Comparison of Cr(VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism
Abstract
1. Introduction
2. Results and Discussion
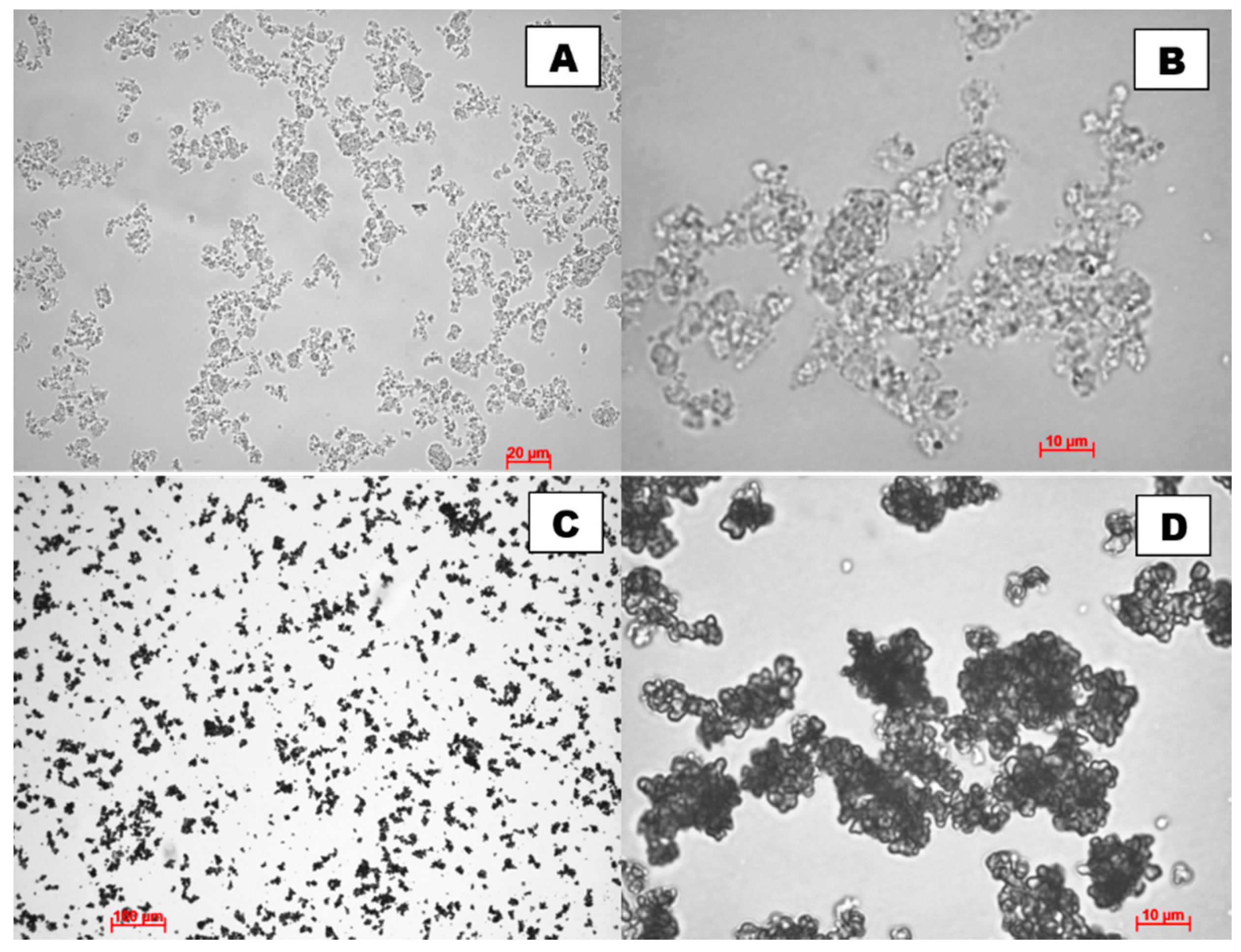
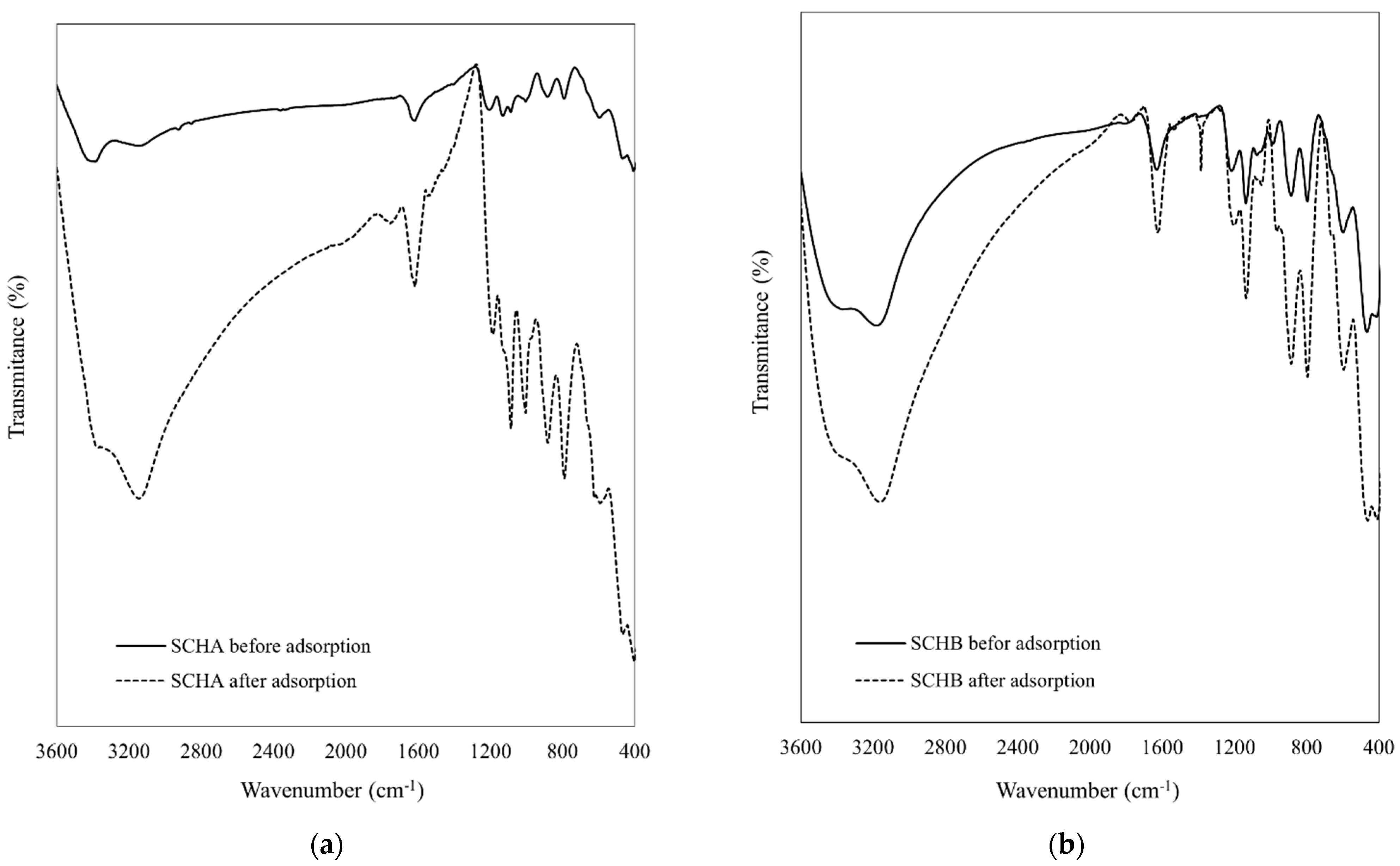
2.1. Characterisation of Adsorbents
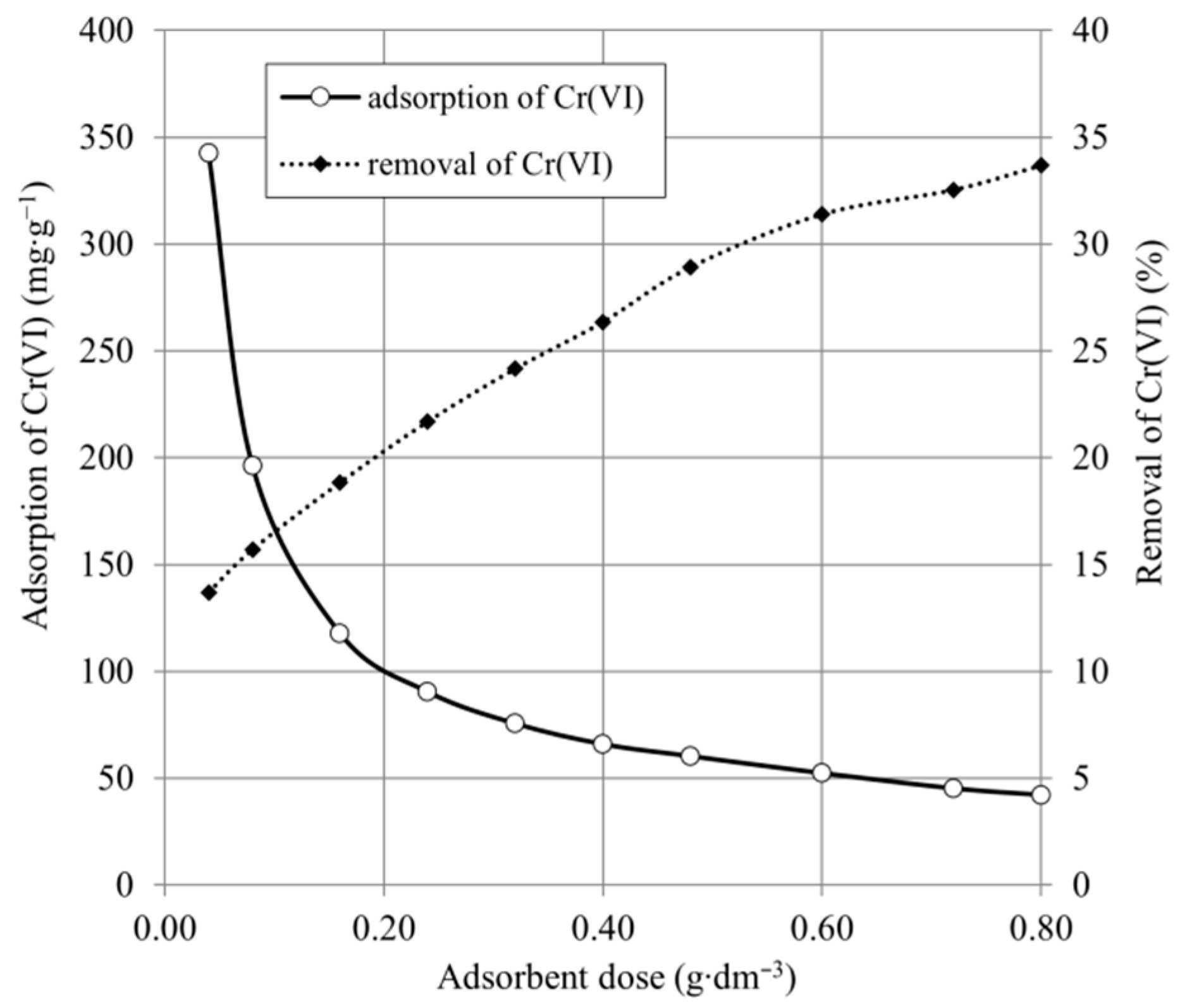
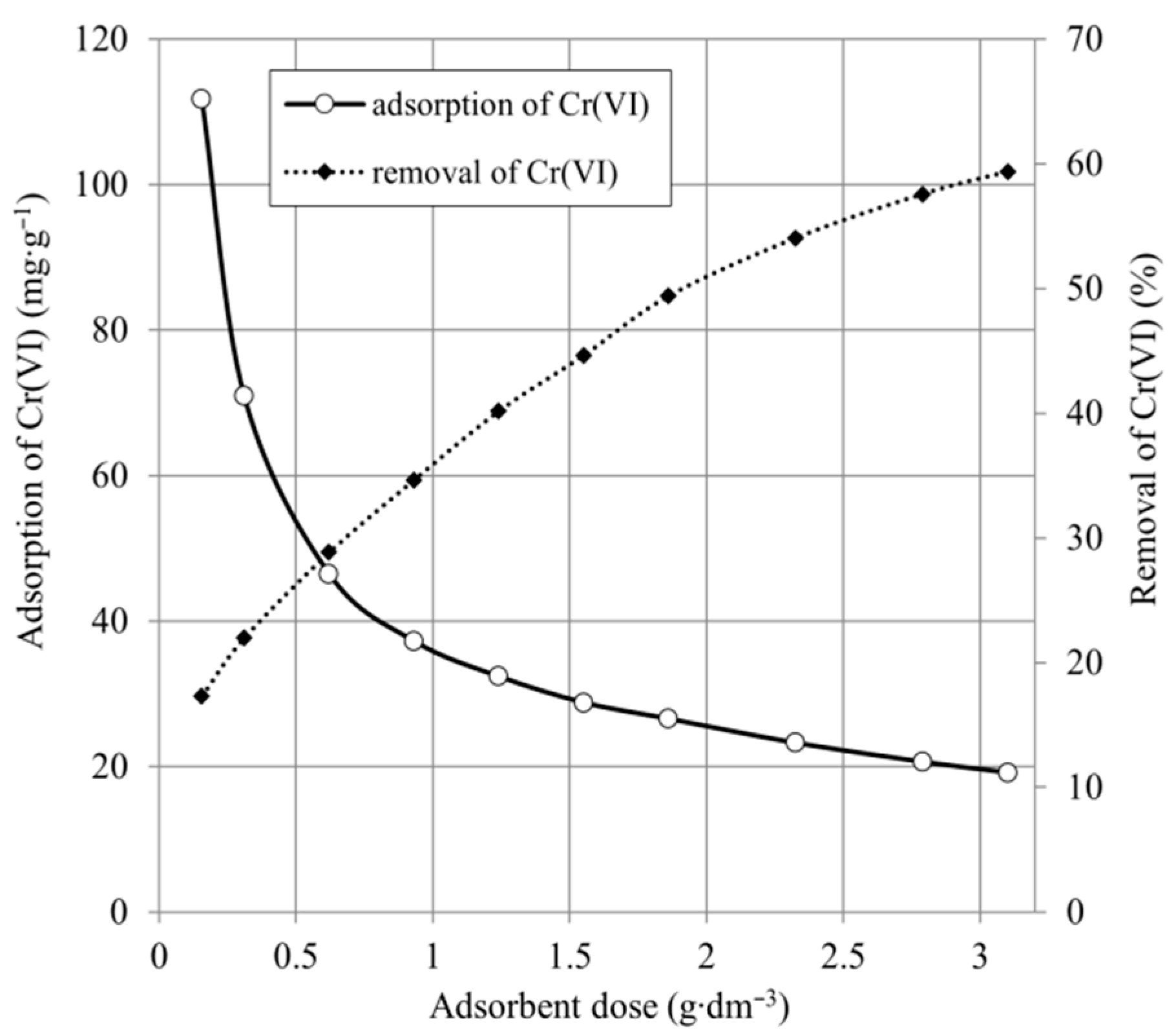
2.2. Effect of Adsorbent Dose
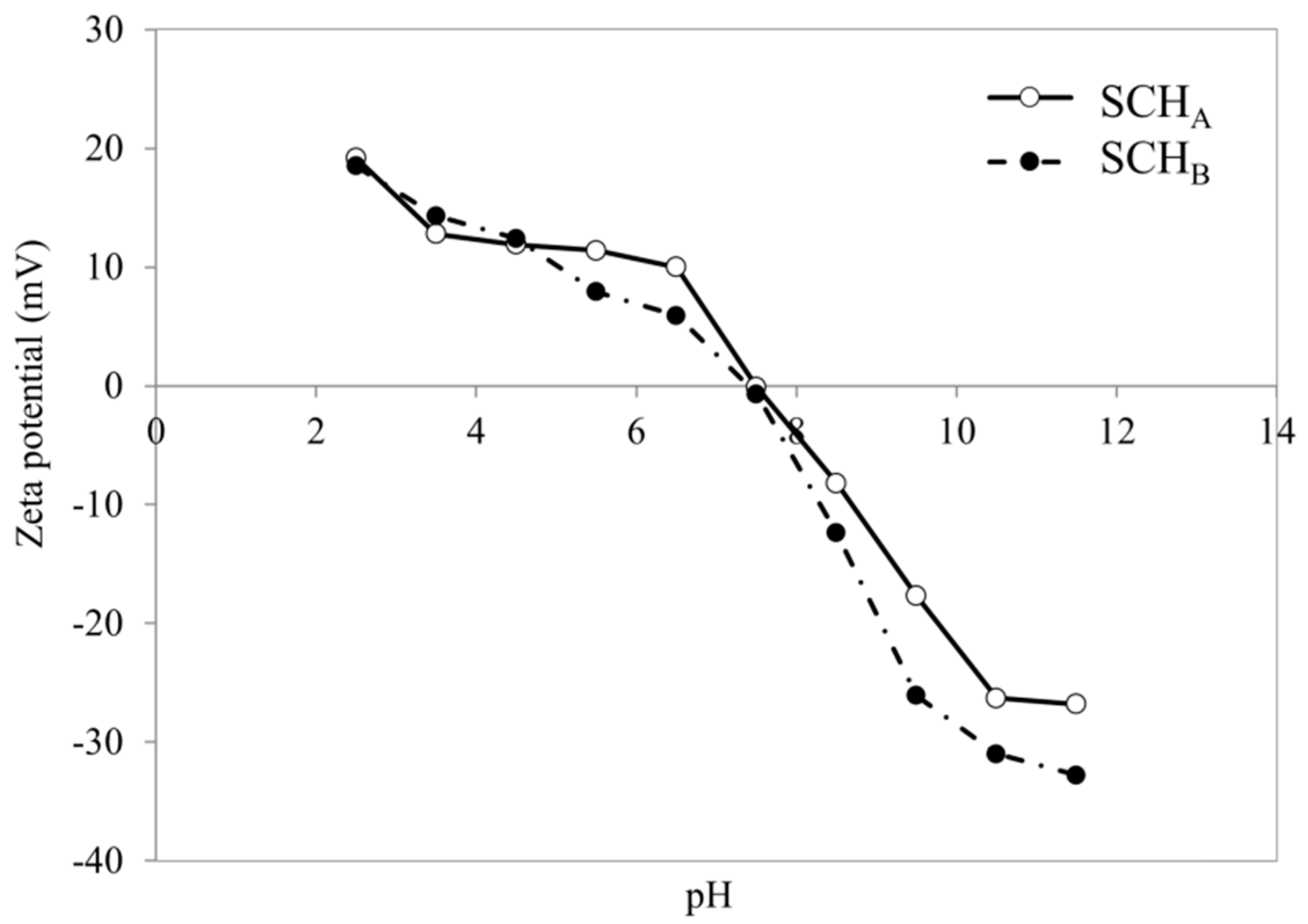
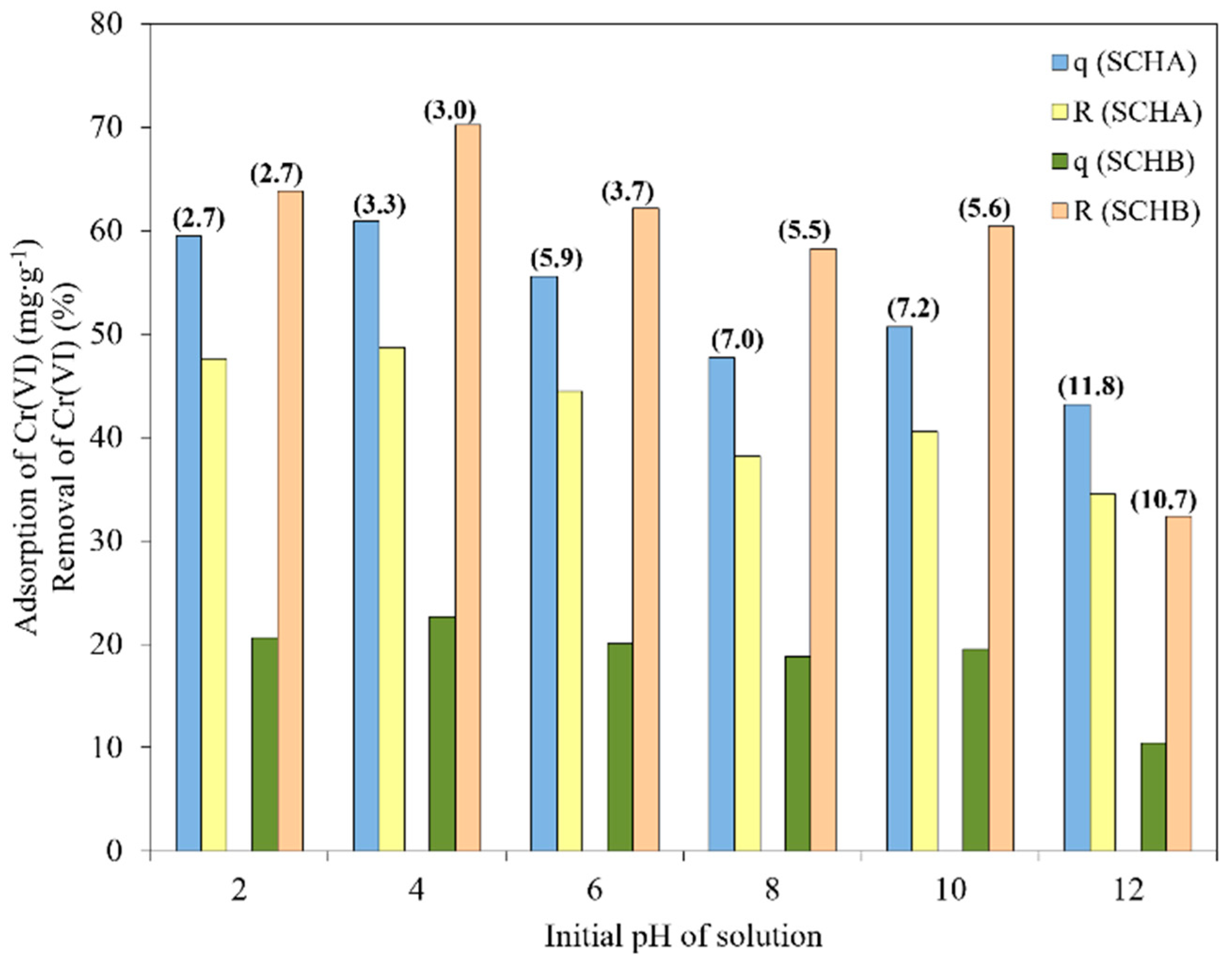
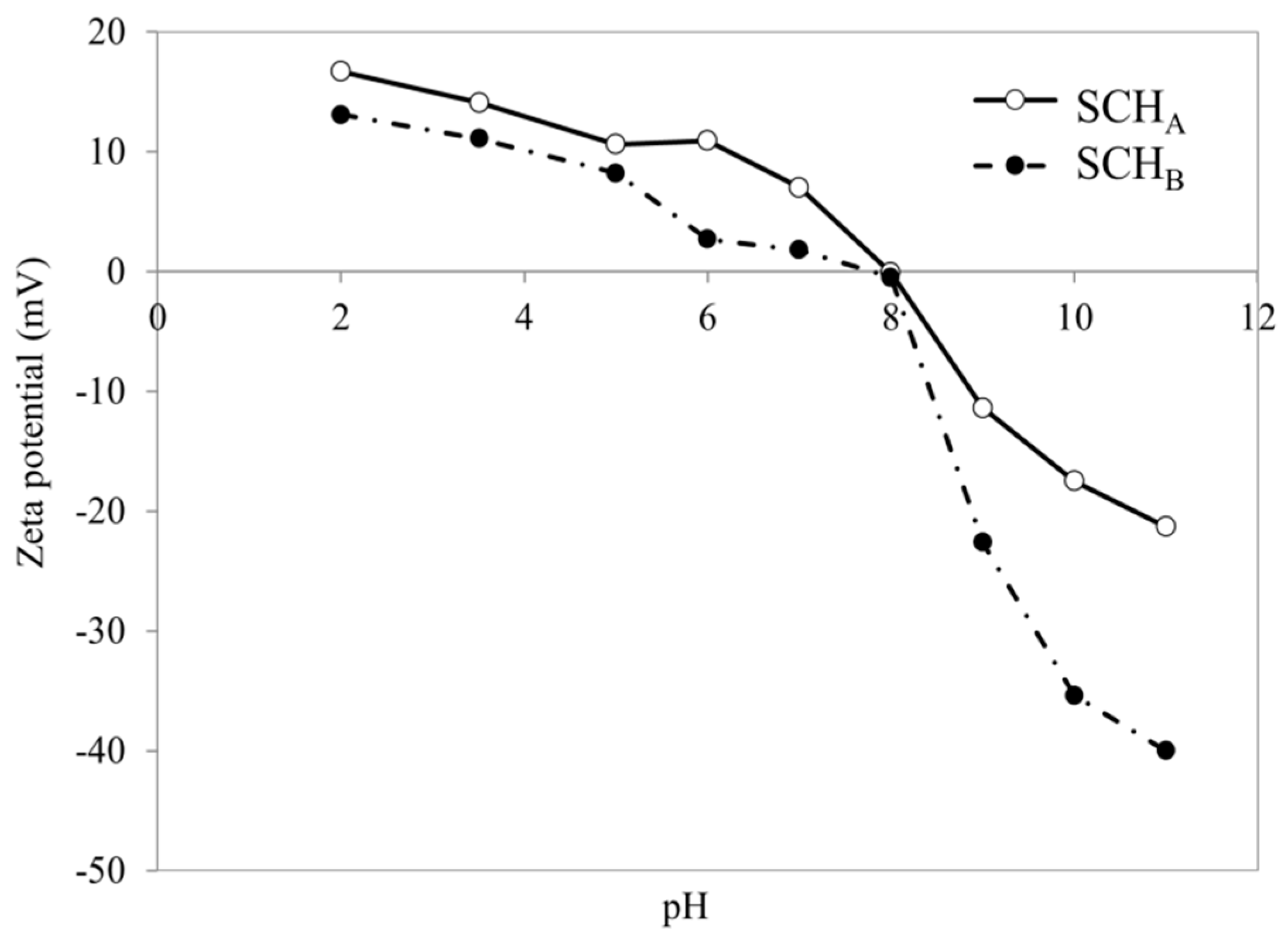
2.3. Effect of pH
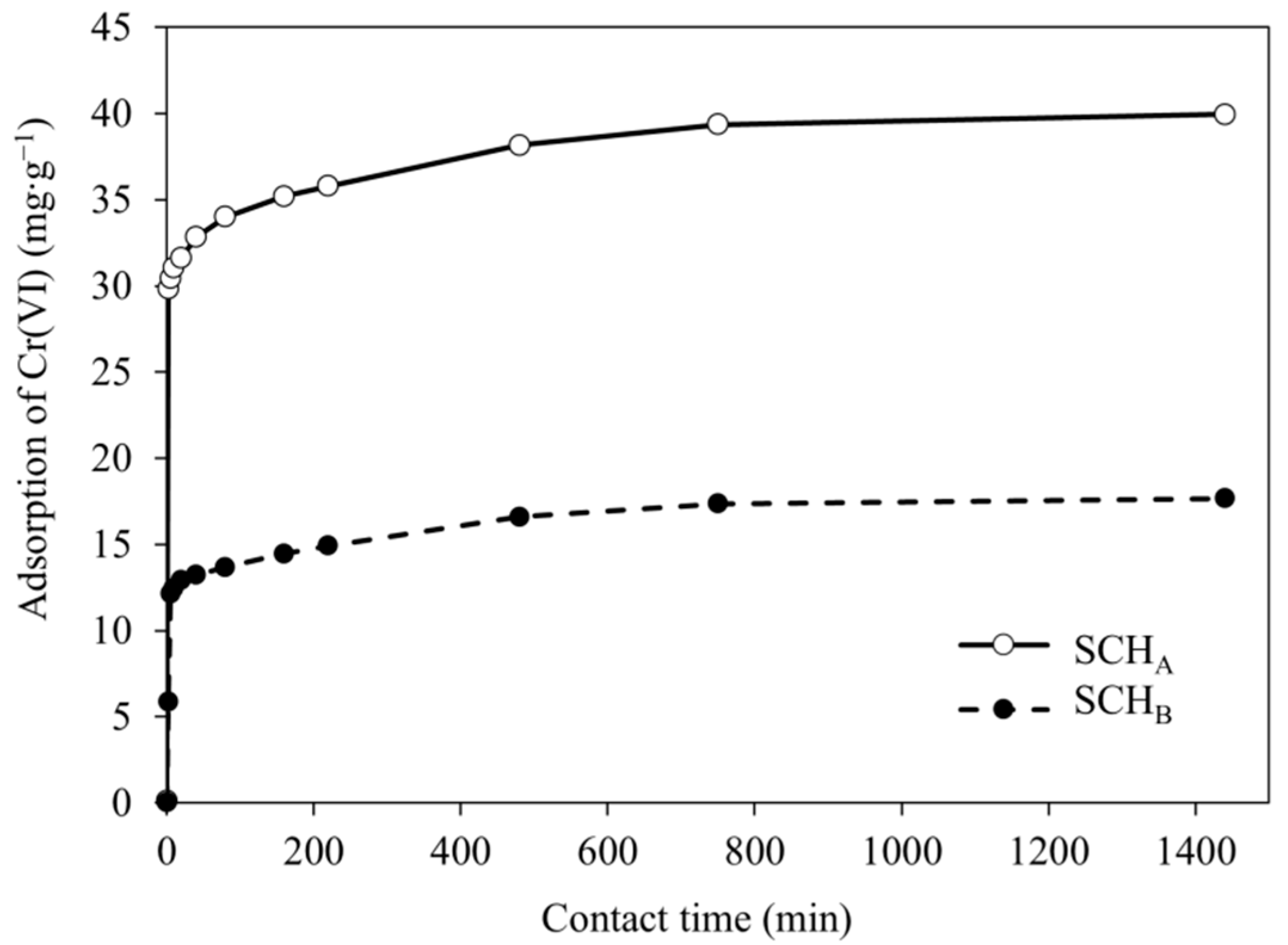
2.4. Effect of Contact Time
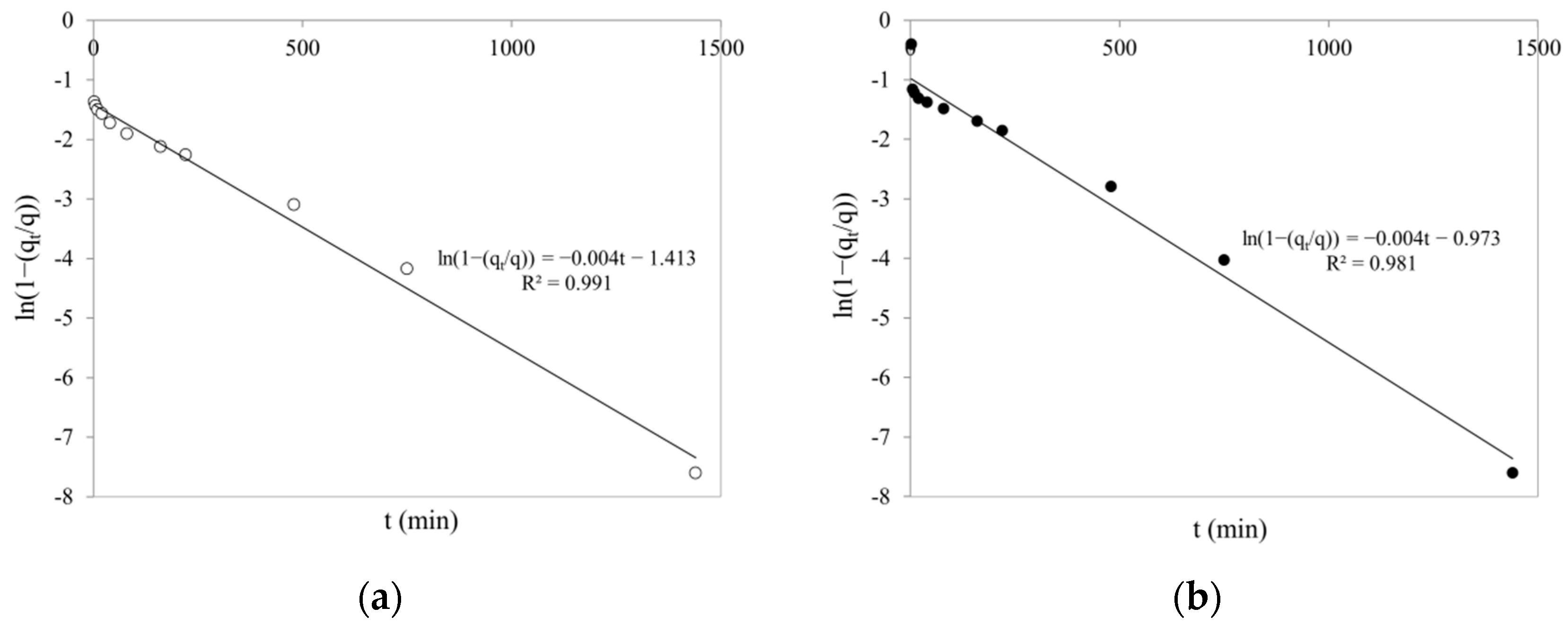
Adsorption Kinetic Models
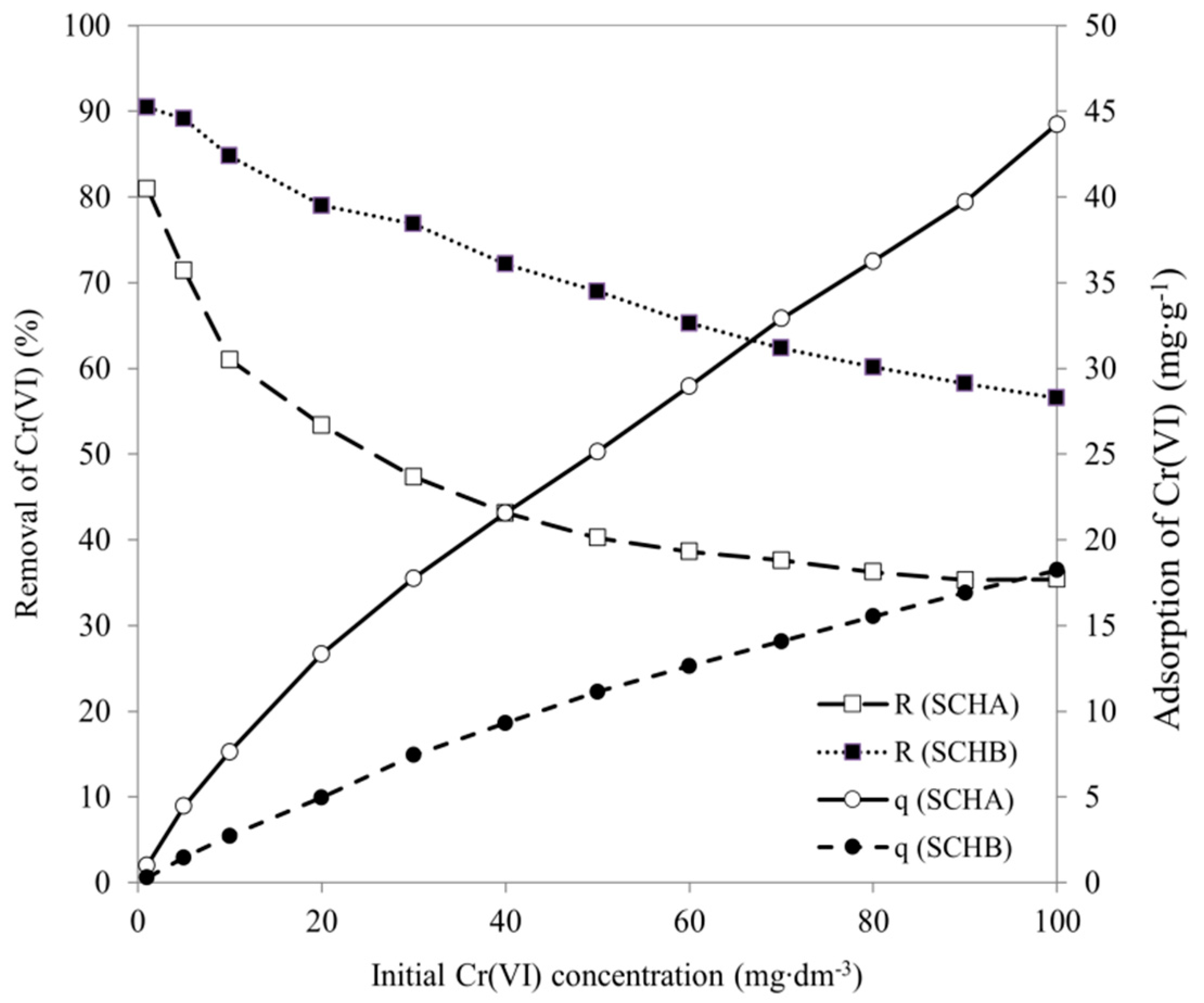
2.5. Effect of Initial Cr(VI) Concentration
Adsorption Isotherm Models
2.6. Comparison with Other Studies
2.7. Possible Cr(VI) Adsorption Mechanism
3. Materials and Methods
3.1. Preparation of Schwertmannite Sorbents
3.2. Characterisation of Schwertmannite
3.3. Adsorption Experiments
4. Conclusions
- Compared with literature data, both SCHA and SCHB are efficient adsorbents for Cr(VI) removal.
- SCHA has a higher adsorption capacity than SCHB, despite its smaller specific surface area.
- The well-developed specific surface area suggests that schwertmannite (SCHA and SCHB) can be a good Cr(VI) sorbents, especially in acidic conditions.
- Adsorption of Cr(VI) on schwertmannite sorbents should be carried out at pH of around 4 as the removal of Cr(VI) is the most efficient under those conditions.
- The IPD model suggested that intraparticle diffusion is not the only rate-limiting step in Cr(VI) adsorption on schwertmannite.
- The PSO model describes the adsorption kinetics of Cr(VI) on SCHA and SCHB better than the PFO model.
- The sorption isotherm was well described by Freundlich and Langmuir models.
- According to the Langmuir model, the maximum adsorption capacity of Cr(VI) at low initial concentration is 42.97 and 17.54 mg·g−1 for SCHA and SCHB, respectively, and at high initial concentrations is 201.8 and 131.8 mg·g−1 for SCHA and SCHB, respectively.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| t | time (min) |
| qe | the amount of Cr(VI) adsorbed at equilibrium (mg·g−1) |
| qt | the amount of Cr(VI) adsorbed at time t (mg·g−1) |
| ce | the equilibrium concentration of Cr(VI) (mg·dm−3) |
| c0 | the initial concentration of Cr(VI) (mg·dm−3) |
| m | the adsorbent mass (g) |
| V | the solution volume (dm3) |
| q1 | the adsorption capacity of Cr(VI) at equilibrium for pseudo-first-order model (mg·g−1) |
| k1 | the rate constant of pseudo-first-order model (min−1) |
| q2 | the adsorption capacity of Cr(VI) at equilibrium for pseudo-second-order model (mg·g−1) |
| k2 | the rate constant of pseudo-second-order model (g·mg−1·min−1) |
| kIPD | the intraparticle diffusion rate constant (mg·g−1·min−1/2) |
| B | the parameter related to the thickness of the boundary layer (mg·g−1) |
| kLFD | the external mass transfer coefficient (min−1) |
| kF | the Freundlich constant indicative of the relative adsorption capacity of the adsorbent ((dm3)1/n·mg(1−1/n)·g−1) |
| n | the Freundlich equation exponent (-) |
| qL | the maximum adsorption capacity in Langmuir model (mg·g−1) |
| kL | the Langmuir constant related to the energy of adsorption (dm3·mg−1) |
| R | the universal gas constant (kJ·mol−1·K−1) |
| T | temperature (K) |
| BT | the Temkin constant related to heat of sorption (kJ·mol−1) |
| kT | the Temkin equilibrium isotherm constant (dm3·g−1) |
| RL | the separation parameter (-) |
| q | the amount of Cr(VI) adsorbed (mg·g−1) |
| c | the concentration of Cr(VI) (mg·dm−3) |
References
- Aris, A.Z.; Lim, W.Y.; Looi, L.J. Natural and Anthropogenic Determinants of Freshwater Ecosystem Deterioration: An Environmental Forensic Study of the Langat River Basin, Malaysia. In Environmental Management of River Basin Ecosystems; Ramkumar, M., Kumaraswamy, K., Mohanraj, R., Eds.; Springer Earth System Sciences: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Markert, B.; Kayser, G.; Korhammer, S.; Oehlmann, J. Distribution and effects of trace substances in soils, plants and animals. Trace Met. Environ. 2000, 4, 3–31. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals, Interdisciplinary Toxicology. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Bochniarz, Z. The Ecological Disaster in Eastern Europe: Background, Current Aspects and Suggestions for the Future. Pol. Rev. 1992, 37, 5–25. Available online: http://www.jstor.org/stable/25778608 (accessed on 9 March 2021).
- Greenwood, N.N.; Earnshaw, A. Chromium, molybdenum and tungsten. Chem. Elem. 1997, 1002–1039. [Google Scholar] [CrossRef]
- Mohanty, M.; Patra, H.K. Effect of Ionic and Chelate Assisted Hexavalent Chromium on Mung Bean Seedlings (Vigna radiata L. wilczek. var k-851) During Seedling Growth. J. Stress Physiol. Biochem. 2013, 9, 232–241. [Google Scholar]
- Gusain, D.; Verma, V.; Uma; Bux, F.; Sharma, Y.C. A novel approach for the removal of chromium (VI) from aqueous solutions using nano iron oxide. Int. J. Environ. Anal. Chem. 2020, 1–16. [Google Scholar] [CrossRef]
- Boddu, S.; Alugunulla, V.N.; Dulla, J.B.; Pilli, R.R.; Khan, A.A. Estimation of biosorption characteristics of chromium (VI) from aqueous and real tannery effluents by treated T. vulgaris: Experimental assessment and statistical modelling. Int. J. Environ. Anal. Chem. 2020, 1–20. [Google Scholar] [CrossRef]
- Minas, F.; Chandravanshi, B.S.; Leta, S. Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem. Int. 2017, 3, 291–305. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Lei, X.; Lu, C.; Liu, J.; Zhang, G.; Zhang, B.; Zhang, Q. A magnetic ion exchange resin with high efficiency of removing Cr(VI). Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125279. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Mia, M.A.S.; Ahmad, F.; Rahman, M.M. An overview of chromium removal techniques from tannery effluent. Appl. Water Sci. 2020, 10, 205. [Google Scholar] [CrossRef]
- Jahangiri, K.; Yousefi, N.; Ghadiri, S.K.; Fekri, R.; Bagheri, A.; Talebi, S.S. Enhancement adsorption of hexavalent chromium onto modified fly ash from aqueous solution; optimization; isotherm, kinetic and thermodynamic study. J. Dispers. Sci. Technol. 2019, 40, 1147–1158. [Google Scholar] [CrossRef]
- Deng, X.; Qi, L.; Zhang, Y. Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash. Water Air Soil Pollut 2018, 229, 18. [Google Scholar] [CrossRef]
- Pengthamkeerati, P.; Satapanajaru, T.; Chularuengoaksorn, P. Chemical modification of coal fly ash for the removal of phosphatefrom aqueous solution. Fuel 2008, 87, 2469–2476. [Google Scholar] [CrossRef]
- Zachara, J.M.; Glrvin, D.C.; Schmidt, R.L.; Resch, T.C. Chromate Adsorption on Amorphous Iron Oxyhydroxide in the Presence of Major Groundwater Ions. Environ. Sci. Technol. 1987, 21, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hingston, F.J.; Posner, A.M.; Quirk, J.P. Competitive adsorption of negatively charged ligands on oxide surfaces. Discuss. Faraday Soc. 1971, 52, 334–342. [Google Scholar] [CrossRef]
- Leckie, J.O.; Benjamin, M.M.; Hayes, K.; Kaufman, G.; Altmann, S. Adsorption/Coprecipitation of Trace Elements from Water with Iron Oxyhydroxide; Environmental Engineering and Science, Stanford University: Stanford, CA, USA, 1980. [Google Scholar] [CrossRef]
- Eary, L.E.; Rai, D. Chromate removal from aqueous wastes by reduction with ferrous ion. Environ. Sci. Technol. 1988, 22, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Stjernström, M.; Banwart, S. Accumulation and remobilization of aqueous chromium(VI) at iron oxide surfaces: Application of a thin-film continuous flow-through reactor. J. Contam. Hydrol. 1996, 21, 141–151. [Google Scholar] [CrossRef]
- Jabasingh, S.A.; Belachew, H.; Yimam, A. Iron oxide induced bagasse nanoparticles for the sequestration of Cr6+ ions from tannery effluent using a modified batch reactor. J. Appl. Polym. Sci. 2018, 135, 46683–46698. [Google Scholar] [CrossRef]
- Chagas, P.M.B.; Caetano, A.A.; Rossi, M.A.; Gonçalves, M.A.; de Castro Ramalho, T.; Corrêa, A.D.; Guimarães, I.R. Chitosan-iron oxide hybrid composite: Mechanism of hexavalent chromium removal by central composite design and theoretical calculations. Environ. Sci. Pollut. Res. 2019, 26, 15973–15988. [Google Scholar] [CrossRef]
- Regenspurg, S.; Peiffer, S. Arsenate and chromate incorporation in schwertmannite. Appl. Geochem. 2005, 20, 1226–1239. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, X.; Li, X.; Zhao, Q.; Chen, H. Schwertmannite: Occurrence, properties, synthesis and application in environmental remediation. RSC Adv. 2018, 8, 33583–33599. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, C.; Li, K.; Sun, T.; Shen, Z.; Jia, J. Arsenic(V) removal behavior of schwertmannite synthesized by KMnO4 rapid oxidation with high adsorption capacity and Fe utilization. Chemosphere 2021, 264, 128398. [Google Scholar] [CrossRef] [PubMed]
- Khamphila, K.; Kodama, R.; Sato, T.; Otake, T. Adsorption and post adsorption behavior of schwertmannite with various oxyanions. J. Miner. Mater. Charact. Eng. 2017, 5, 90–106. [Google Scholar] [CrossRef]
- Gan, M.; Zheng, Z.; Sun, S.; Zhu, J.; Liu, X. The influence of aluminum chloride on biosynthetic schwertmannite and Cu(II)/Cr(VI) adsorption. RSC Adv. 2015, 5, 94500–94512. [Google Scholar] [CrossRef]
- Song, J.; Jia, S.Y.; Ren, H.T.; Wu, S.H.; Han, X. Application of a high-surface-area schwertmannite in the removal of arsenate and arsenite. Int. J. Environ. Sci. Technol. 2015, 12, 1559–1568. [Google Scholar] [CrossRef]
- Goswami, A.; Purkait, M.K. Removal of fluoride from drinking water using nanomagnetite aggregated schwertmannite. J. Water Process Eng. 2014, 1, 91–100. [Google Scholar] [CrossRef]
- Dou, X.; Mohan, D.; Pittman, C.U., Jr. Arsenate adsorption on three types of granular schwertmannite. Water Res. 2013, 47, 2938–2948. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Watling, K.M.; Hocking, R.K.; Sullivan, L.A.; Parker, G.K. Sorption of Arsenic(V) and Arsenic(III) to Schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef]
- Jönsson, J.; Sjöberg, S.; Lövgren, L. Adsorption of Cu(II) to schwertmannite and goethite in presence of dissolved organic matter. Water Res. 2006, 40, 969–974. [Google Scholar] [CrossRef]
- Kawano, M.; Obokata, S.; Tomita, K. Surface reactive sites and ion adsorption modeling of schwertmannite. Clay Sci. 2006, 45, 223–232. [Google Scholar] [CrossRef]
- Merkus, H.G. Particle Size Measurements. Fundamentals, Practice, Quality; Springer Science: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Xie, Y.; Lu, G.; Yang, C.; Qu, L.; Chen, M.; Guo, C.; Dang, Z. Mineralogical characteristics of sediments and heavy metal mobilization along a river watershed affected by acid mine drainage. PLoS ONE 2018, 13, 1–17. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, C.; Zhuang, J.; Zheng, G.; Zhou, L. Assessment of schwertmannite, jarosite and goethite as adsorbents for efficient adsorption of phenanthrene in water and the regeneration of spent adsorbents by heterogeneous fenton-like reaction. Chemosphere 2020, 244, 125523. [Google Scholar] [CrossRef]
- Li, J.; Xie, Y.; Lu, G.; Ye, H.; Yi, X.; Reinfelder, J.R.; Lin, Z.; Dang, Z. Effect of Cu(II) on the stability of oxyanion—Substituted schwertmannite. Environ. Sci. Pollut. Res. 2018, 25, 15492–15506. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zhou, B.; Wang, S.; Fan, L.; Hu, S.; Wu, Y. Adsorption performance and mechanism of synthetic schwertmannite to removal low-concentration fluorine in water. Bull. Environ. Contam. Toxicol. 2021, 1–11. [Google Scholar] [CrossRef]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. As(III) retention kinetics, equilibrium and redox stability on biosynthesized schwertmannite and its fate and control on schwertmannite stability on acidic (pH 3.0) aqueous exposure. Chemosphere 2012, 86, 557–564. [Google Scholar] [CrossRef]
- Reichelt, L.; Bertau, M. Production of ferrihydrite and schwertmannite using a microjet mixer device. Chem. Eng. Res. Des. 2015, 98, 70–80. [Google Scholar] [CrossRef]
- Ying, H.; Feng, X.; Zhu, M.; Lanson, B.; Liu, F.; Wang, X. Formation and transformation of schwertmannite through direct Fe3+ hydrolysis under various geochemical conditions. Environ. Sci. Nano 2020, 7, 2385–2398. [Google Scholar] [CrossRef]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acidic mining lakes. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- Vithana, C.L.; Sullivan, L.A.; Bush, R.T.; Burton, E.D. Schwertmannite in soil materials: Limits of detection of acidified ammonium oxalate method and differential X-ray diffraction. Geoderma 2015, 249–250, 51–60. [Google Scholar] [CrossRef]
- Regenspurg, S. Characterisation of Schwertmannite—Geochemical Interactions with Arsenate and Chromate and Significance in Sediments of Lignite Opencast Lakes. Ph.D. Thesis, Lehrstuhl für Hydrologie der Fakultät für Chemie, Biologie und Geowissenschaften, der Universität Bayreuth, Bayreuth, Germany, 2002. [Google Scholar]
- Liu, F.W.; Zhou, J.; Zhang, S.S.; Liu, L.L.; Zhou, L.X.; Fan, W.H. Schwertmannite Synthesis through Ferrous Ion Chemical Oxidation under Different H2O2 Supply Rates and Its Removal Efficiency for Arsenic from Contaminated Groundwater. PLoS ONE 2015, 10, e0138891. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dou, X.; Mohan, D.; Pittman, C.U., Jr.; Luo, J. Synthesis of graphene oxide/schwertmannite nanocomposites and their application in Sb(V) adsorption from water. Chem. Eng. J. 2015, 270, 205–214. [Google Scholar] [CrossRef]
- Eskandarpour, A.; Onyango, M.S.; Tananhashi, M.; Ochieng, A.; Bando, Y.; Iwai, K.; Okado, M.; Asai, S. Magnetic Fixed-bed Column for Cr(VI) Removal from Aqueous Solution Using Schwertmannite. ISIJ Int. 2008, 48, 240–244. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Mohan, D.; Pittman, C.U., Jr.; Ok, Y.S.; Dou, X.M. Removal of antimonate and antimonite from water by schwertmannite granules. Desalin. Water Treat. 2016, 57, 25639–25652. [Google Scholar] [CrossRef]
- Wang, W.M.; Song, J.; Han, X. Schwertmannite as a new Fenton-like catalyst in the oxidation of phenol by H2O2. J. Hazard. Mater. 2013, 262, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical speciation of chromium in water: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr(VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2017, 60, 012010. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, G.; Li, X.; Zhao, Q.; Bi, X.; Wu, K.; Chen, H. Effects of hydrogen-peroxide supply rate on schwertmannite microstructure and chromium(VI) adsorption performance. J. Hazard. Mater. 2019, 367, 520–528. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der Sogenannten adsorption geloster stoffe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–24. [Google Scholar]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Senturk, E.; Ozkaz, T. Adsorption kinetics for the removal of chromium(VI) from aqueous solutions on the activated carbons prepared from agricultural waste. Water SA 2004, 30, 533–540. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in water pollution research: Removal of biologically resistant pollutants from waste waters by adsorption. In Proceedings of the International Conference on Water Pollution Symposium; Pregamon Press: Oxford, UK, 1962; Volume 2, pp. 231–266. [Google Scholar]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ.-Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites: II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. A comparison of chemisorptions kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76B, 332–340. [Google Scholar] [CrossRef]
- Aroua, M.K.; Daud, W.M.A.W.; Yin, C.Y.; Adinata, D.J.S. Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep. Purif. Technol. 2008, 62, 609–613. [Google Scholar] [CrossRef]
- Gorzin, F.; Bahri Rasht Abadi, M.M. Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Al-Ghouki, M.A.; Daana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Samarghandi, M.R.; McKay, G. Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: Study of residual errors. Chem. Eng. J. 2010, 160, 408–416. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundamen. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hammed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Antelo, J.; Fiol, S.; Gondar, D.; López, R.; Arce, F. Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs. anion-exchange. J Colloid Interface Sci. 2012, 386, 338–343. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Wu, X.; Lan, Y.; Zhou, L. Cr(VI) removal by biogenic schwertmannite in continous flow column. Geochem. J. 2014, 48, 1–7. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Wang, H. Initial pH and K+ concentrations jointly determine the types of biogenic ferric hydroxysulfate minerals and their effect on adsorption removal of Cr(VI) in simulated acid mine drainage. Water Sci. Technol. 2018, 78, 2183–2191. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Gan, M. Controllable biosynthesis of nanoscale schwertmannite and the application in heavy metal effective removal. Appl. Surf. Sci. 2020, 529, 147012. [Google Scholar] [CrossRef]
- Paikaray, S.; Schröder, C.; Peiffer, S. Schwertmannite stability in anoxic Fe(II)—Rich aqueous solution. Geochim. Cosmochim. Acta 2017, 217, 292–305. [Google Scholar] [CrossRef]
- Boily, J.F.; Gassman, P.L.; Pevetyahko, T.; Szanyi, J.; Zachora, J.M. FTIR spectral components of schwertmannite. Environ. Sci. Technol. 2010, 44, 1185–1190. [Google Scholar] [CrossRef]
- Hoffman, M.M.; Darab, J.G.; Fulton, J.L. An infrared and X–ray absorption study of the equilibria and structures of chromate, bichromate, and dichromate in ambient aqueous solutions. J. Phys. Chem. A 2001, 105, 1772–1782. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Xia, L.; Kendig, M.W.; McCreery, R.L. Raman spectroscopic analysis of the speciation of dilute chromate solutions. Corros. Sci. 2001, 43, 1557–1572. [Google Scholar] [CrossRef]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent applications of vibrational mid-Infrared (IR) spectroscopy for studying soil components: A review. J. Cent. Eur. Agric. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Bigham, J.M.; Schwertmann, U.; Carlson, I.; Murad, E. A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in acid mine waters. Geochim. Cosmochim. Acta 1990, 54, 2743–2758. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]




| Model | Equation | Parameters | Relationship | Reference |
|---|---|---|---|---|
| Pseudo-first-order (PFO) | log(qe − qt) = log(q1) − (k1/2.303)·t (2) | q1 (mg·g−1) k1 (min−1) | log(qe − qt) vs. t | [53,54] |
| Pseudo-second-order (PSO) | (t/qt) = (1/(k2·q22)) + (1/q2)·t (3) | q2 (mg·g−1) k2 (g·mg−1·min−1) | t/qt vs. t | [55,56] |
| Intraparticle diffusion (IPD) | qt = kIPD·t1/2 + B (4) | kIPD (mg·g−1·min−1/2) B (mg·g−1) | qt vs. t1/2 | [57,58] |
| Liquid film diffusion (LFD) | ln(1 − qt/qe) = − kLFD·t (5) | kLFD (min−1) | ln(1 − qt/qe) vs. t | [58,59] |
| Adsorbent | PFO | PSO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qexp (mg·g−1) | k1 (min−1) | q1 (mg·g−1) | R2 | SD | k2 (g·mg−1·min−1) | q2 (mg·g−1) | R2 | SD | |
| SCHA | 39.98 | 0.0043 | 11.49 | 0.949 | 20.1 | 0.0026 | 39.97 | 0.999 | 0.007 |
| SCHB | 17.69 | 0.0046 | 7.49 | 0.964 | 7.21 | 0.0007 | 17.72 | 0.999 | 0.022 |
| Adsorbent | IPD | LFD | ||||||
|---|---|---|---|---|---|---|---|---|
| kIPD1 (mg·g−1·min−0.5) | B1 (mg·g−1) | R2 | kIPD2 (mg·g−1·min−0.5) | B2 (mg·g−1) | R2 | kLFD (min−1) | R2 | |
| SCHA | 0.1458 | 30.04 | 0.957 | 0.0084 | 34.88 | 0.798 | 0.0041 | 0.991 |
| SCHB | 0.0587 | 12.14 | 0.907 | 0.0057 | 14.30 | 0.776 | 0.0044 | 0.981 |
| Isotherm Model | Equation | Parameters | Reference |
|---|---|---|---|
| Freundlich | qe = kF·ce1/n (6) | kF ((dm3)1/n·mg(1−1/n)·g−1) n (−) | [63,64] |
| Langmuir | qe = (qL·kL·ce)/(1 + kL·ce) (7) | qL (mg·g−1) kL (dm3·mg−1) | [64,65] |
| Temkin | qe = (R·T/BT)·ln(kT·ce) (8) | BT (kJ·mol−1) kT (dm3·g−1) | [64,66] |
| Low Concentration of Cr(VI) (1–100 mg·dm−3) | High Concentration of Cr(VI) (10–1000 mg·dm−3) | |||
|---|---|---|---|---|
| SCHA | SCHB | SCHA | SCHB | |
| Freundlich | ||||
| n | 1.68 | 1.77 | 1.03 | 1.29 |
| 1/n | 0.595 | 0.565 | 0.971 | 0.775 |
| kF ((dm3)1/n mg(1–1/n)·g−1) | 3.49 | 2.24 | 1.91 | 1.17 |
| R2 | 0.998 | 0.996 | 0.985 | 0.982 |
| Langmuir | ||||
| qL (mg·g−1) | 42.97 | 17.54 | 201.8 | 131.8 |
| kL (dm3·mg−1) | 0.054 | 0.116 | 0.0127 | 0.0055 |
| R2 | 0.984 | 0.976 | 0.966 | 0.961 |
| Temkin | ||||
| bT (J·mol−1) | 244.6 | 823.7 | 21.8 | 56.9 |
| kT (dm3·g−1) | 0.584 | 3.50 | 0.0733 | 0.0395 |
| R2 | 0.887 | 0.870 | 0.621 | 0.788 |
| Method of Synthesis | qmax (mg·g−1) | Reference |
|---|---|---|
| Fe2+ oxidation | 17.5 * | This paper |
| 131.8 ** | ||
| 219 | [52] | |
| Fe3+ hydrolysis | 43.0 * | This paper |
| 201.8 ** | ||
| 83.5 | [25] | |
| 105 | [71] | |
| 178.6 | [46] | |
| Biosynthesis | 38.8 | [26] |
| 35.3 | [72] | |
| 19.0 | [73] | |
| 58.2 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulatowska, J.; Stala, Ł.; Polowczyk, I. Comparison of Cr(VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism. Int. J. Mol. Sci. 2021, 22, 8175. https://doi.org/10.3390/ijms22158175
Ulatowska J, Stala Ł, Polowczyk I. Comparison of Cr(VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism. International Journal of Molecular Sciences. 2021; 22(15):8175. https://doi.org/10.3390/ijms22158175
Chicago/Turabian StyleUlatowska, Justyna, Łukasz Stala, and Izabela Polowczyk. 2021. "Comparison of Cr(VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism" International Journal of Molecular Sciences 22, no. 15: 8175. https://doi.org/10.3390/ijms22158175
APA StyleUlatowska, J., Stala, Ł., & Polowczyk, I. (2021). Comparison of Cr(VI) Adsorption Using Synthetic Schwertmannite Obtained by Fe3+ Hydrolysis and Fe2+ Oxidation: Kinetics, Isotherms and Adsorption Mechanism. International Journal of Molecular Sciences, 22(15), 8175. https://doi.org/10.3390/ijms22158175






