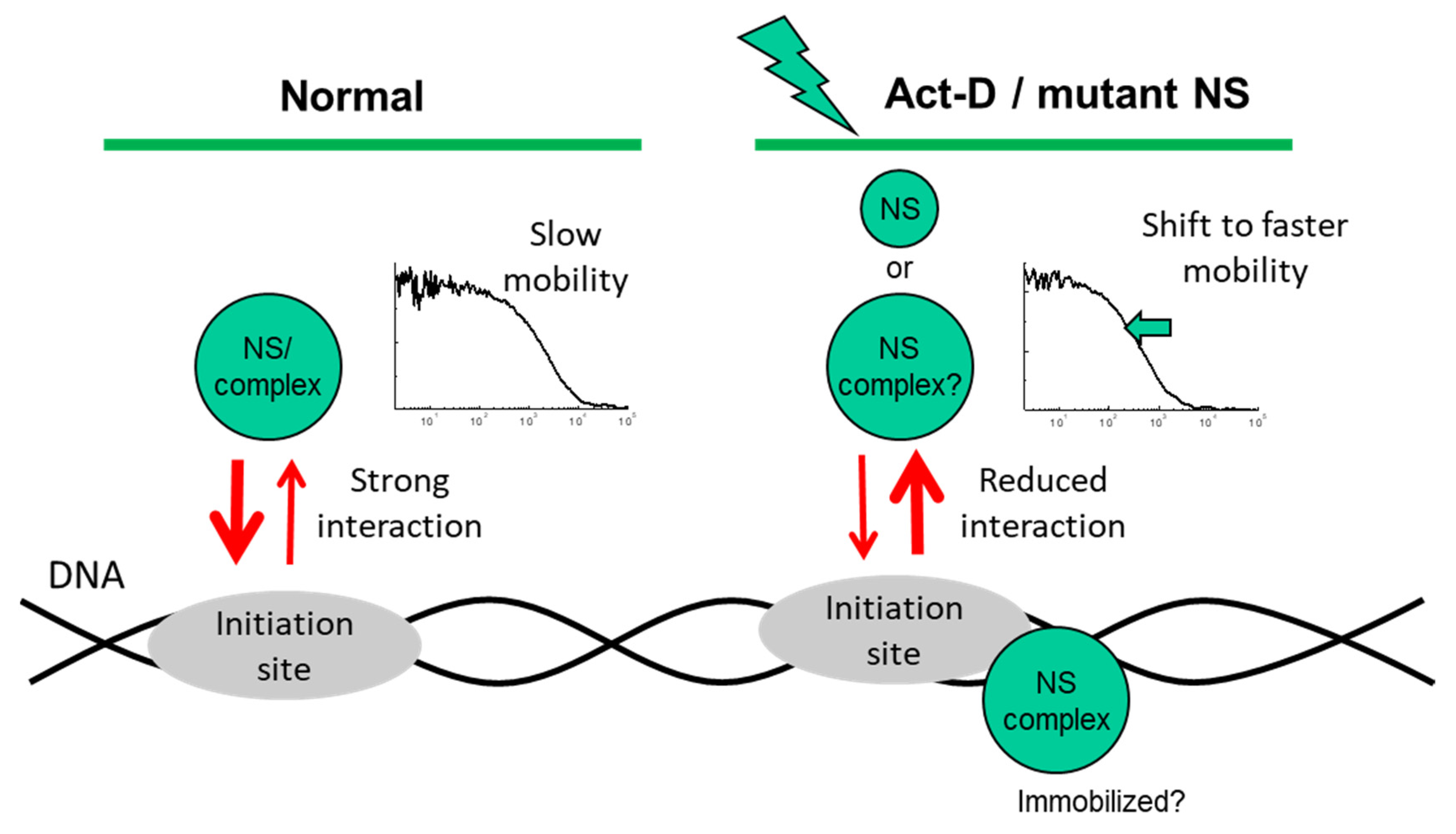
3. Discussion
4. Materials and Methods
4.1. Inhibitors
4.2. Plasmids
4.3. Cell Culture and Treatments
4.4. Cell Lysis
4.5. Live Cell Imaging
4.6. Fluorescence Correlation Spectroscopy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Politz, J.C.R.; Polena, I.; Trask, I.; Bazett-Jones, D.P.; Pederson, T. A Nonribosomal Landscape in the Nucleolus Revealed by the Stem Cell Protein Nucleostemin. Mol. Biol. Cell 2005, 16, 3401–3410. [Google Scholar] [CrossRef]
- Tsai, R.Y.L.; McKay, R.D.G. A Multistep, GTP-Driven Mechanism Controlling the Dynamic Cycling of Nucleostemin. J. Cell Biol. 2005, 168, 179–184. [Google Scholar] [CrossRef]
- Ma, H.; Pederson, T. Nucleophosmin Is a Binding Partner of Nucleostemin in Human Osteosarcoma Cells. Mol. Biol. Cell 2008, 19, 2870–2875. [Google Scholar] [CrossRef]
- Tsai, R.Y.L. Turning a New Page on Nucleostemin and Self-Renewal. J. Cell Sci. 2014, 127, 3885–3891. [Google Scholar] [CrossRef]
- Tsai, R.Y.L. A Nucleolar Mechanism Controlling Cell Proliferation in Stem Cells and Cancer Cells. Genes Dev. 2002, 16, 2991–3003. [Google Scholar] [CrossRef]
- Yang, H.-X. Screening and Identification of Proteins Interacting with Nucleostemin. World J. Gastroenterol. 2005, 11, 4812. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yasumoto, H.; Tsai, R.Y.L. Multiple Controls Regulate Nucleostemin Partitioning between Nucleolus and Nucleoplasm. J. Cell Sci. 2006, 119, 5124–5136. [Google Scholar] [CrossRef]
- Zhu, Q.; Yasumoto, H.; Tsai, R.Y.L. Nucleostemin Delays Cellular Senescence and Negatively Regulates TRF1 Protein Stability. Mol. Cell. Biol. 2006, 26, 9279–9290. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hsu, J.K.; Zhu, Q.; Lin, T.; Tsai, R.Y.L. Nucleostemin Inhibits TRF1 Dimerization and Shortens Its Dynamic Association with the Telomere. J. Cell Sci. 2011, 124, 3706–3714. [Google Scholar] [CrossRef]
- Ma, H.; Pederson, T. Depletion of the Nucleolar Protein Nucleostemin Causes G1 Cell Cycle Arrest via the P53 Pathway. Mol. Biol. Cell 2007, 18, 2630–2635. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.-S.; Sun, X.-X.; Lu, H. Aberrant Expression of Nucleostemin Activates P53 and Induces Cell Cycle Arrest via Inhibition of MDM2. Mol. Cell. Biol. 2008, 28, 4365–4376. [Google Scholar] [CrossRef]
- Meng, L.; Lin, T.; Tsai, R.Y.L. Nucleoplasmic Mobilization of Nucleostemin Stabilizes MDM2 and Promotes G2-M Progression and Cell Survival. J. Cell Sci. 2008, 121, 4037–4046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rigler, R.; Mets, Ü.; Widengren, J.; Kask, P. Fluorescence Correlation Spectroscopy with High Count Rate and Low Background: Analysis of Translational Diffusion. Eur. Biophys. J. 1993, 22, 7. [Google Scholar] [CrossRef]
- Vukojević, V.; Pramanik, A.; Yakovleva, T.; Rigler, R.; Terenius, L.; Bakalkin, G. Study of Molecular Events in Cells by Fluorescence Correlation Spectroscopy. Cell. Mol. Life Sci. 2005, 62, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging Intracellular Viscosity of a Single Cell during Photoinduced Cell Death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef]
- Lee, W.; Lee, Y.-I.; Lee, J.; Davis, L.M.; Deininger, P.; Soper, S.A. Cross-Talk-Free Dual-Color Fluorescence Cross-Correlation Spectroscopy for the Study of Enzyme Activity. Anal. Chem. 2010, 82, 1401–1410. [Google Scholar] [CrossRef][Green Version]
- Pack, C.; Saito, K.; Tamura, M.; Kinjo, M. Microenvironment and Effect of Energy Depletion in the Nucleus Analyzed by Mobility of Multiple Oligomeric EGFPs. Biophys. J. 2006, 91, 3921–3936. [Google Scholar] [CrossRef]
- Park, H.; Han, S.; Sako, Y.; Pack, C. Dynamic and Unique Nucleolar Microenvironment Revealed by Fluorescence Correlation Spectroscopy. FASEB J. 2015, 29, 837–848. [Google Scholar] [CrossRef]
- Pack, C.-G. Confocal Laser Scanning Microscopy and Fluorescence Correlation Methods for the Evaluation of Molecular Interactions. In Advanced Imaging and Bio Techniques for Convergence Science; Kim, J.K., Kim, J.K., Pack, C.-G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1310, pp. 1–30. ISBN 978-981-336-063-1. [Google Scholar]
- Guy, A.L.; Taylor, J.H. Actinomycin D Inhibits Initiation of DNA Replication in Mammalian Cells. Proc. Natl. Acad. Sci. USA 1978, 75, 6088–6092. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Lee, Y.-L.; Lai, Y.-H.; Chen, J.J.W.; Wu, W.-L.; Yuann, J.-M.P.; Su, W.-L.; Chuang, S.-M.; Hou, M.-H. Spermine Attenuates the Action of the DNA Intercalator, Actinomycin D, on DNA Binding and the Inhibition of Transcription and DNA Replication. PLoS ONE 2012, 7, e47101. [Google Scholar] [CrossRef]
- Müller, W.; Crothers, D.M. Studies of the Binding of Actinomycin and Related Compounds to DNA. J. Mol. Biol. 1968, 35, 251–290. [Google Scholar] [CrossRef]
- Wells, R.D.; Larson, J.E. Studies on the Binding of Actinomycin D to DNA and DNA Model Polymers. J. Mol. Biol. 1970, 49, 319–342. [Google Scholar] [CrossRef]
- Toth, K.F. Trichostatin A-Induced Histone Acetylation Causes Decondensation of Interphase Chromatin. J. Cell Sci. 2004, 117, 4277–4287. [Google Scholar] [CrossRef]
- Recher, L.; Briggs, L.G.; Parry, N.T. A Reevaluation of Nuclear and Nucleolar Changes Induced in Vitro by Actinomycin D. Cancer Res. 1971, 31, 140–151. [Google Scholar] [PubMed]
- Recher, L.; Parry, N.T.; Briggs, L.G.; Whitescarver, J. Difference in Effects of Proflavine and Actinomycin D on Mammalian Cell Nucleoli. Cancer Res. 1971, 31, 1915–1922. [Google Scholar] [PubMed]
- Chen, D.; Huang, S. Nucleolar Components Involved in Ribosome Biogenesis Cycle between the Nucleolus and Nucleoplasm in Interphase Cells. J. Cell Biol. 2001, 153, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hihara, S.; Pack, C.-G.; Kaizu, K.; Tani, T.; Hanafusa, T.; Nozaki, T.; Takemoto, S.; Yoshimi, T.; Yokota, H.; Imamoto, N.; et al. Local Nucleosome Dynamics Facilitate Chromatin Accessibility in Living Mammalian Cells. Cell Rep. 2012, 2, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Ho, D.-R.; Chen, F.-Y.; Chen, C.-R.; Ke, Y.-D.; Su, J.-G.J. AKT Mediates Actinomycin D-Induced P53 Expression. Oncotarget 2014, 5, 693–703. [Google Scholar] [CrossRef]
- Hsu, J.K.; Lin, T.; Tsai, R.Y.L. Nucleostemin Prevents Telomere Damage by Promoting PML-IV Recruitment to SUMOylated TRF1. J. Cell Biol. 2012, 197, 613–624. [Google Scholar] [CrossRef]
- Meng, L.; Lin, T.; Peng, G.; Hsu, J.K.; Lee, S.; Lin, S.-Y.; Tsai, R.Y.L. Nucleostemin Deletion Reveals an Essential Mechanism That Maintains the Genomic Stability of Stem and Progenitor Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 11415–11420. [Google Scholar] [CrossRef]
- Lin, T.; Ibrahim, W.; Peng, C.-Y.; Finegold, M.J.; Tsai, R.Y.L. A Novel Role of Nucleostemin in Maintaining the Genome Integrity of Dividing Hepatocytes during Mouse Liver Development and Regeneration. Hepatology 2013, 58, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Lis, J.T.; Zhou, S.; Tjian, R. A TRF1:BRF Complex Directs Drosophila RNA Polymerase III Transcription. Cell 2000, 101, 459–469. [Google Scholar] [CrossRef][Green Version]
- Reina, J.H.; Hernandez, N. On a Roll for New TRF Targets. Genes Dev. 2007, 21, 2855–2860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bindra, R.S.; Schaffer, P.J.; Meng, A.; Woo, J.; Måseide, K.; Roth, M.E.; Lizardi, P.; Hedley, D.W.; Bristow, R.G.; Glazer, P.M. Down-Regulation of Rad51 and Decreased Homologous Recombination in Hypoxic Cancer Cells. Mol. Cell. Biol. 2004, 24, 8504–8518. [Google Scholar] [CrossRef]
- Sugaya, K.; Vigneron, M.; Cook, P. Mammalian Cell Lines Expressing Functional RNA Polymerase II Tagged with the Green Fluorescent Protein. J. Cell Sci. 2000, 113, 2679–2683. [Google Scholar] [CrossRef]
- Saito, K.; Wada, I.; Tamura, M.; Kinjo, M. Direct Detection of Caspase-3 Activation in Single Live Cells by Cross-Correlation Analysis. Biochem. Biophys. Res. Commun. 2004, 324, 849–854. [Google Scholar] [CrossRef]
- Saito, K.; Ito, E.; Takakuwa, Y.; Tamura, M.; Kinjo, M. In Situ Observation of Mobility and Anchoring of PKCβI in Plasma Membrane. FEBS Lett. 2003, 541, 126–131. [Google Scholar] [CrossRef]
- Weisshart, K.; Jungel, V.; Briddon, S.J. The LSM 510 META—ConfoCor 2 System: An Integrated Imaging and Spectroscopic Platform for Single-Molecule Detection. Curr. Pharm. Biotechnol. 2004, 5, 135–154. [Google Scholar] [CrossRef]
- Pack, C.-G.; Yukii, H.; Toh-e, A.; Kudo, T.; Tsuchiya, H.; Kaiho, A.; Sakata, E.; Murata, S.; Yokosawa, H.; Sako, Y.; et al. Quantitative Live-Cell Imaging Reveals Spatio-Temporal Dynamics and Cytoplasmic Assembly of the 26S Proteasome. Nat. Commun. 2014, 5, 3396. [Google Scholar] [CrossRef]
- Pack, C.-G.; Ahn, S.-G. Heat Shock-Induced Interactions among Nuclear HSFs Detected by Fluorescence Cross-Correlation Spectroscopy. Biochem. Biophys. Res. Commun. 2015, 463, 303–308. [Google Scholar] [CrossRef]
- Shin, H.-S.; Okamoto, A.; Sako, Y.; Kim, S.W.; Kim, S.Y.; Pack, C.-G. Characterization of the Triplet State of Hybridization-Sensitive DNA Probe by Using Fluorescence Correlation Spectroscopy. J. Phys. Chem. A 2013, 117, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kawai-Noma, S.; Ayano, S.; Pack, C.-G.; Kinjo, M.; Yoshida, M.; Yasuda, K.; Taguchi, H. Dynamics of Yeast Prion Aggregates in Single Living Cells. Genes Cells 2006, 11, 1085–1096. [Google Scholar] [CrossRef] [PubMed]

| Protein | Fast Component | Slow Component | ||
|---|---|---|---|---|
| (Expressed in the Nucleoplasm Unless Specified) | D (µm2 s−1) | y1 (%) | D (µm2 s−1) | y2 (%) |
| NS-GFP | 8.7 ± 1.5 | 58 | 0.34 ± 0.08 * | 42 ± 4 * |
| NS-GFP in cytosol | 12.8 ± 2.2 | 91 | 0.06 ± 0.03 | 9 ± 6 |
| NS-GFP/ActD 1 | 11.6 ± 1.8 | 80 | 0.41 ± 0.1 | 20 ± 3 * |
| NS-GFP/DRB 1 | 12.2 ± 3.6 | 55 | 0.60 ± 0.3 | 45 ± 6 |
| NS-GFP/TSA 1 | 11.3 ± 3.4 | 59 | 0.28 ± 0.2 | 41 ± 4 |
| G256V-GFP | 15.0 ± 2.1 | 61 | 1.3 ± 0.3 * | 39 ± 5 |
| N176I-GFP | 11.0 ± 1.6 | 64 | 0.6 ± 0.1 * | 36 ± 5 |
| GFP-B23 | 11.7 ± 3.1 | 30 | 3.0 ± 0.5 | 70 ± 8 |
| Dimeric GFP | 14.2 ± 1.9 | 100 | N.D. 2 | |
| In Lysed Solution | Fast Component (D, µm2 s−1) (y1, Fraction%) | Slow Component (D, µm2 s−1) (y2, Fraction%) |
|---|---|---|
| NS-GFP | 38.0 ± 2.9 (64) | 4.9 ± 0.4 (36 ± 5) |
| G256V-GFP | 51.0 ± 2.3 (100) | N.D. 1 |
| GFP-B23 | 28.4 ± 0.7 (100) | N.D. 1 |
| Dimeric GFP | 49.5 ± 1.0 (100) | N.D. 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pack, C.-G.; Jung, K.; Paulson, B.; Kim, J.K. Mobility of Nucleostemin in Live Cells Is Specifically Related to Transcription Inhibition by Actinomycin D and GTP-Binding Motif. Int. J. Mol. Sci. 2021, 22, 8293. https://doi.org/10.3390/ijms22158293
Pack C-G, Jung K, Paulson B, Kim JK. Mobility of Nucleostemin in Live Cells Is Specifically Related to Transcription Inhibition by Actinomycin D and GTP-Binding Motif. International Journal of Molecular Sciences. 2021; 22(15):8293. https://doi.org/10.3390/ijms22158293
Chicago/Turabian StylePack, Chan-Gi, Keehoon Jung, Bjorn Paulson, and Jun Ki Kim. 2021. "Mobility of Nucleostemin in Live Cells Is Specifically Related to Transcription Inhibition by Actinomycin D and GTP-Binding Motif" International Journal of Molecular Sciences 22, no. 15: 8293. https://doi.org/10.3390/ijms22158293
APA StylePack, C.-G., Jung, K., Paulson, B., & Kim, J. K. (2021). Mobility of Nucleostemin in Live Cells Is Specifically Related to Transcription Inhibition by Actinomycin D and GTP-Binding Motif. International Journal of Molecular Sciences, 22(15), 8293. https://doi.org/10.3390/ijms22158293








