Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE)
Abstract
:1. Introduction
2. Results
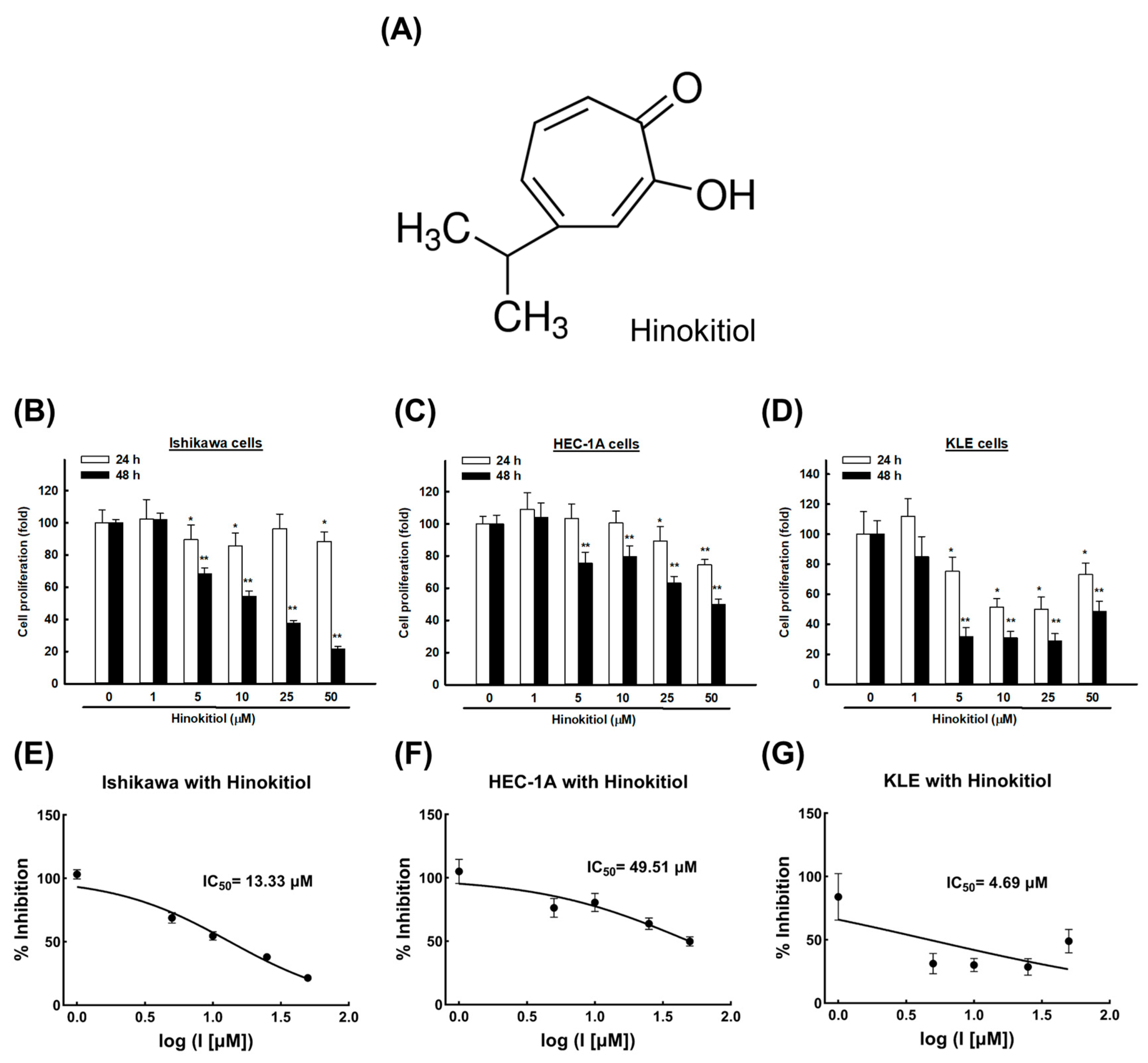
2.1. Hinokitiol Induces an Anti-Proliferative Effect on Endometrial Cancer Cells
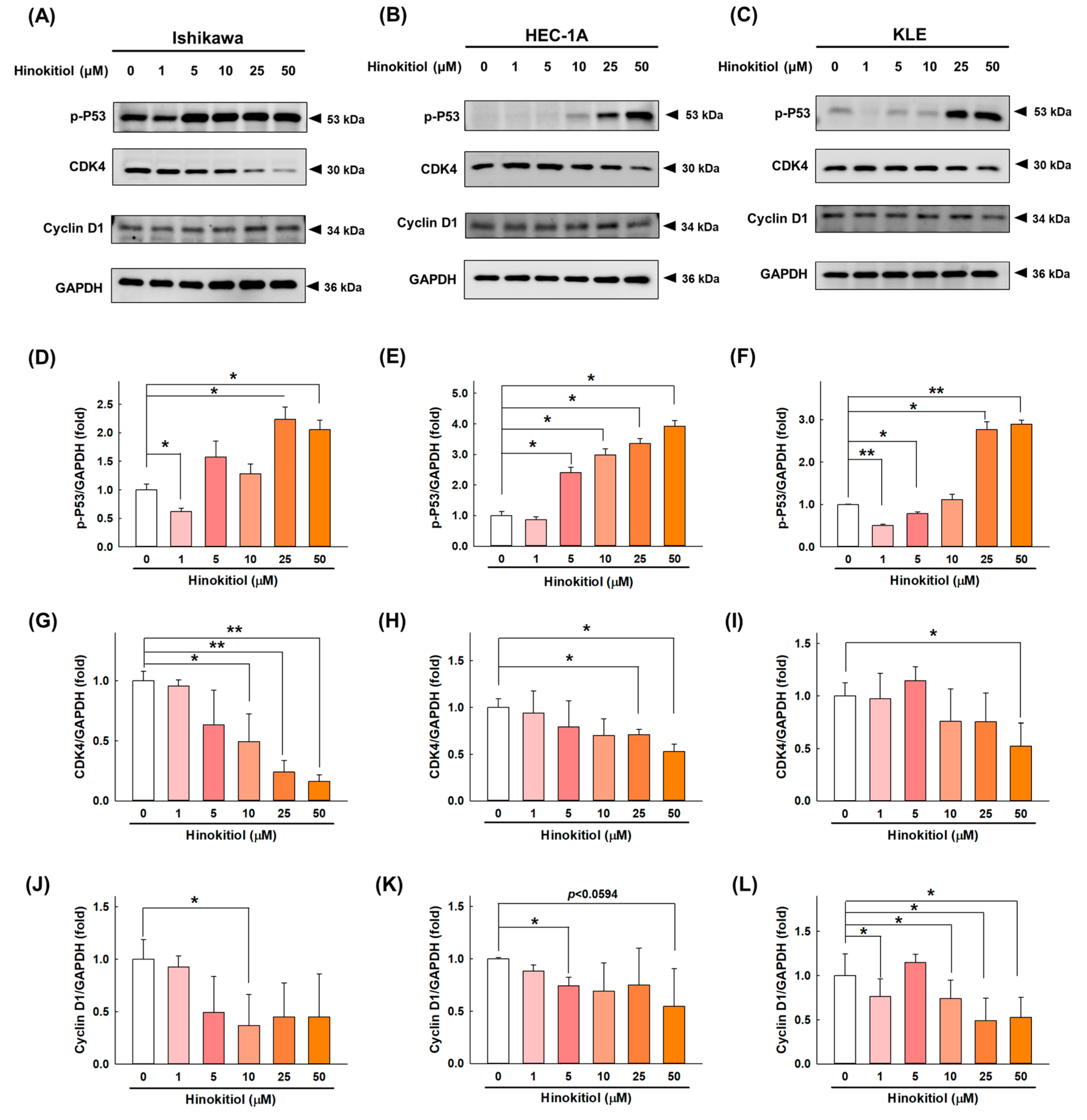
2.2. Hinokitiol Promotes Cell-Cycle Arrest at the G0/G1 Phase in Endometrial Cancer Cells
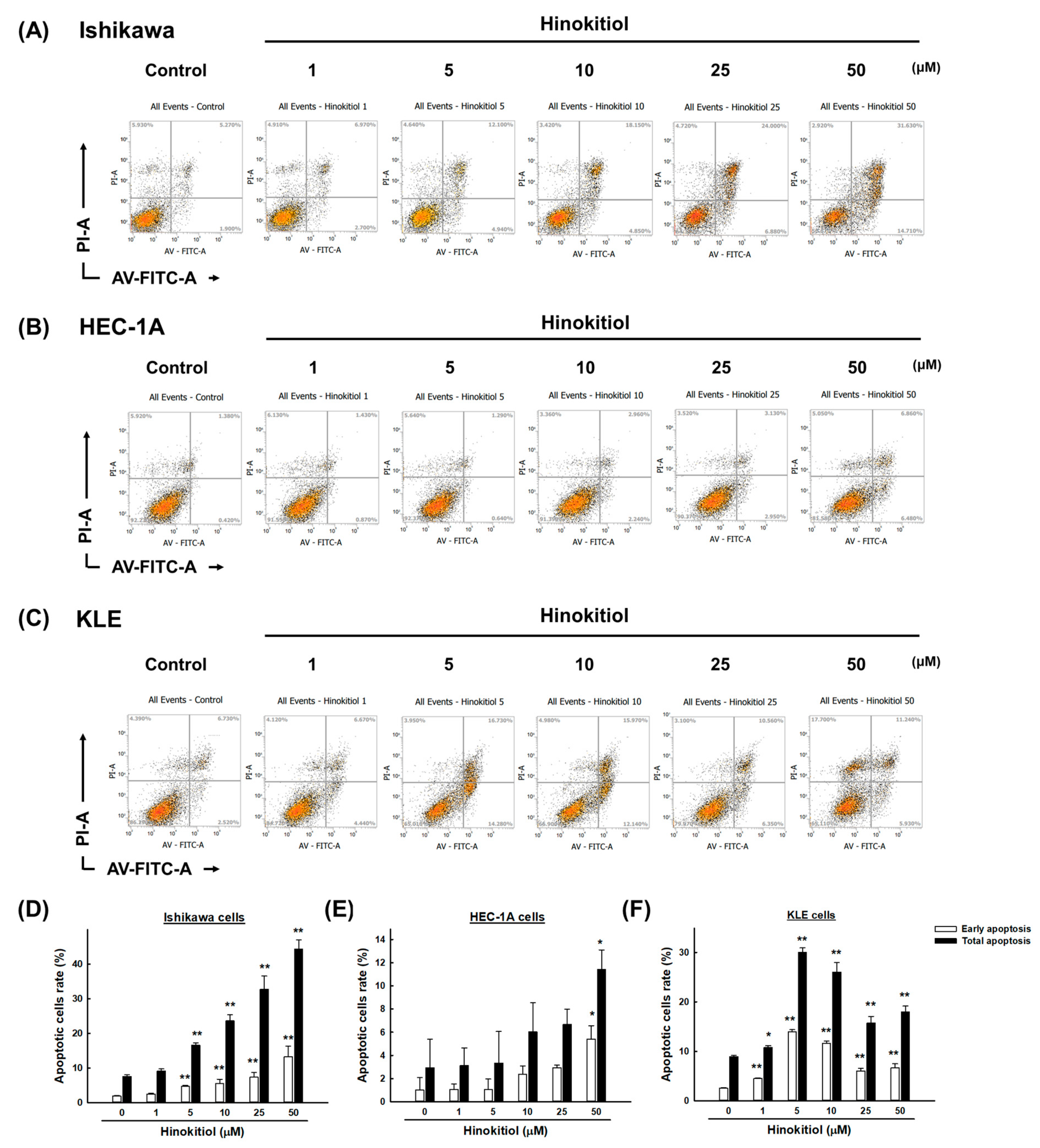
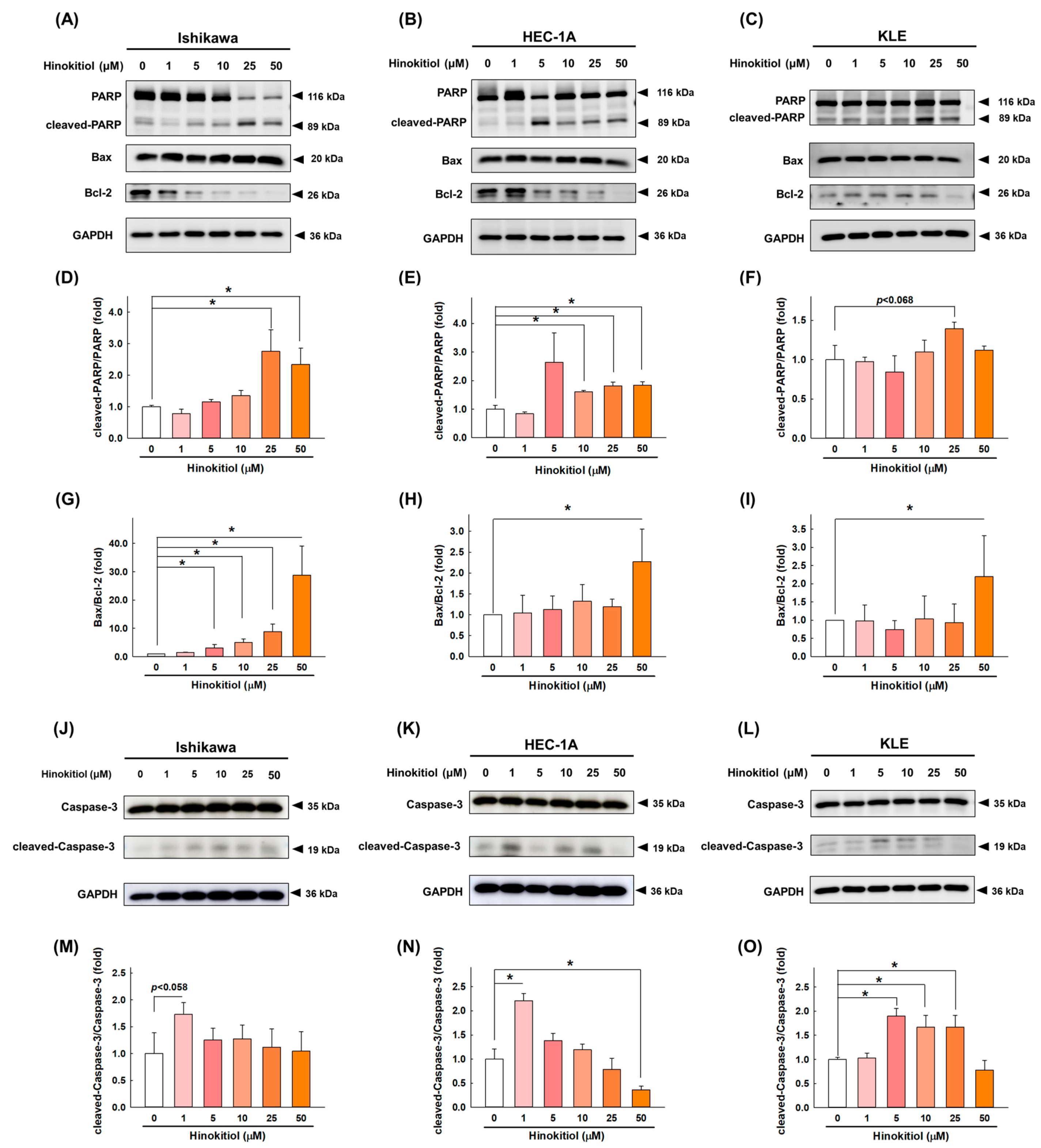
2.3. Hinokitiol Induces Cell Apoptosis via the Caspase Apoptotic Pathway in Endometrial Cancer Cells
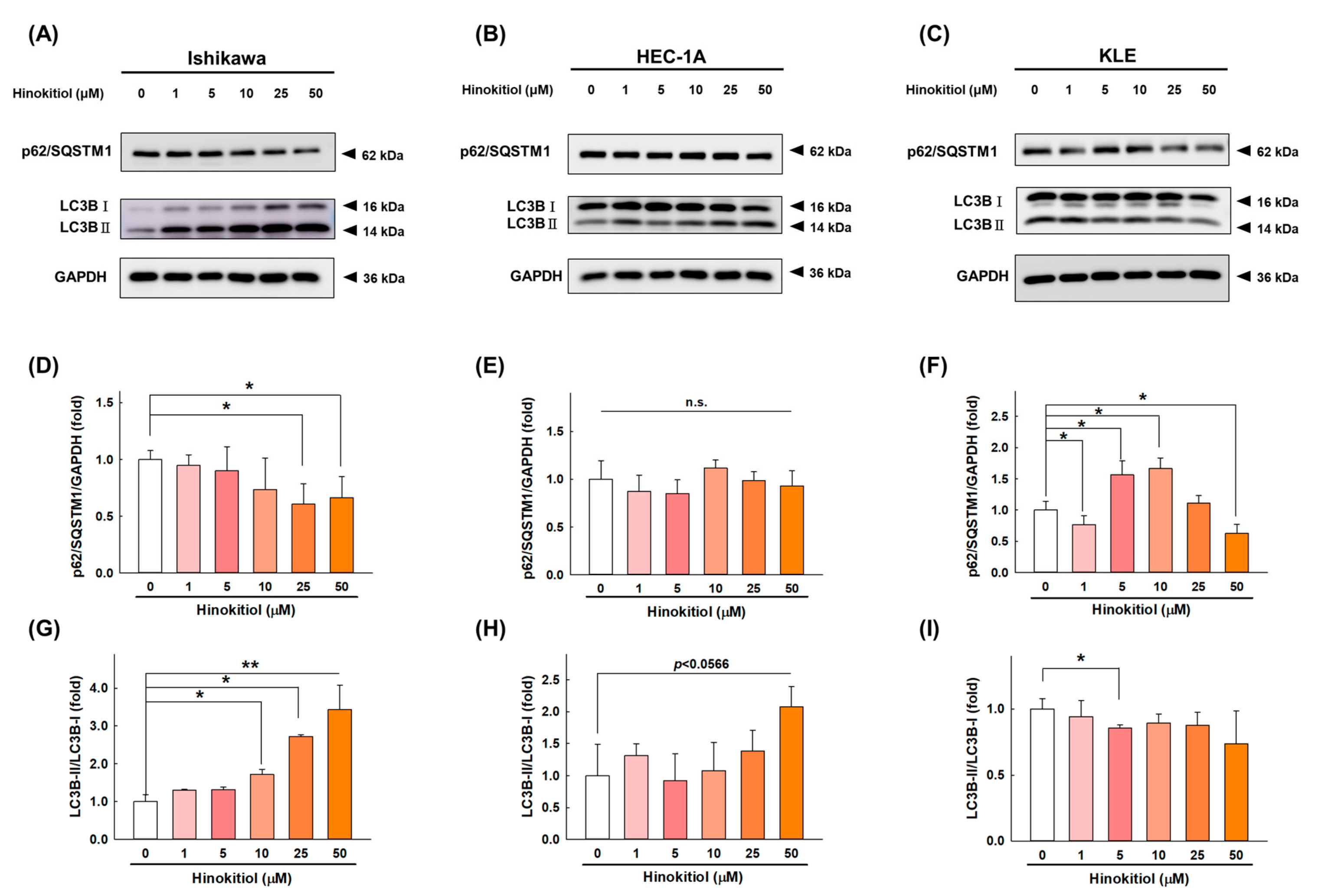
2.4. Hinokitiol Induces Cell Autophagy in Endometrial Cancer Cells
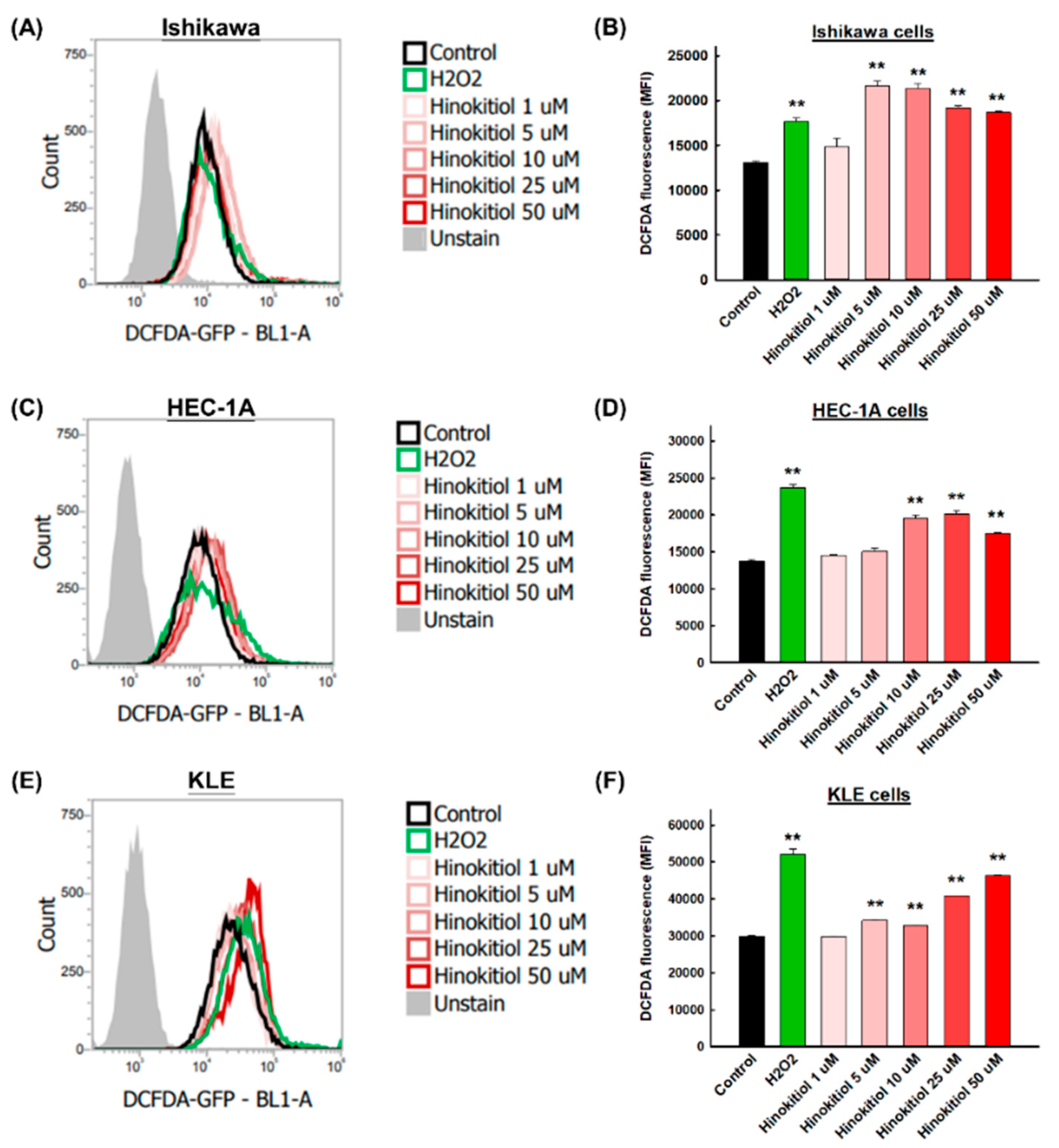
2.5. Hinokitiol Induces Intracellular Reactive Oxygen Species (ROSs) in Endometrial Cancer Cells
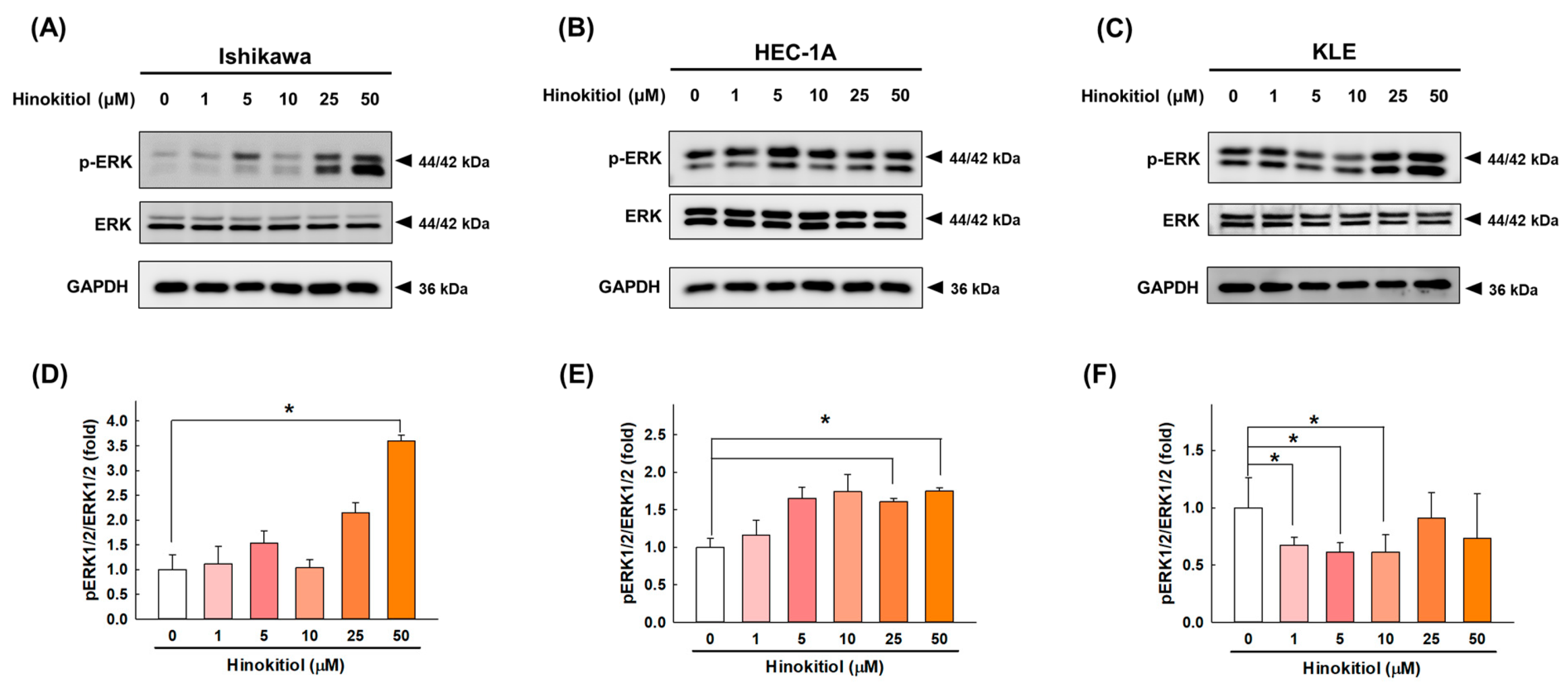
2.6. Hinokitiol Regulates the ERK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Preparation of Hinokitiol
4.2. Cell Lines and Culture
4.3. Cell Viability Assays
4.4. Western Blotting Analysis
4.5. Flow Cytometry Analysis
4.6. Measurement of Intracellular Reactive Oxygen Species (ROSs)
4.7. Statistical Analysis
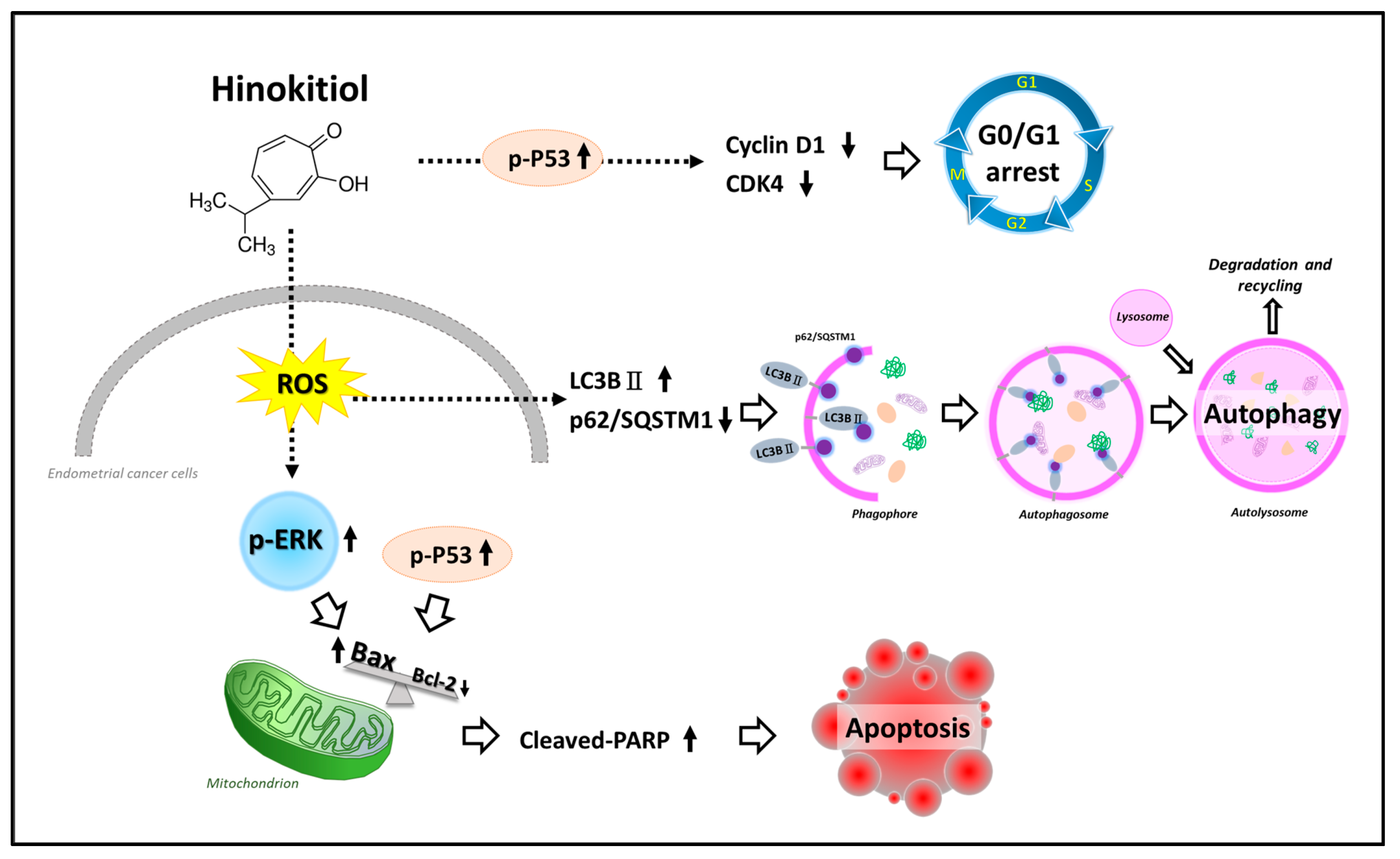
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.-Y.; Weng, C.-S.; Huang, S.-M.; Huang, N.; Chou, Y.-J.; Wang, C.-C.; Wang, K.-L. Incidence and lifetime risk of uterine corpus cancer in Taiwanese women from 1991 to 2010. Taiwan. J. Obstet. Gynecol. 2017, 56, 68–72. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Skok, K.; Maver, U.; Gradišnik, L.; Kozar, N.; Takač, I.; Arko, D. Endometrial cancer and its cell lines. Mol. Biol. Rep. 2020, 47, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Abdulfatah, E.; Wakeling, E.; Sakr, S.; Al-Obaidy, K.; Bandyopadhyay, S.; Morris, R.; Feldman, G.; Ali-Fehmi, R. Molecular classification of endometrial carcinoma applied to endometrial biopsy specimens: Towards early personalized patient management. Gynecol. Oncol. 2019, 154, 467–474. [Google Scholar] [CrossRef]
- Muller, P.A.; Vousden, K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.Y.; Chiang, Y.F.; Huang, J.S.; Huang, T.C.; Shih, Y.H.; Wang, K.L.; Ali, M.; Hong, Y.H.; Shieh, T.M.; Hsia, S.M. Isoliquiritigenin Reverses Epithelial-Mesenchymal Transition Through Modulation of the TGF-β/Smad Signaling Pathway in Endometrial Cancer. Cancers 2021, 13, 1236. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on natural compounds in chemoprevention and treatment of cancer: An update with new promising compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Jayakumar, T.; Hsu, W.H.; Yen, T.L.; Luo, J.Y.; Kuo, Y.C.; Fong, T.H.; Sheu, J.R. Hinokitiol, a natural tropolone derivative, offers neuroprotection from thromboembolic stroke in vivo. Evid. Based Complementary Altern. Med. 2013, 2013, 840487. [Google Scholar] [CrossRef] [PubMed]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial activity of hinokitiol against both antibiotic-resistant and -susceptible pathogenic bacteria that predominate in the oral cavity and upper airways. Microbiol. Immunol. 2019, 63, 213–222. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Lee, Y.J.; Park, S.Y. SIRT1, a Class III Histone Deacetylase, Regulates LPS-Induced Inflammation in Human Keratinocytes and Mediates the Anti-Inflammatory Effects of Hinokitiol. J. Investig. Dermatol. 2017, 137, 1257–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.J.; Hsu, W.J.; Wu, L.H.; Liou, H.P.; Pangilinan, C.R.; Tyan, Y.C.; Lee, C.H. Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase B pathway. Int. J. Med. Sci. 2020, 17, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, T.; Liu, C.H.; Wu, G.Y.; Lee, T.Y.; Manubolu, M.; Hsieh, C.Y.; Yang, C.H.; Sheu, J.R. Hinokitiol Inhibits Migration of A549 Lung Cancer Cells via Suppression of MMPs and Induction of Antioxidant Enzymes and Apoptosis. Int. J. Mol. Sci. 2018, 19, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Chen, B.K.; Chen, P.H.; Chen, L.C. Hinokitiol induces cell death and inhibits epidermal growth factor-induced cell migration and signaling pathways in human cervical adenocarcinoma. Taiwan. J. Obstet. Gynecol. 2020, 59, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yamauchi, H. Hinokitiol, a metal chelator derived from natural plants, suppresses cell growth and disrupts androgen receptor signaling in prostate carcinoma cell lines. Biochem. Biophys. Res. Commun. 2006, 351, 26–32. [Google Scholar] [CrossRef]
- Wei, K.-C.; Chen, R.-F.; Chen, Y.-F.; Lin, C.-H. Hinokitiol suppresses growth of B16 melanoma by activating ERK/MKP3/proteosome pathway to downregulate survivin expression. Toxicol. Appl. Pharmacol. 2019, 366, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Chang, K.W.; Hsia, S.M.; Yu, C.C.; Fuh, L.J.; Chi, T.Y.; Shieh, T.M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Tanaka, S.; Tak, Y.S.; Araki, H. The role of CDK in the initiation step of DNA replication in eukaryotes. Cell Div. 2007, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Suryadinata, R.; Sadowski, M.; Sarcevic, B. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci. Rep. 2010, 30, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ko, J.; Kim, J.T.; Cho, J.S.; Qiu, S.; Kim, G.D.; Auh, J.H.; Lee, H.J. β-Thujaplicin inhibits basal-like mammary tumor growth by regulating glycogen synthase kinase-3β/β-catenin signaling. Food Funct. 2019, 10, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; O’Connor, L.; Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000, 69, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, J.; Ye, X.; Zhu, J.; Hu, X.; Shen, M.; Ma, Y.; Mao, Z.; Song, H.; Chen, F. β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuffa, R.; Lamark, T.; Tarafder, A.K.; Guesdon, A.; Rybina, S.; Hagen, W.J.; Johansen, T.; Sachse, C. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 2015, 11, 748–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Miao, Q.; Jin, K.; Lou, L.; Ye, X.; Xi, Y.; Ye, J. Protective Role of Hinokitiol Against H2O2-Induced Injury in Human Corneal Epithelium. Curr. Eye Res. 2017, 42, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.Y.; Sun, Y.S.; Ma, R.H.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Asparanin A from Asparagus officinalis L. Induces G0/G1 Cell Cycle Arrest and Apoptosis in Human Endometrial Carcinoma Ishikawa Cells via Mitochondrial and PI3K/AKT Signaling Pathways. J. Agric. Food Chem. 2020, 68, 213–224. [Google Scholar] [CrossRef]
- Li, X.L.; Ma, R.H.; Ni, Z.J.; Thakur, K.; Cespedes-Acuña, C.L.; Wang, S.; Zhang, J.G.; Wei, Z.J. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial-dependent signaling pathway. Food Chem. Toxicol. 2021, 148, 111941. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004, 3, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-Y.; Cheng, W.-P.; Chiang, Y.-F.; Hong, Y.-H.; Ali, M.; Huang, T.-C.; Wang, K.-L.; Shieh, T.-M.; Chang, H.-Y.; Hsia, S.-M. Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE). Int. J. Mol. Sci. 2021, 22, 8268. https://doi.org/10.3390/ijms22158268
Chen H-Y, Cheng W-P, Chiang Y-F, Hong Y-H, Ali M, Huang T-C, Wang K-L, Shieh T-M, Chang H-Y, Hsia S-M. Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE). International Journal of Molecular Sciences. 2021; 22(15):8268. https://doi.org/10.3390/ijms22158268
Chicago/Turabian StyleChen, Hsin-Yuan, Wen-Pin Cheng, Yi-Fen Chiang, Yong-Han Hong, Mohamed Ali, Tsui-Chin Huang, Kai-Lee Wang, Tzong-Ming Shieh, Hsin-Yi Chang, and Shih-Min Hsia. 2021. "Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE)" International Journal of Molecular Sciences 22, no. 15: 8268. https://doi.org/10.3390/ijms22158268
APA StyleChen, H.-Y., Cheng, W.-P., Chiang, Y.-F., Hong, Y.-H., Ali, M., Huang, T.-C., Wang, K.-L., Shieh, T.-M., Chang, H.-Y., & Hsia, S.-M. (2021). Hinokitiol Exhibits Antitumor Properties through Induction of ROS-Mediated Apoptosis and p53-Driven Cell-Cycle Arrest in Endometrial Cancer Cell Lines (Ishikawa, HEC-1A, KLE). International Journal of Molecular Sciences, 22(15), 8268. https://doi.org/10.3390/ijms22158268










