Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment
Abstract
:1. Introduction
2. Results
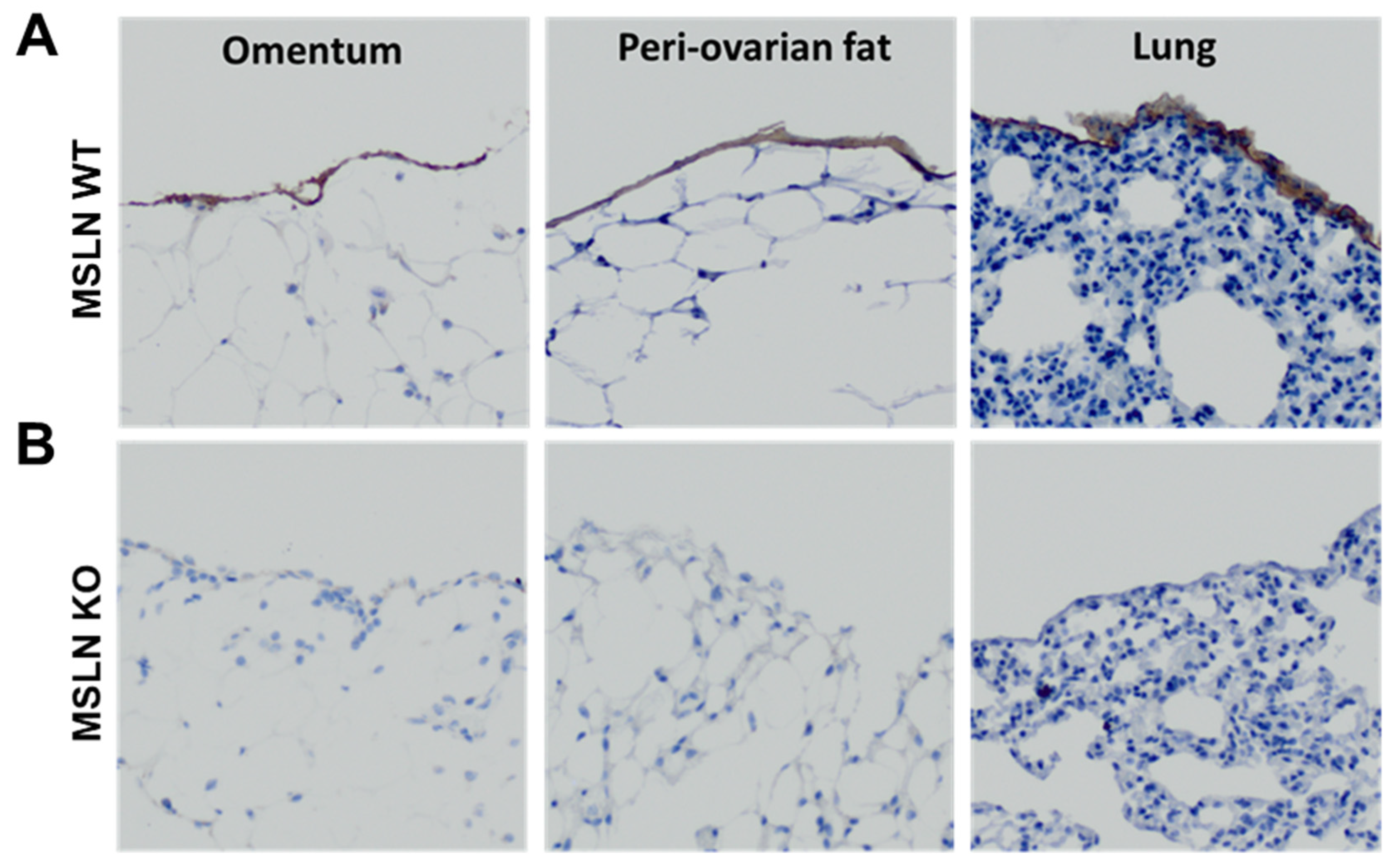
2.1. Host MSLN Expression in Wild Type and Knockout Mesothelial Cells
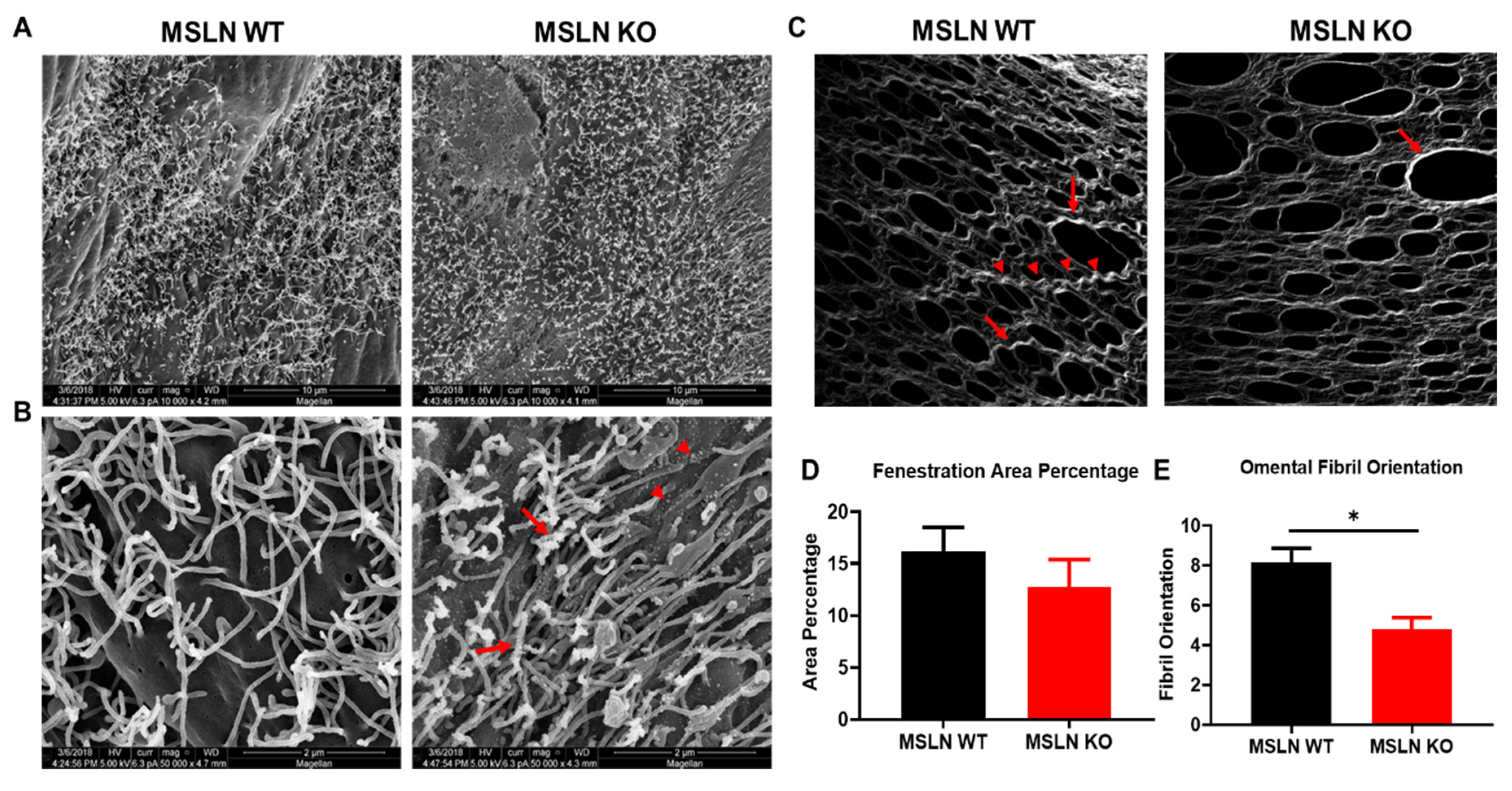
2.2. Alterations in the Peritoneal Ultrastructure of MSLNKO Mice
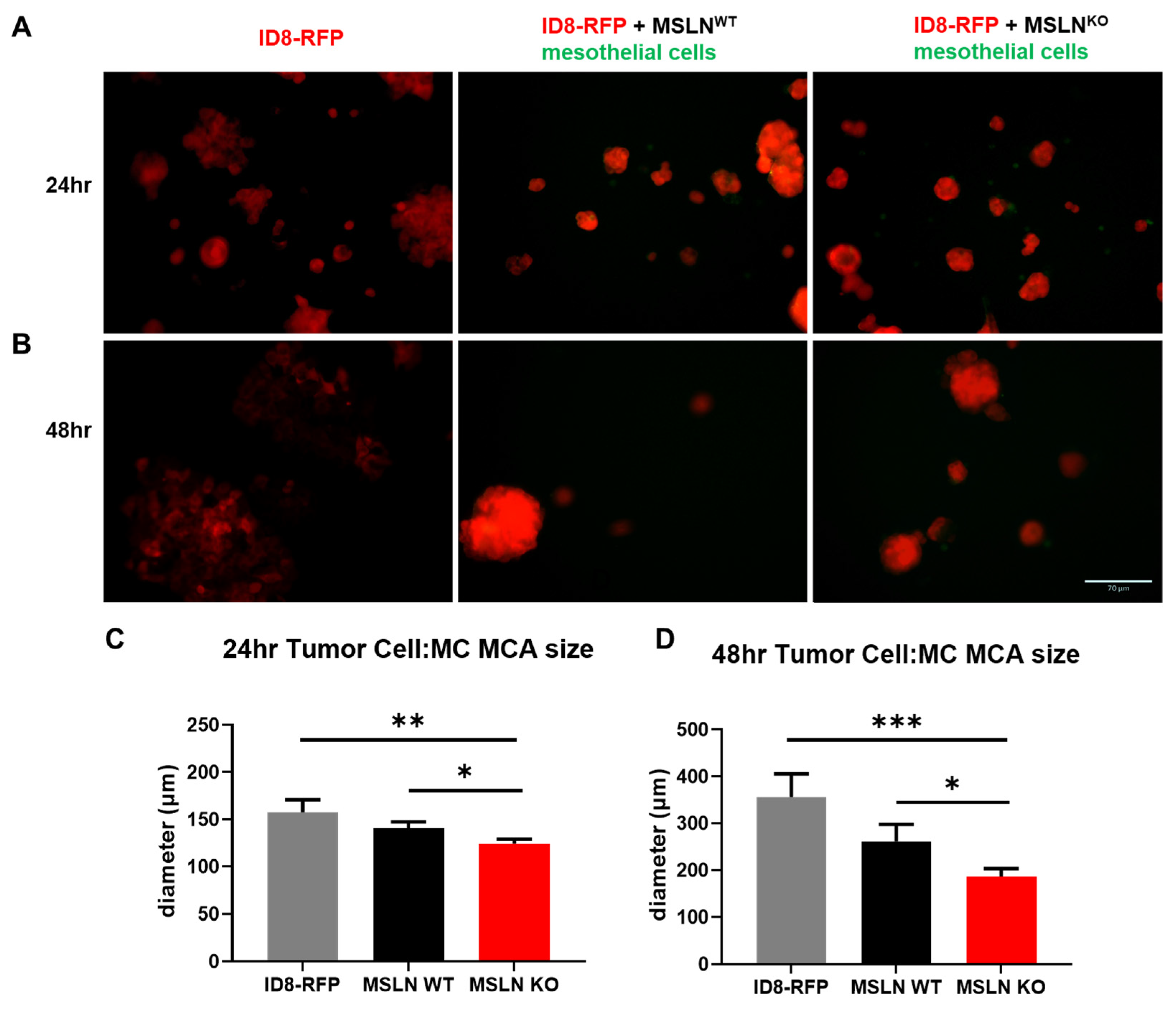
2.3. Host MSLN Expression Regulates In Vitro Multicellular Aggregate Size
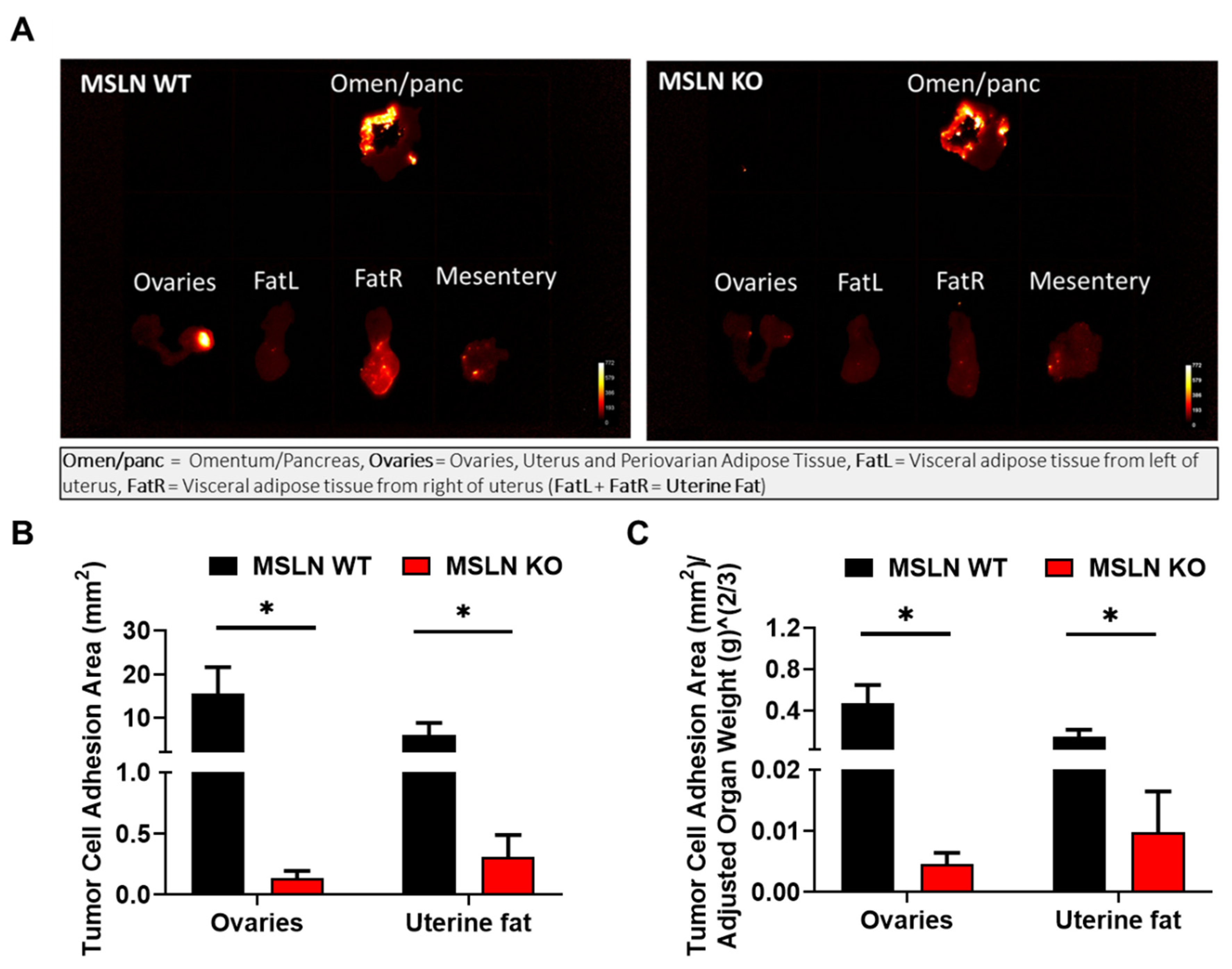
2.4. Lack of Host MSLN Expression Alters In Vivo Adhesion to Abdominal Adipose
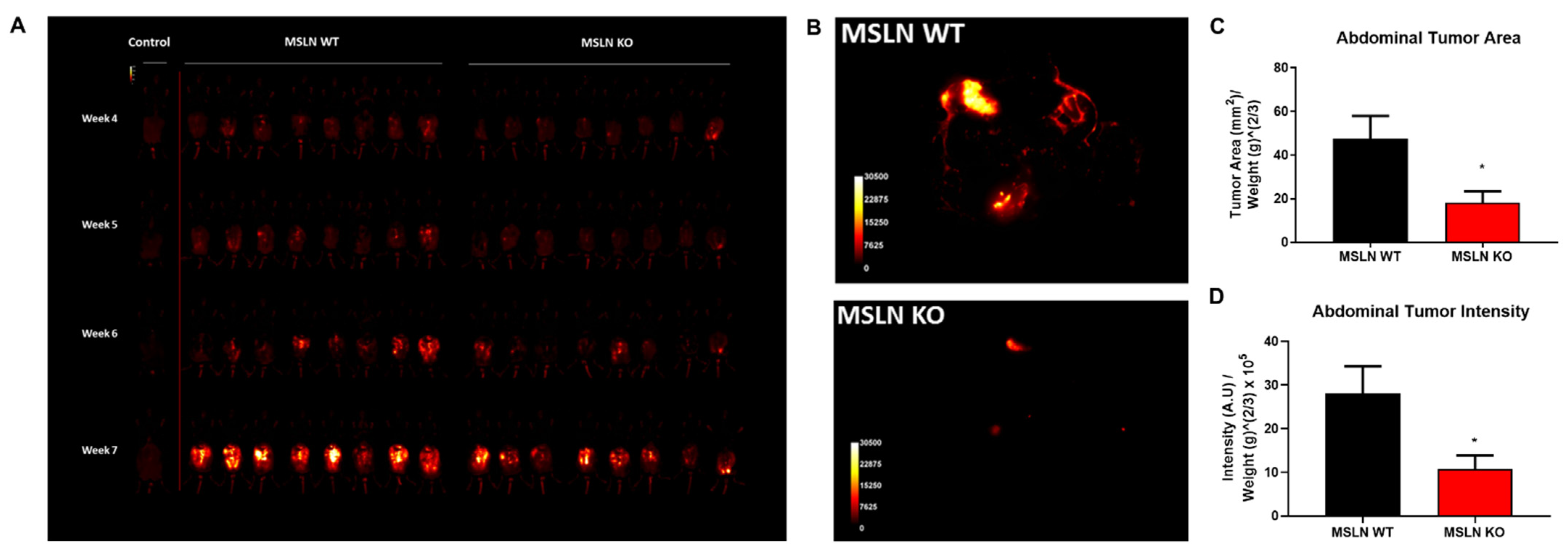
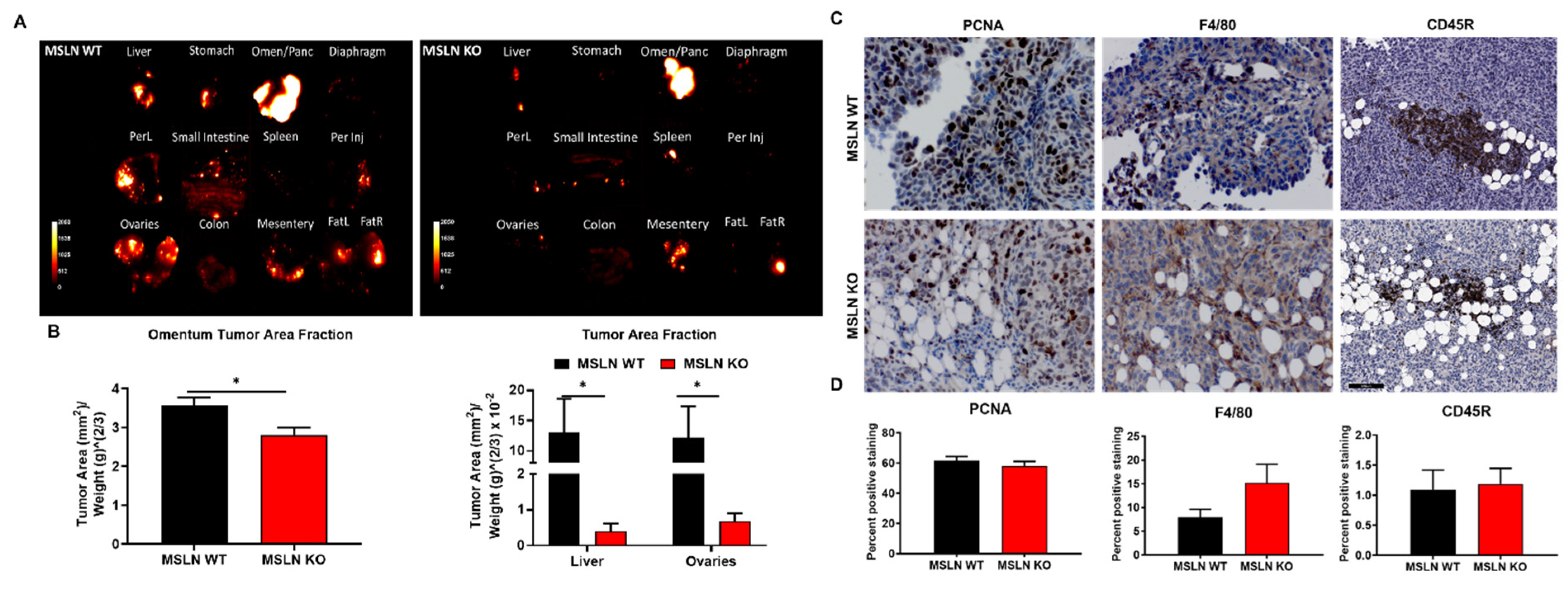
2.5. Deletion of Host MSLN Impacts Tumor Metastasis and Peritoneal Dissemination
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mesothelin Mouse Model
4.3. Scanning Electron Microscopy (SEM) Tissue Processing and Analysis
4.4. Second Harmonic Generation Microscopy
4.5. Isolation and Propagation of Mesothelial Cells
4.6. Multicellular Aggregate Hanging Drop Culture
4.7. In Vivo Adhesion Assay
4.8. Murine Allograft Model of Ovarian Cancer Metastasis
4.9. Histology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.C.; Chen, C.A.; Chen, P.J.; Chiang, Y.C.; Chen, Y.L.; Mao, T.L.; Lin, H.W.; Lin Chiang, W.H.; Cheng, W.F. Mesothelin enhances invasion of ovarian cancer by inducing mmp-7 through mapk/erk and jnk pathways. Biochem. J. 2012, 442, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bharadwaj, U.; Zhang, R.; Zhang, S.; Mu, H.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol. Cancer Ther. 2008, 7, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-muc16 binding is a high affinity, n-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Joo, M.; Kim, H.; Chang, S. Mesothelin expression in gastric adenocarcinoma and its relation to clinical outcomes. J. Pathol. Transl. Med. 2017, 51, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Bodempudi, V.; Liu, Z.; Borrego-Diaz, E.; Yamoutpoor, F.; Meyer, A.; Woo, R.A.; Pan, W.; Dudek, A.Z.; Olyaee, M.S.; et al. Inhibition of mesothelin as a novel strategy for targeting cancer cells. PLoS ONE 2012, 7, e33214. [Google Scholar] [CrossRef]
- Yen, M.J.; Hsu, C.Y.; Mao, T.L.; Wu, T.C.; Roden, R.; Wang, T.L.; Shih Ie, M. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin. Cancer Res. 2006, 12, 827–831. [Google Scholar] [CrossRef] [Green Version]
- Hilliard, T.S. The impact of mesothelin in the ovarian cancer tumor microenvironment. Cancers 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen ca125/muc16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.F.; Huang, C.Y.; Chang, M.C.; Hu, Y.H.; Chiang, Y.C.; Chen, Y.L.; Hsieh, C.Y.; Chen, C.A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melaiu, O.; Stebbing, J.; Lombardo, Y.; Bracci, E.; Uehara, N.; Bonotti, A.; Cristaudo, A.; Foddis, R.; Mutti, L.; Barale, R.; et al. Msln gene silencing has an anti-malignant effect on cell lines overexpressing mesothelin deriving from malignant pleural mesothelioma. PLoS ONE 2014, 9, e85935. [Google Scholar]
- Coelho, R.; Ricardo, S.; Amaral, A.L.; Huang, Y.L.; Nunes, M.; Neves, J.P.; Mendes, N.; Lopez, M.N.; Bartosch, C.; Ferreira, V.; et al. Regulation of invasion and peritoneal dissemination of ovarian cancer by mesothelin manipulation. Oncogenesis 2020, 9, 61. [Google Scholar] [CrossRef]
- Bera, T.K.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell. Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive epithelial ovarian cancer survival by histotype and disease stage. J. Natl. Cancer Inst. 2018, 111, 60–68. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Oliphant, M.; Berne, A.S.; Feldberg, M.A. The peritoneal ligaments and mesenteries: Pathways of intraabdominal spread of disease. Radiology 1987, 163, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Coakley, F.V.; Hricak, H. Imaging of peritoneal and mesenteric disease: Key concepts for the clinical radiologist. Clin. Radiol. 1999, 54, 563–574. [Google Scholar] [CrossRef]
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef]
- Penet, M.F.; Krishnamachary, B.; Wildes, F.B.; Mironchik, Y.; Hung, C.F.; Wu, T.C.; Bhujwalla, Z.M. Ascites volumes and the ovarian cancer microenvironment. Front. Oncol. 2018, 8, 595. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Okla, K.; Surowka, J.; Fraszczak, K.; Czerwonka, A.; Kalawaj, K.; Wawruszak, A.; Kotarski, J.; Wertel, I. Assessment of the clinicopathological relevance of mesothelin level in plasma, peritoneal fluid, and tumor tissue of epithelial ovarian cancer patients. Tumour Biol. 2018, 40, 1010428318804937. [Google Scholar] [CrossRef] [Green Version]
- Yeung, T.L.; Leung, C.S.; Yip, K.P.; Au Yeung, C.L.; Wong, S.T.; Mok, S.C. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: Cell and molecular processes in cancer metastasis. Am. J. Physiol. Cell Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef] [Green Version]
- Kenny, H.A.; Chiang, C.Y.; White, E.A.; Schryver, E.M.; Habis, M.; Romero, I.L.; Ladanyi, A.; Penicka, C.V.; George, J.; Matlin, K.; et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J. Clin. Investig. 2014, 124, 4614–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klymenko, Y.; Bos, B.; Campbell, L.; Loughran, E.; Liu, Y.; Yang, J.; Kim, O.; Stack, M.S. Lysophosphatidic acid modulates ovarian cancer multicellular aggregate assembly and metastatic dissemination. Sci. Rep. 2020, 10, 10877. [Google Scholar] [CrossRef] [PubMed]
- Shishido, A.; Mori, S.; Yokoyama, Y.; Hamada, Y.; Minami, K.; Qian, Y.; Wang, J.; Hirose, H.; Wu, X.; Kawaguchi, N.; et al. Mesothelial cells facilitate cancer stemlike properties in spheroids of ovarian cancer cells. Oncol. Rep. 2018, 40, 2105–2114. [Google Scholar] [PubMed] [Green Version]
- Matte, I.; Legault, C.M.; Garde-Granger, P.; Laplante, C.; Bessette, P.; Rancourt, C.; Piche, A. Mesothelial cells interact with tumor cells for the formation of ovarian cancer multicellular spheroids in peritoneal effusions. Clin. Exp. Metastasis 2016, 33, 839–852. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Mesothelial cells. Perit. Dial. Int. 2007, 27, 110–115. [Google Scholar] [CrossRef]
- Madison, L.D.; Bergstrom-Porter, B.; Torres, A.R.; Shelton, E. Regulation of surface topography of mouse peritoneal cells. Formation of microvilli and vesiculated pits on omental mesothelial cells by serum and other proteins. J. Cell Biol. 1979, 82, 783–797. [Google Scholar] [CrossRef]
- Grosse-Gehling, P.; Fargeas, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, L.A. Cd133 as a biomarker for putative cancer stem cells in solid tumours: Limitations, problems and challenges. J. Pathol. 2013, 229, 355–378. [Google Scholar] [CrossRef]
- Thamm, K.; Simaite, D.; Karbanova, J.; Bermudez, V.; Reichert, D.; Morgenstern, A.; Bornhauser, M.; Huttner, W.B.; Wilsch-Brauninger, M.; Corbeil, D. Prominin-1 (cd133) modulates the architecture and dynamics of microvilli. Traffic 2019, 20, 39–60. [Google Scholar] [CrossRef]
- Basil-Jones, M.M.; Edmonds, R.L.; Norris, G.E.; Haverkamp, R.G. Collagen fibril alignment and deformation during tensile strain of leather: A small-angle X-ray scattering study. J. Agric. Food Chem. 2012, 60, 1201–1208. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Basil-Jones, M.M.; Edmonds, R.L.; Cooper, S.M.; Kirby, N.; Hawley, A.; Haverkamp, R.G. Collagen orientation and leather strength for selected mammals. J. Agric. Food Chem. 2013, 61, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel, C.H., 3rd; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H.; et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, S.; Foreman, K.M.; Soriano, M.I.; Rossen, N.S.; Shehade, H.; Fregoso, D.R.; Eggold, J.T.; Krishnan, V.; Dorigo, O.; Krieg, A.J.; et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res. 2019, 79, 2271–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar]
- Casey, R.C.; Burleson, K.M.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R., Jr.; Ruff, L.E.; Skubitz, A.P. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am. J. Pathol. 2001, 159, 2071–2080. [Google Scholar] [CrossRef]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef] [Green Version]
- Gunay, G.; Kirit, H.A.; Kamatar, A.; Baghdasaryan, O.; Hamsici, S.; Acar, H. The effects of size and shape of the ovarian cancer spheroids on the drug resistance and migration. Gynecol. Oncol. 2020, 159, 563–572. [Google Scholar] [CrossRef]
- Al Habyan, S.; Kalos, C.; Szymborski, J.; McCaffrey, L. Multicellular detachment generates metastatic spheroids during intra-abdominal dissemination in epithelial ovarian cancer. Oncogene 2018, 37, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Sodek, K.L.; Ringuette, M.J.; Brown, T.J. Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int. J. Cancer 2009, 124, 2060–2070. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Shi, Z.; Tan, X.; Jin, N.; O’Brien, C.; Ott, C.; Grisoli, A.; Lee, E.; Volk, K.; et al. In vivo selection of highly metastatic human ovarian cancer sublines reveals role for amigo2 in intra-peritoneal metastatic regulation. Cancer Lett. 2021, 503, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE 2015, 10, e0130348. [Google Scholar]
- Ordonez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef]
- Coelho, R.; Marcos-Silva, L.; Ricardo, S.; Ponte, F.; Costa, A.; Lopes, J.M.; David, L. Peritoneal dissemination of ovarian cancer: Role of muc16-mesothelin interaction and implications for treatment. Expert Rev. Anticancer 2018, 18, 177–186. [Google Scholar] [CrossRef]
- Asem, M.; Young, A.M.; Oyama, C.; De La Zerda, A.C.; Liu, Y.; Yang, J.; Hilliard, T.S.; Johnson, J.; Harper, E.I.; Guldner, I.; et al. Host wnt5a potentiates microenvironmental regulation of ovarian cancer metastasis. Cancer Res. 2020, 80, 1156–1170. [Google Scholar] [CrossRef]
- Liu, Y.; Metzinger, M.N.; Lewellen, K.A.; Cripps, S.N.; Carey, K.D.; Harper, E.I.; Shi, Z.; Tarwater, L.; Grisoli, A.; Lee, E.; et al. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of m1 macrophages. Cancer Res. 2015, 75, 5046–5057. [Google Scholar] [CrossRef] [Green Version]
- Loughran, E.A.; Phan, R.C.; Leonard, A.K.; Tarwater, L.; Asem, M.; Liu, Y.; Yang, J.; Klymenko, Y.; Johnson, J.; Shi, Z.; et al. Multiparity activates interferon pathways in peritoneal adipose tissue and decreases susceptibility to ovarian cancer metastasis in a murine allograft model. Cancer Lett. 2017, 411, 74–81. [Google Scholar] [CrossRef]
- Loughran, E.A.; Leonard, A.K.; Hilliard, T.S.; Phan, R.C.; Yemc, M.G.; Harper, E.; Sheedy, E.; Klymenko, Y.; Asem, M.; Liu, Y.; et al. Aging increases susceptibility to ovarian cancer metastasis in murine allograft models and alters immune composition of peritoneal adipose tissue. Neoplasia 2018, 20, 621–631. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Powell, D.J., Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Bohm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A strong b-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Yull, F.; Khabele, D. Bipolar tumor-associated macrophages in ovarian cancer as targets for therapy. Cancers 2018, 10, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.; Li, P. Mesothelin as a biomarker for targeted therapy. Biomark. Res. 2019, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Weekes, C.D.; Lamberts, L.E.; Borad, M.J.; Voortman, J.; McWilliams, R.R.; Diamond, J.R.; de Vries, E.G.; Verheul, H.M.; Lieu, C.H.; Kim, G.P.; et al. Phase i study of dmot4039a, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol. Cancer Ther. 2016, 15, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Wickstroem, K.; Hagemann, U.B.; Cruciani, V.; Wengner, A.M.; Kristian, A.; Ellingsen, C.; Siemeister, G.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; et al. Synergistic effect of a mesothelin-targeted (227)th conjugate in combination with DNA damage response inhibitors in ovarian cancer xenograft models. J. Nucl. Med. 2019, 60, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholler, N.; Sharma, K.; Yin, C.; Kamat, K.; Fernando, R.I.; Sei, S.; Shoemaker, R.H.; Stein, P.L.; Sambucetti, L. Development of a mesothelin-based prophylactic vaccine against ovarian cancer. J. Immunol. 2018, 200, 181.23. [Google Scholar]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A live-attenuated listeria vaccine (anz-100) and a live-attenuated listeria vaccine expressing mesothelin (crs-207) for advanced cancers: Phase i studies of safety and immune induction. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Klymenko, Y.; Kim, O.; Loughran, E.; Yang, J.; Lombard, R.; Alber, M.; Stack, M.S. Cadherin composition and multicellular aggregate invasion in organotypic models of epithelial ovarian cancer intraperitoneal metastasis. Oncogene 2017, 36, 5840–5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudaoud, A.; Burian, A.; Borowska-Wykret, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. Fibriltool, an imagej plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Worthmuller-Rodriguez, J.; Wu, L.; Vrugt, B.; de Perrot, M.; Schwaller, B. Establishment of immortalized murine mesothelial cells and a novel mesothelioma cell line. Vitr. Cell Dev. Biol. Anim. 2015, 51, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lewellen, K.A.; Metzinger, M.N.; Liu, Y.; Stack, M.S. Quantitation of intra-peritoneal ovarian cancer metastasis. J. Vis. Exp. 2016, 113, e53316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilliard, T.S.; Kowalski, B.; Iwamoto, K.; Agadi, E.A.; Liu, Y.; Yang, J.; Asem, M.; Klymenko, Y.; Johnson, J.; Shi, Z.; et al. Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment. Int. J. Mol. Sci. 2021, 22, 12443. https://doi.org/10.3390/ijms222212443
Hilliard TS, Kowalski B, Iwamoto K, Agadi EA, Liu Y, Yang J, Asem M, Klymenko Y, Johnson J, Shi Z, et al. Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment. International Journal of Molecular Sciences. 2021; 22(22):12443. https://doi.org/10.3390/ijms222212443
Chicago/Turabian StyleHilliard, Tyvette S., Brooke Kowalski, Kyle Iwamoto, Elizabeth A. Agadi, Yueying Liu, Jing Yang, Marwa Asem, Yuliya Klymenko, Jeff Johnson, Zonggao Shi, and et al. 2021. "Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment" International Journal of Molecular Sciences 22, no. 22: 12443. https://doi.org/10.3390/ijms222212443
APA StyleHilliard, T. S., Kowalski, B., Iwamoto, K., Agadi, E. A., Liu, Y., Yang, J., Asem, M., Klymenko, Y., Johnson, J., Shi, Z., Marfowaa, G., Yemc, M. G., Petrasko, P., & Stack, M. S. (2021). Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment. International Journal of Molecular Sciences, 22(22), 12443. https://doi.org/10.3390/ijms222212443





