An Experimental Workflow for Studying Barrier Integrity, Permeability, and Tight Junction Composition and Localization in a Single Endothelial Cell Monolayer: Proof of Concept
Abstract
1. Introduction
2. Results
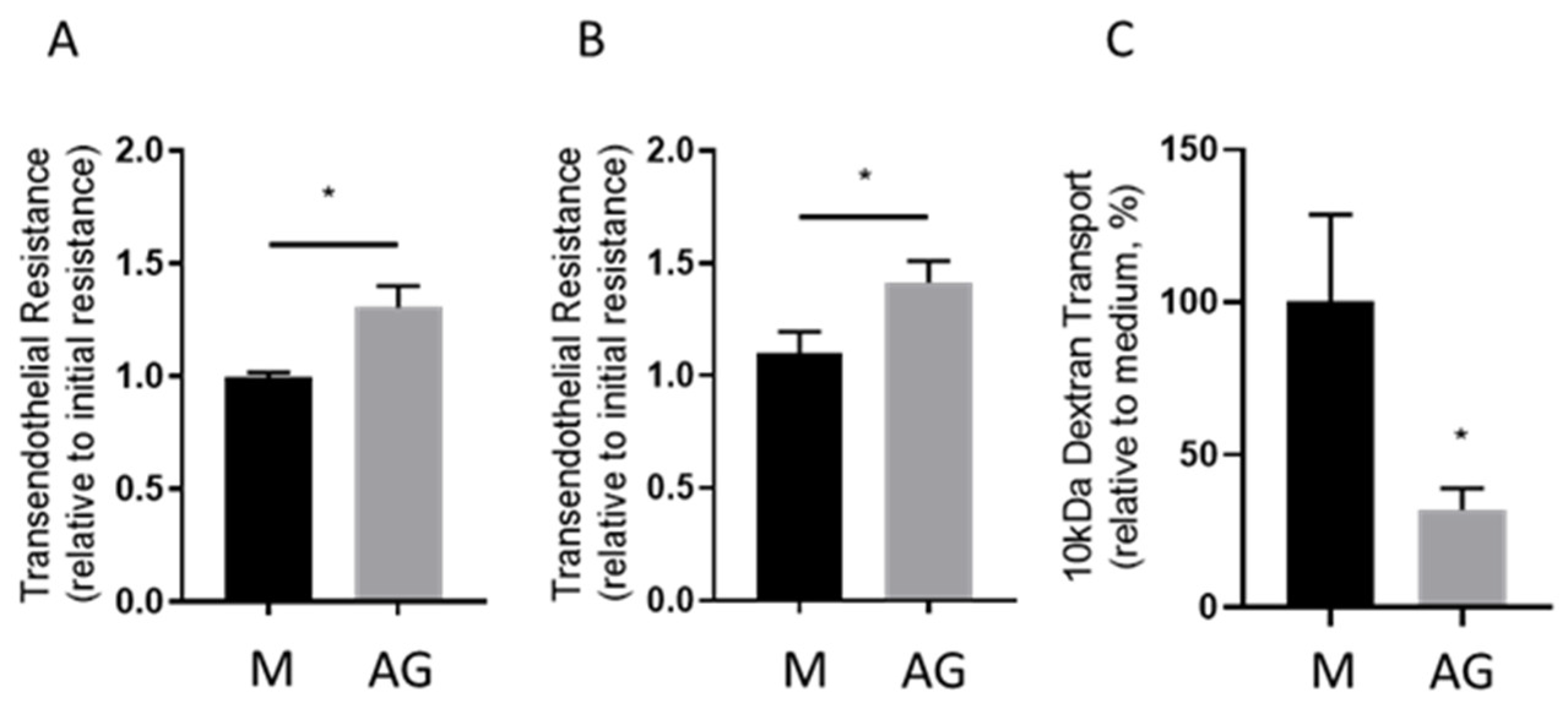
2.1. Transendothelial Resistance and Paracellular Dextran Permeability Assay
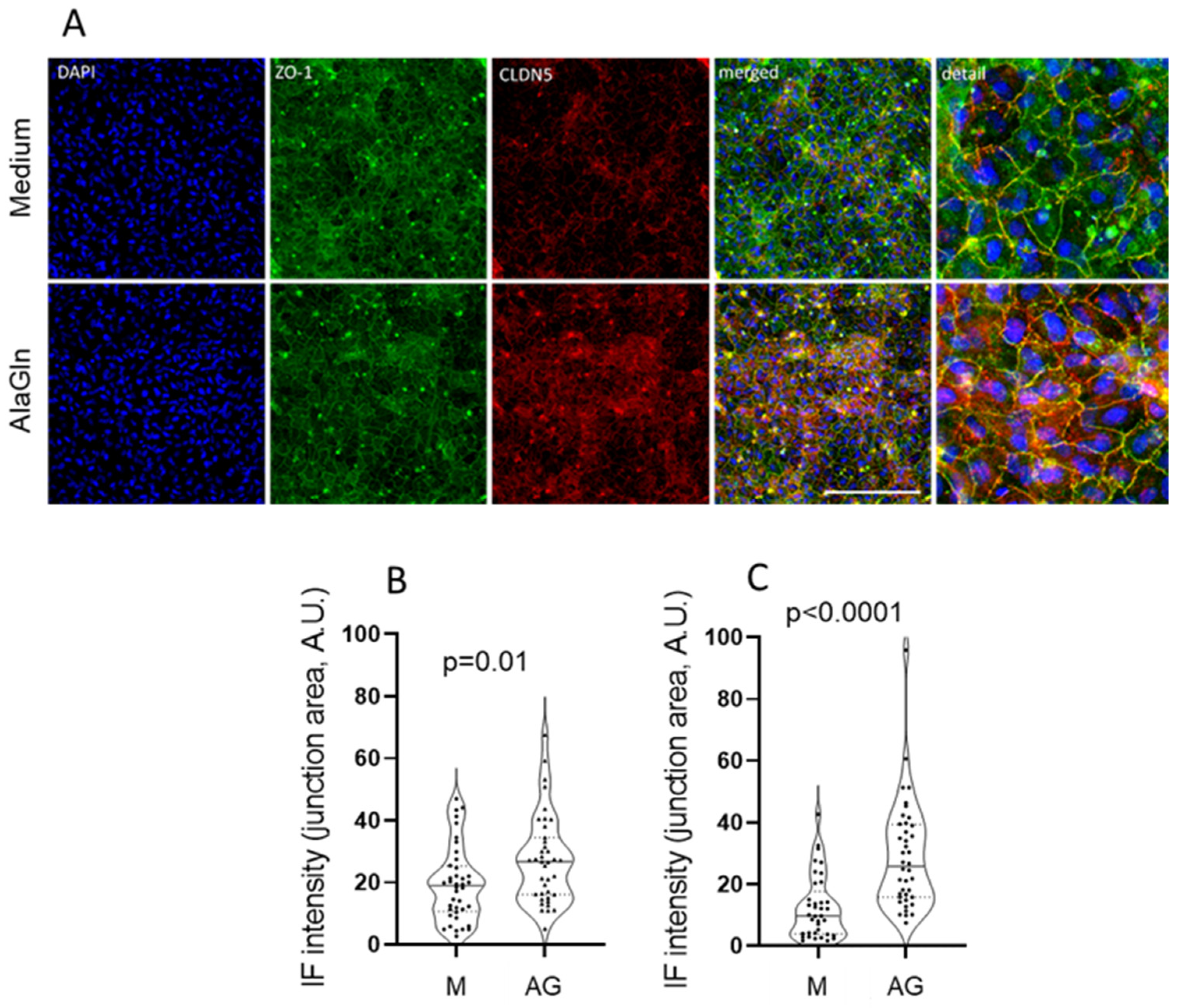
2.2. Immunofluorescence Probing for ZO-1 and CLDN5
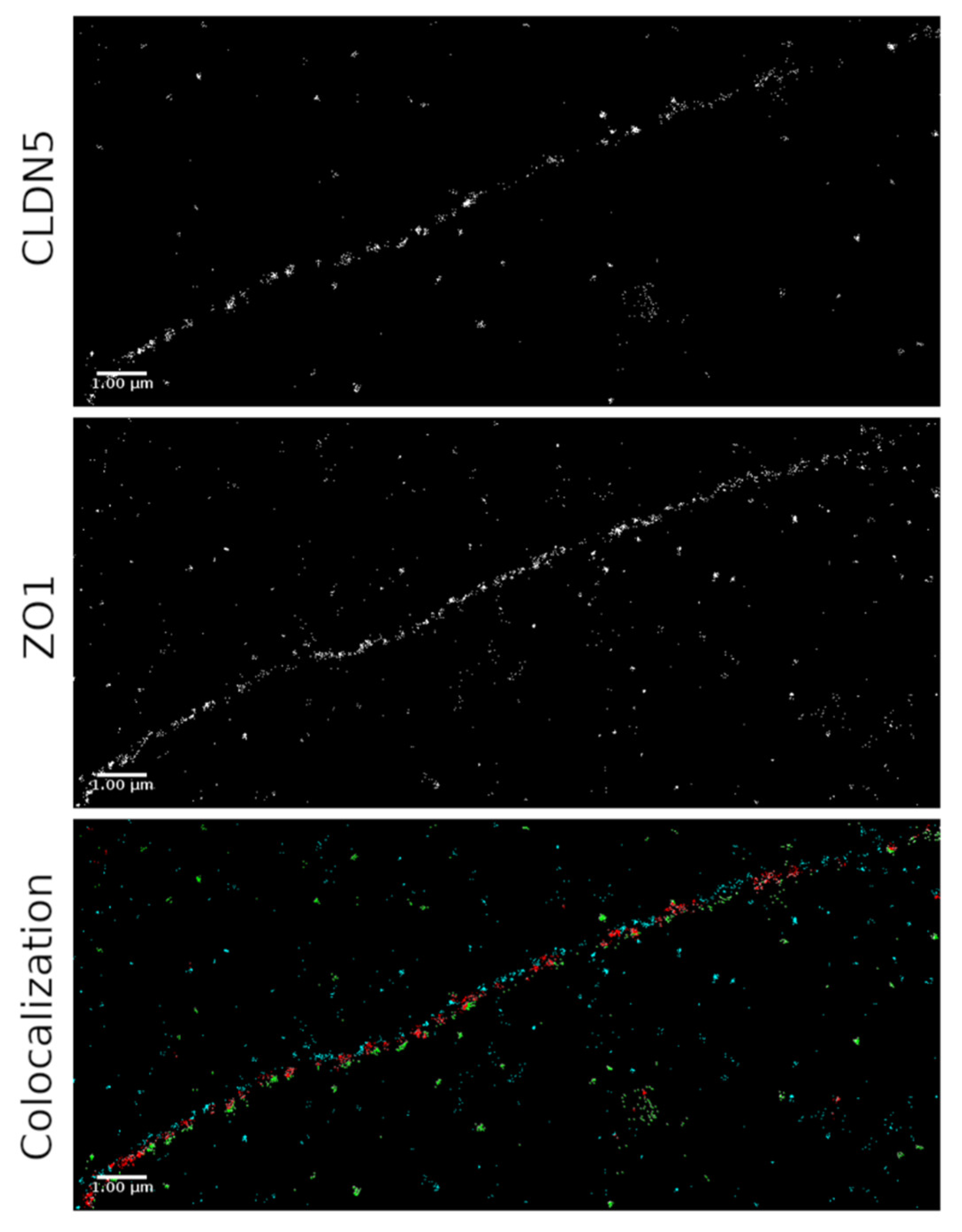
2.3. SMLM for Localization of ZO-1 and CLDN5 Clustering
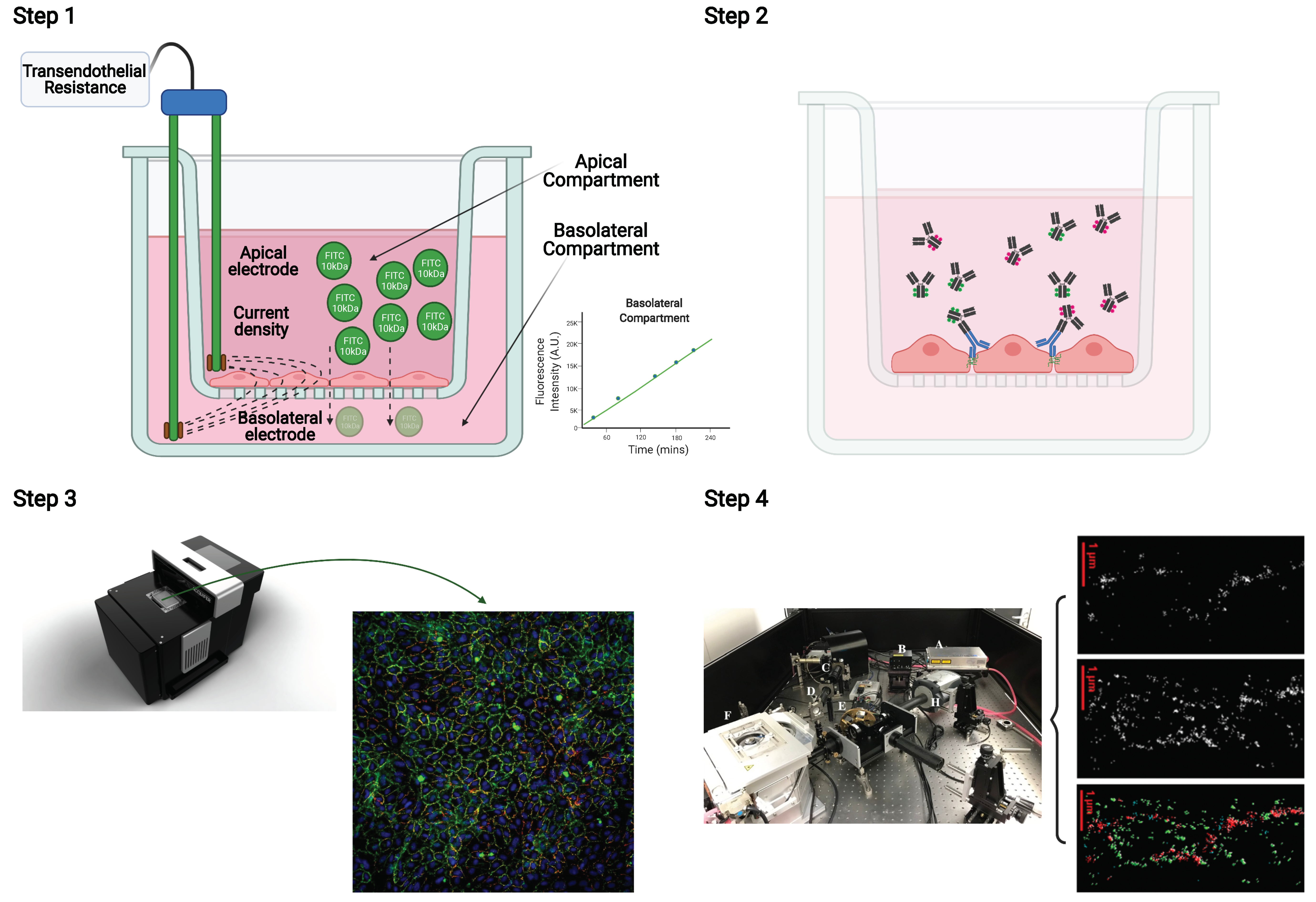
2.4. Integration of the Multiple Stepwise Tight Junction Analyses of a Single Experiment
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Trans-Endothelial Resistance
4.3. Paracellular Endothelial Barrier Dextran Transport Assessment
4.4. Immunostaining
4.5. Automated Imaging and Image Analysis
4.6. Single Molecule Localization Microscopy (SMLM)
4.7. SMLM Data Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- France, M.M.; Turner, J.R. The mucosal barrier at a glance. J. Cell Sci. 2017, 130, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kapus, A.; Szászi, K. Coupling between apical and paracellular transport processes. Biochem. Cell Biol. 2006, 84, 870–880. [Google Scholar] [CrossRef]
- Cereijido, M.; Shoshani, L.; Contreras, R.G. The polarized distribution of Na+, K+-ATPase and active transport across epithelia. J. Membr. Biol. 2001, 184, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef]
- Van Driessche, W.; Kreindler, J.L.; Malik, A.B.; Margulies, S.; Lewis, S.A.; Kim, K.-J. Interrelations/cross talk between transcellular transport function and paracellular tight junctional properties in lung epithelial and endothelial barriers. Am. J. Physiol. Cell. Mol. Physiol. 2007, 293, L520–L524. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Tokumasu, R.; Kimura, H.; Tsukita, S. Role of claudin species–specific dynamics in reconstitution and remodeling of the zonula occludens. Mol. Biol. Cell 2011, 22, 1495–1504. [Google Scholar] [CrossRef]
- Tsukita, S.; Katsuno, T.; Yamazaki, Y.; Umeda, K.; Tamura, A.; Tsukita, S. Roles of ZO-1 and ZO-2 in Establishment of the Belt-like Adherens and Tight Junctions with Paracellular Permselective Barrier Function. Ann. N. Y. Acad. Sci. 2009, 1165, 44–52. [Google Scholar] [CrossRef]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2, a002907. [Google Scholar] [CrossRef]
- Piontek, J.; Fritzsche, S.; Cording, J.; Richter, S.; Hartwig, J.; Walter, M.; Yu, D.; Turner, J.R.; Gehring, C.; Rahn, H.P.; et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell. Mol. Life Sci. 2011, 68, 3903–3918. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Holmes, J.; Anderson, J.M. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 2010, 123, 2844–2852. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Farkas, A.E.; Hilgarth, R.S.; Krug, S.M.; Wolf, M.F.; Benedik, J.K.; Fromm, M.; Koval, M.; Parkos, C.; Nusrat, A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol. Biol. Cell 2014, 25, 2710–2719. [Google Scholar] [CrossRef]
- Meyer, T.N.; Schwesinger, C.; Ye, J.; Denker, B.M.; Nigam, S.K. Reassembly of the Tight Junction after Oxidative Stress Depends on Tyrosine Kinase Activity. J. Biol. Chem. 2001, 276, 22048–22055. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Arsenopoulou, Z.V.; Taitzoglou, I.A.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C.; Zarogiannis, S.G. Silver nanoparticles alter the permeability of sheep pleura and of sheep and human pleural mesothelial cell monolayers. Environ. Toxicol. Pharmacol. 2017, 50, 212–215. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hashimoto, K.; Sato, M.; Watanabe, M.; Tomikawa, N.; Kanno, S.; Kawasaki, Y.; Momoi, N.; Hosoya, M. Establishment of a method for evaluating endothelial cell injury by TNF-α in vitro for clarifying the pathophysiology of virus-associated acute encephalopathy. Pediatr. Res. 2017, 81, 942–947. [Google Scholar] [CrossRef]
- Gillespie, J.L.; Anyah, A.; Taylor, J.M.; Marlin, J.W.; Taylor, T.A. A Versatile Method for Immunofluorescent Staining of Cells Cultured on Permeable Membrane Inserts. Med. Sci. Monit. Basic Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Matsui, T.; Nakayama, M.; Furuse, E.; Sasaki, H.; Furuse, M.; Tsukita, S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004, 279, 44785–44794. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, M.; Herzog, R.; Ridinger, D.; Levai, E.; Jenei, H.; Zhang, C.; González Mateo, G.T.; Marinovic, I.; Hackert, T.; Bestvater, F.; et al. Alanyl-Glutamine Restores Tight Junction Organization after Disruption by a Conventional Peritoneal Dialysis Fluid. Biomolecules 2020, 10, 1178. [Google Scholar] [CrossRef]
- Gonschior, H.; Haucke, V.; Lehmann, M. Super-resolution imaging of tight and adherens junctions: Challenges and open questions. Int. J. Mol. Sci. 2020, 21, 744. [Google Scholar] [CrossRef]
- Gehrig, J. Fiji Tool for Batch Stitching of ACQUIFER Imaging Machine Data. 2020. Available online: https://zenodo.org/record/3631724#.YQOQr0ARWUk (accessed on 27 July 2021).
- Hausmann, M.; Ilić, N.; Pilarczyk, G.; Lee, J.H.; Logeswaran, A.; Borroni, A.P.; Krufczik, M.; Theda, F.; Waltrich, N.; Bestvater, F.; et al. Challenges for super-resolution localization microscopy and biomolecular fluorescent nano-probing in cancer research. Int. J. Mol. Sci. 2017, 18, 2066. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Sigurbjörnsdóttir, S.; Mathew, R.; Leptin, M. Molecular mechanisms of de novo lumen formation. Nat. Rev. Mol. Cell Biol. 2014, 15, 665–676. [Google Scholar] [CrossRef]
- Lizama, C.O.; Zovein, A.C. Polarizing pathways: Balancing endothelial polarity, permeability, and lumen formation. Exp. Cell Res. 2013, 319, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef]
- Looney, M.R.; Matthay, M.A. Bench-to-bedside review: The role of activated protein C in maintaining endothelial tight junction function and its relationship to organ injury. Crit. Care 2006, 10, 239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Mu, S.; Li, X.; Liang, Y.; Wang, L.; Ma, X. Unfractionated Heparin Alleviates Sepsis-Induced Acute Lung Injury by Protecting Tight Junctions. J. Surg. Res. 2019, 238, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Radeva, M.Y.; Waschke, J. Mind the gap: Mechanisms regulating the endothelial barrier. Acta Physiol. 2018, 222, e12860. [Google Scholar] [CrossRef]
- Yamamoto, N.; Yamashita, Y.; Yoshioka, Y.; Nishiumi, S.; Ashida, H. Rapid Preparation of a Plasma Membrane Fraction: Western Blot Detection of Translocated Glucose Transporter 4 from Plasma Membrane of Muscle and Adipose Cells and Tissues. Curr. Protoc. Protein Sci. 2016, 85, 29.18.1–29.18.12. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J. Rigor and Reproducibility in Confocal Fluorescence Microscopy. Cytom. Part A 2020, 97, 113–115. [Google Scholar] [CrossRef]
- Stevens, J.R.; Herrick, J.S.; Wolff, R.K.; Slattery, M.L. Power in pairs: Assessing the statistical value of paired samples in tests for differential expression. BMC Genom. 2018, 19, 953. [Google Scholar] [CrossRef]
- Ledolter, J.; Kardon, R.H. Focus on data: Statistical design of experiments and sample size selection using power analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 11. [Google Scholar] [CrossRef]
- Uhal, B.D.; Flowers, K.M.; Rannels, D.E. Type II pneumocyte proliferation in vitro: Problems and future directions. Am. J. Physiol. Cell. Mol. Physiol. 1991, 261, L110–L117. [Google Scholar] [CrossRef]
- Elaut, G.; Henkens, T.; Papeleu, P.; Snykers, S.; Vinken, M.; Vanhaecke, T.; Rogiers, V. Molecular Mechanisms Underlying the Dedifferentiation Process of Isolated Hepatocytes and Their Cultures. Curr. Drug Metab. 2006, 7, 629–660. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Pongkorpsakol, P.; Turner, J.R.; Zuo, L. Culture of Intestinal Epithelial Cell Monolayers and Their Use in Multiplex Macromolecular Permeability Assays for In Vitro Analysis of Tight Junction Size Selectivity. Curr. Protoc. Immunol. 2020, 131, e112. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Eryilmaz, M.; Schmitt, E.; Krufczik, M.; Theda, F.; Lee, J.H.; Cremer, C.; Bestvater, F.; Schaufler, W.; Hausmann, M.; Hildenbrand, G. Localization microscopy analyses of MRE11 clusters in 3D-conserved cell nuclei of different cell lines. Cancers 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Wagner, E.; Lee, J.H.; Schrock, G.; Schaufler, W.; Krufczik, M.; Papenfuß, F.; Port, M.; Bestvater, F.; Scherthan, H. Super-resolution localization microscopy of radiation-induced histone H2AX-phosphorylation in relation to H3K9-trimethylation in HeLa cells. Nanoscale 2018, 10, 4320–4331. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, P.; Gunkel, M.; Baddeley, D.; Kaufmann, R.; Urich, A.; Weiland, Y.; Reymann, J.; Müller, P.; Hausmann, M.; Cremer, C. SPDM: Light microscopy with single-molecule resolution at the nanoscale. Appl. Phys. B Lasers Opt. 2008, 93, 1–12. [Google Scholar] [CrossRef]


| Quantification of Immunofluorescence Intensity. | |||||
|---|---|---|---|---|---|
| ZO-1 (Conjugated) | CLDN5 (Unconjugated) | ||||
| monoclonal | monoclonal | monoclonal | polyclonal | ||
| 555 nm | 647 nm | 555 nm | 647 nm | ||
| Whole | Medium | 0.32 (±0.16) | 0.25 (±0.15) | 0.11 (±0.06) | 0.6 (±0.3) |
| 24 mM AlaGln | 0.6 (±0.3) | 0.59 (±0.19) | 0.65 (±0.29) | 0.6 (±0.3) | |
| p-value | 0.0013 | 0.005 | 0.001 | 0.85 | |
| Junction | Medium | 19 (±12) | 20 (±12) | 12.7 (±7.5) | 30 (±19) |
| 24 mM AlaGln | 35 (±20) | 25 (±11) | 29.5 (±13.6) | 26 (±14) | |
| p-value | 0.048 | 0.095 | <0.0001 | 0.67 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartosova, M.; Ridinger, D.; Marinovic, I.; Heigwer, J.; Zhang, C.; Levai, E.; Westhoff, J.H.; Schaefer, F.; Terjung, S.; Hildenbrand, G.; et al. An Experimental Workflow for Studying Barrier Integrity, Permeability, and Tight Junction Composition and Localization in a Single Endothelial Cell Monolayer: Proof of Concept. Int. J. Mol. Sci. 2021, 22, 8178. https://doi.org/10.3390/ijms22158178
Bartosova M, Ridinger D, Marinovic I, Heigwer J, Zhang C, Levai E, Westhoff JH, Schaefer F, Terjung S, Hildenbrand G, et al. An Experimental Workflow for Studying Barrier Integrity, Permeability, and Tight Junction Composition and Localization in a Single Endothelial Cell Monolayer: Proof of Concept. International Journal of Molecular Sciences. 2021; 22(15):8178. https://doi.org/10.3390/ijms22158178
Chicago/Turabian StyleBartosova, Maria, David Ridinger, Iva Marinovic, Jana Heigwer, Conghui Zhang, Eszter Levai, Jens H. Westhoff, Franz Schaefer, Stefan Terjung, Georg Hildenbrand, and et al. 2021. "An Experimental Workflow for Studying Barrier Integrity, Permeability, and Tight Junction Composition and Localization in a Single Endothelial Cell Monolayer: Proof of Concept" International Journal of Molecular Sciences 22, no. 15: 8178. https://doi.org/10.3390/ijms22158178
APA StyleBartosova, M., Ridinger, D., Marinovic, I., Heigwer, J., Zhang, C., Levai, E., Westhoff, J. H., Schaefer, F., Terjung, S., Hildenbrand, G., Krunic, D., Bestvater, F., Hausmann, M., Schmitt, C. P., & Zarogiannis, S. G. (2021). An Experimental Workflow for Studying Barrier Integrity, Permeability, and Tight Junction Composition and Localization in a Single Endothelial Cell Monolayer: Proof of Concept. International Journal of Molecular Sciences, 22(15), 8178. https://doi.org/10.3390/ijms22158178







