N-Glycomics of Human Erythrocytes
Abstract
1. Introduction
2. Results
2.1. RCB N-Glycoprofiles by MALDI-MS
2.1.1. Oligomannose N-Glycans
2.1.2. Hybrid N-Glycans
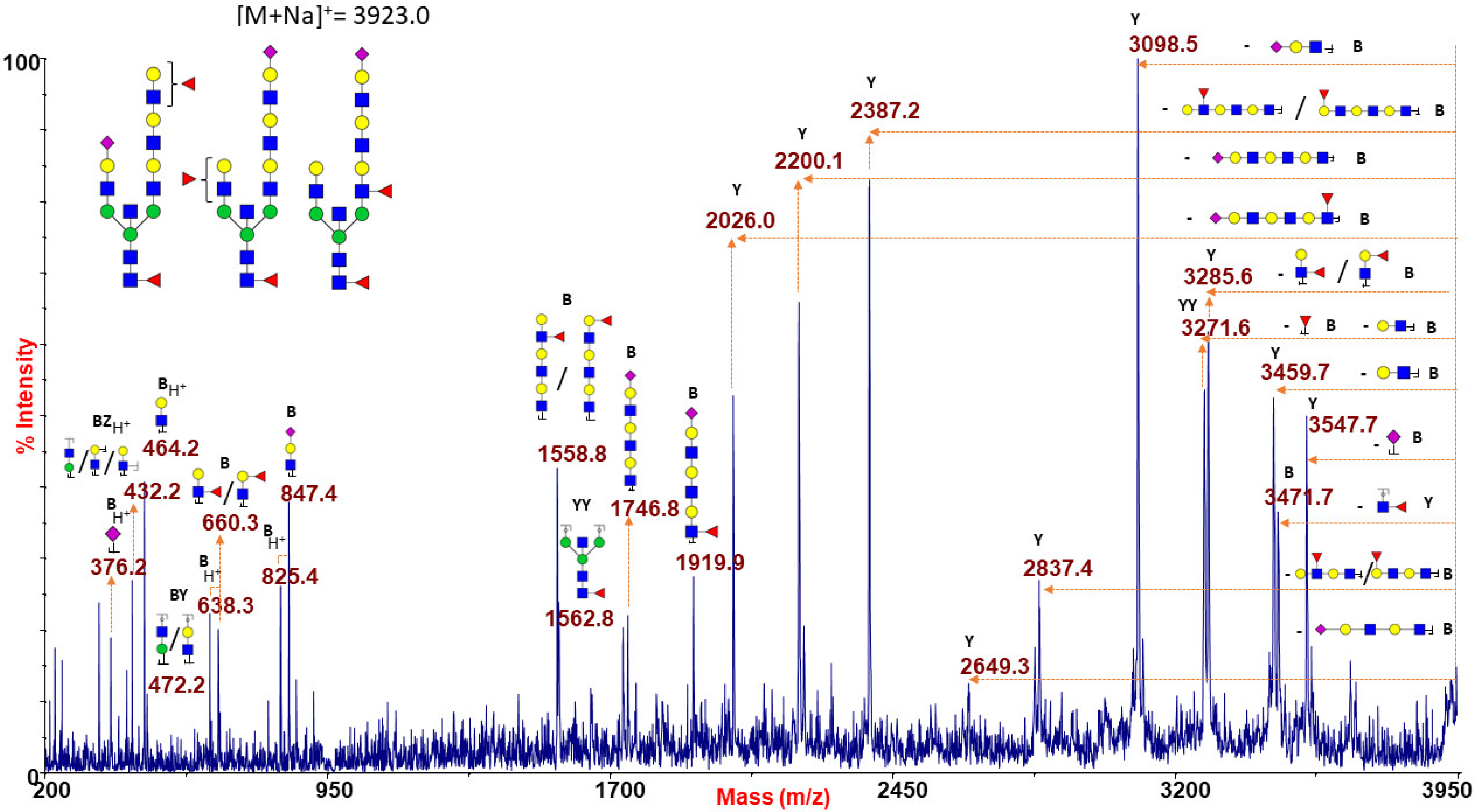
2.1.3. Bisected N-Glycans
2.1.4. Poly-LacNAc N-Glycans
2.1.5. Sialylated N-Glycans
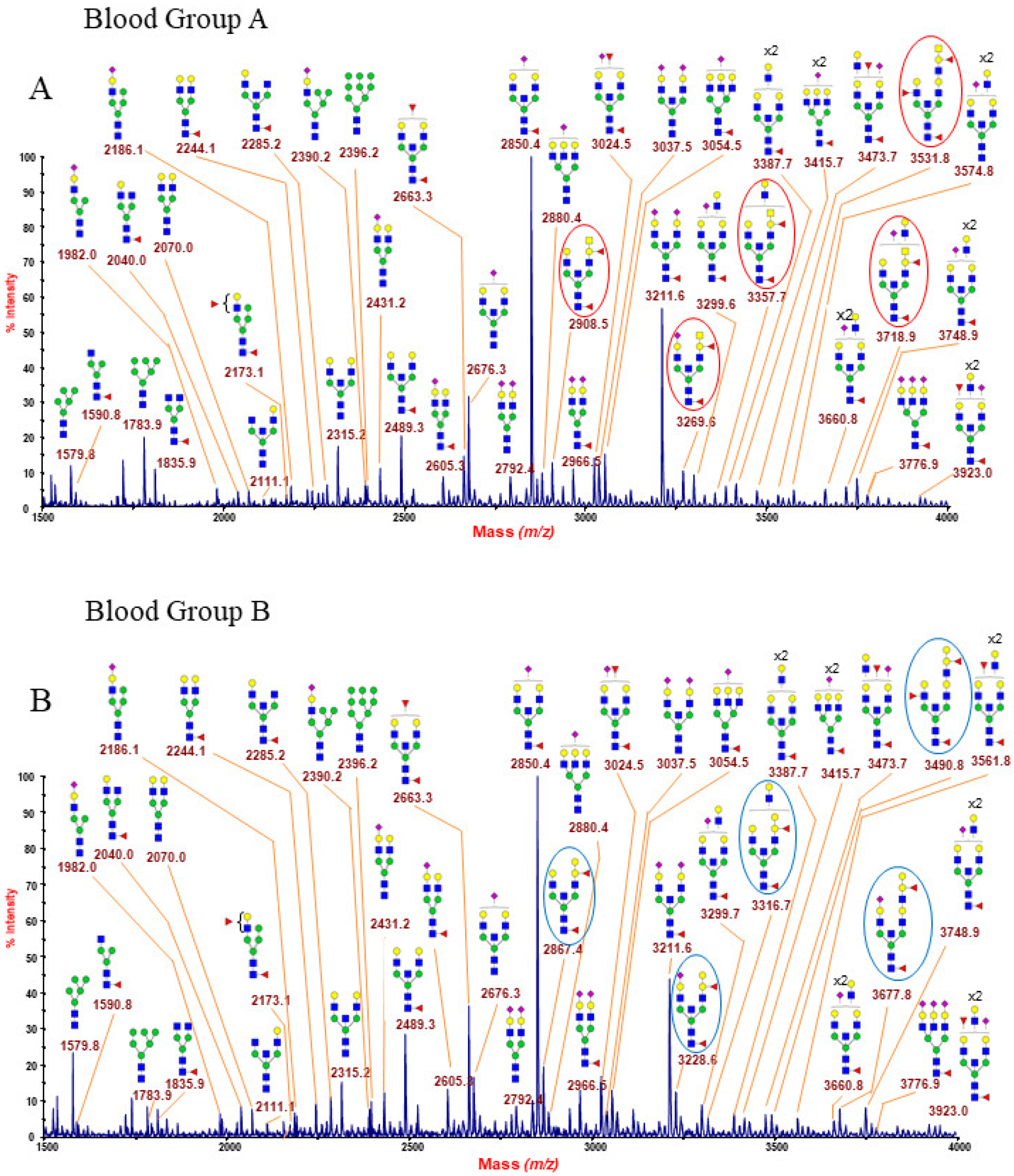
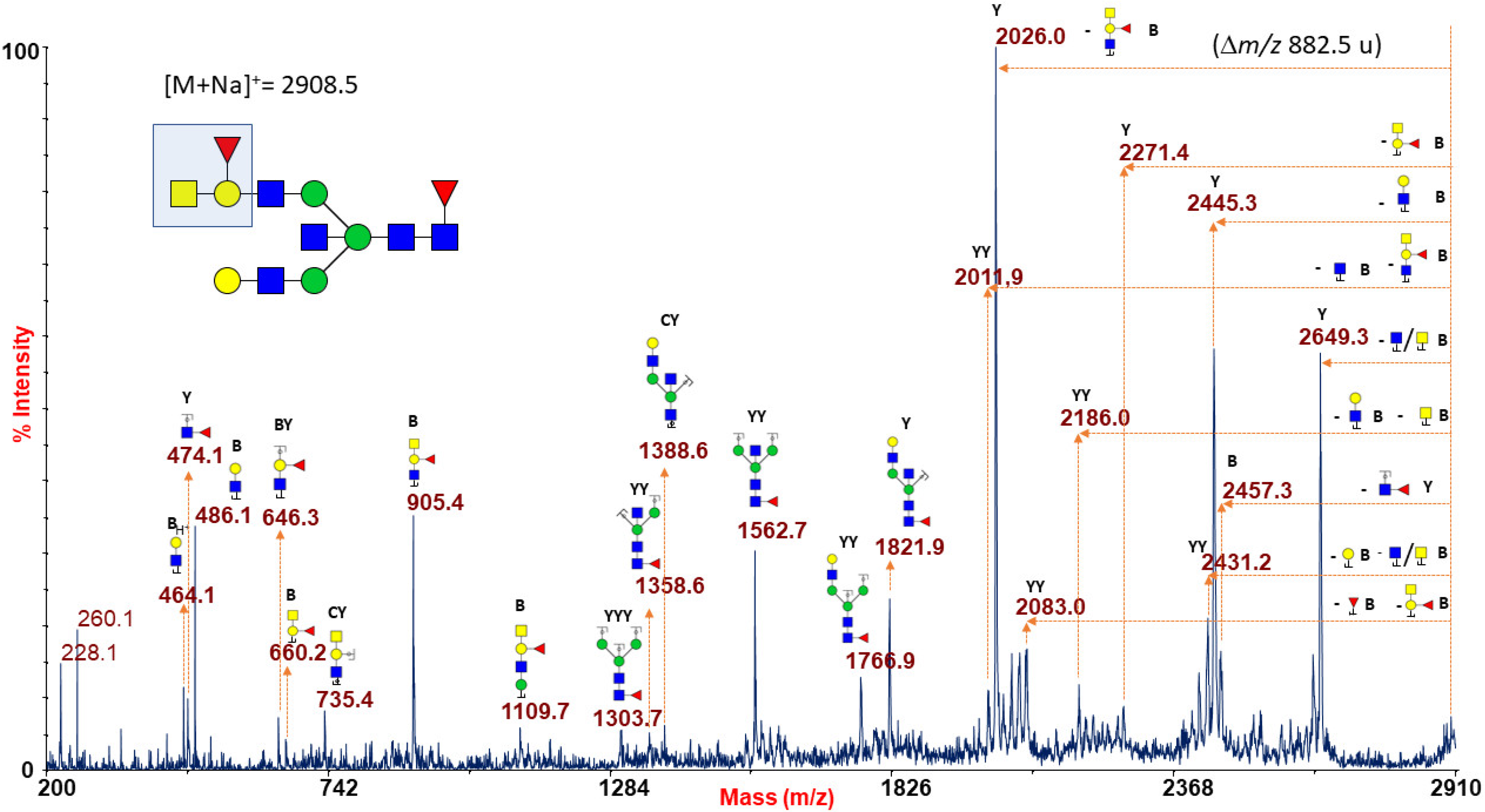
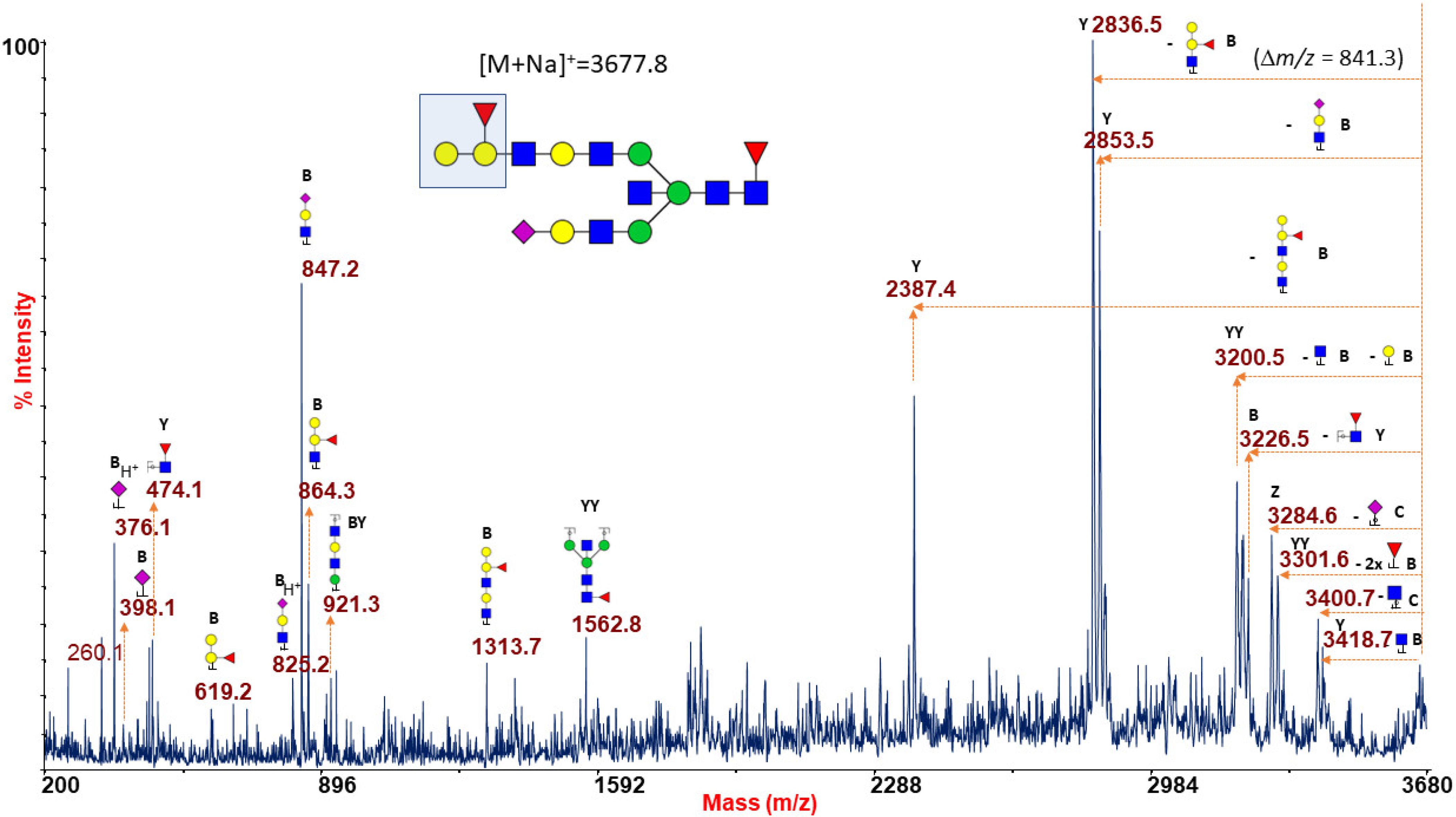
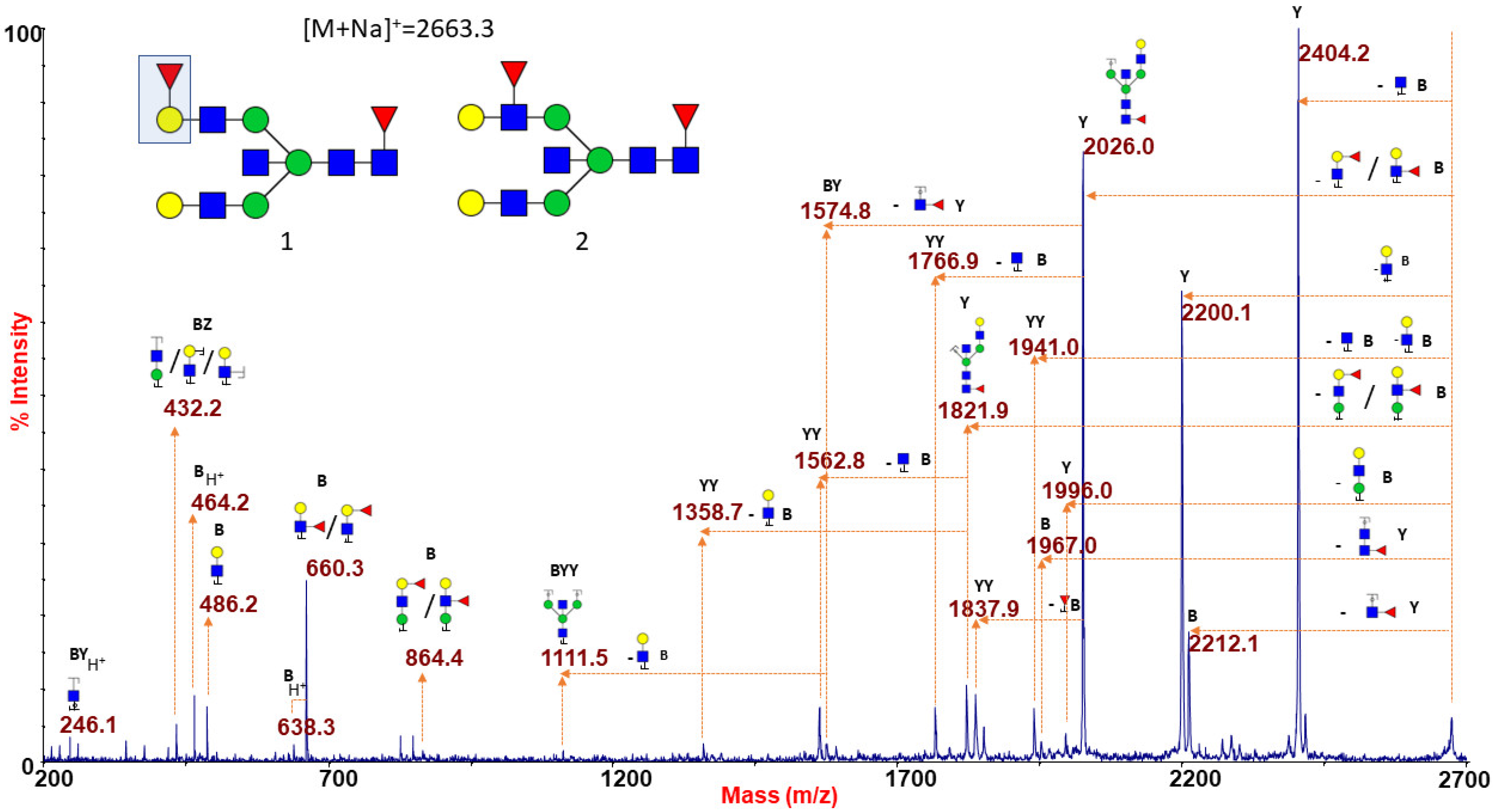
2.1.6. ABO(H) Antigen-Bearing N-Glycans
3. Discussion
4. Materials and Methods
4.1. Erythrocyte Isolation
4.2. Hemolysis and Extraction of Membrane Proteins
4.3. MALDI TOF MS and MS/MS Analysis
4.4. Data Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, P.; Murata-hori, M.; Lodish, H.F. Formation of mammalian erythrocytes: Chromatin condensation and enucleation. Trends Cell Biol. 2011, 21, 409–415. [Google Scholar] [CrossRef]
- Goodman, S.R.; Kurdia, A.; Ammann, L.; Kakhniashvili, D.; Daescu, O. The human red blood cell proteome and interactome. Exp. Biol. Med. 2007, 232, 1391–1408. [Google Scholar] [CrossRef]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- Phillips, D.R.; Morrison, M. The arrangement of Proteins in the human erythrocyte membrane. Biochem. Biophys. Res. Commun. 1970, 40, 284–289. [Google Scholar] [CrossRef]
- De Oliveira, S.; Saldanha, C. An overview about erythrocyte membrane. Clin. Hemorheol. Microcirc. 2010, 44, 63–74. [Google Scholar] [CrossRef]
- Delaunay, J. The molecular basis of hereditary red cell membrane disorders. Blood Rev. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Hochmuth, R. Erythrocyte Membrane Elasticity and Viscosity. Annu. Rev. Physiol. 1987, 49, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Stevens, J.; Blixt, O.; Tumpey, T.M.; Taubenberger, J.K.; Paulson, J.C.; Wilson, I.A. Structure and Receptor Specificity of the Hemagglutin from an H5N1 Influenza Virus. Science 2006, 312, 404–410. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. Aberrant Glycosylation in Cancer: A Novel Molecular Mechanism Controlling Metastasis. Cancer Cell 2017, 31, 733–735. [Google Scholar] [CrossRef]
- Shade, K.T.C.; Anthony, R. Antibody Glycosylation and Inflammation. Antibodies 2013, 2, 392–414. [Google Scholar] [CrossRef]
- Albrecht, S.; Unwin, L.; Muniyappa, M.; Rudd, P.M. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark. 2014, 14, 17–28. [Google Scholar] [CrossRef]
- Hwang, H.; Zhaang, J.; Chung, K.A.; Leverenz, J.B.; Zabetian, C.P.; Peskind, E.R.; Jankovic, J.; Su, Z.; Hancock, A.M.; Pan, C.; et al. Glycoproteomics in neurodegenerative diseases. Mass Spectrom. Rev. 2010, 29, 79–125. [Google Scholar] [CrossRef]
- Palmigiano, A.; Barone, R.; Sturiale, L.; Sanfilippo, C.; Bua, R.O.; Romeo, D.A.; Messina, A.; Capuana, M.L.; Maci, T.; Le Pira, F.; et al. CSF N-glycoproteomics for early diagnosis in Alzheimer’s disease. J. Proteom. 2016, 131, 29–37. [Google Scholar] [CrossRef]
- Jaeken, J. Congenital disorders of glycosylation. Ann. N. Y. Acad. Sci. 2010, 1214, 190–198. [Google Scholar] [CrossRef]
- Barone, R.; Sturiale, L.; Garozzo, D. Mass spectrometry in the characterization of human genetic N-glycosylation defects. Mass Spectrom. Rev. 2009, 28, 517–542. [Google Scholar] [CrossRef]
- Aoki, T. A Comprehensive Review of Our Current Understanding of Red Blood Cell (RBC) Glycoproteins. Membranes 2017, 7, 56. [Google Scholar] [CrossRef]
- Fukuda, M.; Dell, A.; Oates, J.E.; Fukuda, M.N. Structure of Branched Lactosaminoglycan, the Carbohydrate Moiety of Band 3 Isolated from Adult Human Erythrocytes. J. Biol. Chem. 1984, 259, 8260–8273. [Google Scholar] [CrossRef]
- Tsuji, T.; Irimura, T.; Osawa, T. The carbohydrate moiety of band 3 glycoprotein of human erythrocyte membranes. Structures of lower molecular weight oligosaccharides. J. Biol. Chem. 1981, 256, 10497–10502. [Google Scholar] [CrossRef]
- Yoshima, H.; Furthmayr, H.; Kobata, A. Structures of the asparagine-linked sugar chains of glycophorin A. J. Biol. Chem. 1980, 255, 9713–9718. [Google Scholar] [CrossRef]
- Irimura, T.; Tsuji, T.; Tagami, S.; Yamamoto, K.; Osawa, T. Structure of a Complex-Type Sugar Chain of Human Glycophorin A. Biochemistry 1981, 20, 560–566. [Google Scholar] [CrossRef]
- Wilczynska, Z.; Miller-Podraza, H.; Koscielak, J. The contribution of different glycoconjugates to the total ABH blood group activity of human erythrocytes. FEBS Lett. 1980, 112, 277–279. [Google Scholar] [CrossRef]
- Krusius, T.; Finne, J.; Rauvala, H. The Poly(glycosy1) Chains of Glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur. J. Biochem. 1978, 92, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, S.Å.; Podbielska, M.; Nilsson, B.; Lisowska, E.; Krotkiewski, H. ABH blood group antigens in N-glycan of human glycophorin A. Arch. Biochem. Biophys. 2010, 498, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sundlov, M.M.; Stowell, S.R.; Hoffmeister, K.M. Multifaceted role of glycosylation in transfusion medicine, platelets, and red blood cells. J. Thromb. Haemost. 2020, 18, 1535–1547. [Google Scholar] [CrossRef]
- Stowell, S.R.; Stowell, C.P. Biologic roles of the ABH and Lewis histo-blood group antigens part II: Thrombosis, cardiovascular disease and metabolism. Vox Sang. 2019, 114, 535–552. [Google Scholar] [CrossRef]
- Siransy, L.K.; Nanga, Z.Y.; Zaba, F.S.; Tufa, N.Y.; Dasse, S.R. ABO/Rh Blood Groups and Risk of HIV Infection and Hepatitis B among Blood Donors of Abidjan, Côte D’Ivoire. Eur. J. Microbiol. Immunol. 2015, 5, 205–209. [Google Scholar] [CrossRef]
- Degarege, A.; Gebrezgi, M.T.; Ibanez, G.; Wahlgren, M.; Madhivanan, P. Effect of the ABO blood group on susceptibility to severe malaria: A systematic review and meta-analysis. Blood Rev. 2019, 33, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Nguyen, N.Y.; Dunstan, S.J.; Baillie, J.K. The role of host genetics in susceptibility to influenza: A systematic review. PLoS ONE 2012, 7, e0033180. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Qi, J.; Zhang, Q.; Wang, H.; Pang, L.; Sun, X.; Duan, Z. Human group A rotavirus P[25] VP8 * specifically binds to A-type histo-blood group antigen. Virology 2021, 555, 56–63. [Google Scholar] [CrossRef]
- Tyrrell, D.A.J.; Sparrow, P.; Beare, A.S. Relation between Blood Groups and Resistance to Infection with Influenza and some Picornaviruses. Nature 1968, 220, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.M.; Hendrickse, H.L.; Matsha, T.E. Membrane Influence Human Immunodeficiency Virus Infection? Cells 2020, 9, 845. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef]
- E Fernandes, T.D.S. Chaotic model for COVID-19 growth factor. Res. Biomed. Eng. 2020, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Häussinger, L.; Steinacker, J.M.; Dinse-Lambracht, A. Association between the dynamics of the COVID-19 epidemic and ABO blood type distribution. Epidemiol. Infect. 2021, 149, E19. [Google Scholar] [CrossRef] [PubMed]
- Pendu, J.L.; Breiman, A.; Rocher, J.; Dion, M.; Ruvoën-Clouet, N. ABO Blood Types and COVID-19: Spurious, Anecdotal, or Truly Important Relationships? A Reasoned Review of Available Data. Viruses 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J. Proteomic analysis of glycosylation: Structural determination of N- and O-linked glycans by mass spectrometry. Expert Rev. Proteom. 2005, 2, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Banazadeh, A.; Veillon, L.; Wooding, K.M.; Zabet, M. Recent Advances in Mass Spectrometric Analysis of Glycoproteins. Electrophoresis 2017, 38, 162–189. [Google Scholar] [CrossRef] [PubMed]
- North, S.J.; von Gunten, S.; Antonopoulos, A.; Trollope, A.; MacGlashan, D.W., Jr.; Jang-lee, J.; Dell, A.; Metcalfe, D.D.; Kirshenbaum, A.S.; Bochner, B.S.; et al. Glycomic analysis of human mast cells, eosinophils and basophils. Glycobiology 2012, 22, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Karamanska, R.; Chan, R.W.Y.; Chan, M.C.W.; Jia, N.; Air, G.; Hopton, C.; Wong, M.P.; Dell, A.; Peiris, J.S.M.; et al. Glycomic Analysis of Human Respiratory Tract Tissues and Correlation with Influenza Virus Infection. PLoS Pathog. 2013, 9, e1003223. [Google Scholar] [CrossRef]
- Vreeker, G.C.M.; Bladergroen, M.R.; Nicolardi, S.; Mesker, W.E.; Tollenaar, R.A.E.M.; van der Burgt, Y.E.M.; Wuhrer, M. Dried blood spot N-glycome analysis by MALDI mass spectrometry. Talanta 2019, 205, 120104. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.N.; Dell, A.; Scartezzinill, P. Primary Defect of Congenital Dyserythropoietic Anemia Type II. Failure in glycosylation of erythrocyte lactosaminoglycan proteins caused by lowered N-acetylglucosaminyltransferase II. J. Biol. Chem. 1987, 262, 7195–7206. [Google Scholar] [CrossRef]
- Denecke, J.; Kranz, C.; Nimtz, M.; Conradt, H.S.; Brune, T.; Heimpel, H.; Marquardt, T. Characterization of the N-glycosylation phenotype of erythrocyte membrane proteins in congenital dyserythropoietic anemia type II (CDA II/HEMPAS). Glycoconj. J. 2008, 25, 375–382. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Daniels, G. ABO, H, and Lewis Systems. In Human Blood Groups; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 11–95. ISBN 9781444333244. [Google Scholar]
- Pisano, A.; Redmond, J.W.; Williams, K.L.; Gooley, A.A. Glycosylation sites identified by solid-phase edman degradation: O-linked glycosylation motifs on human glycophorin A. Glycobiology 1993, 3, 429–435. [Google Scholar] [CrossRef]
- Zdebska, E.; Kościelak, J. A single-sample method for determination of carbohydrate and protein contents glycoprotein bands separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 1999, 275, 171–179. [Google Scholar] [CrossRef]
- Climer, L.K.; Dobretsov, M.; Lupashin, V. Defects in the COG complex and COG-related trafficking regulators affect neuronal Golgi function. Front. Neurosci. 2015, 9, 405. [Google Scholar] [CrossRef]
- Palmigiano, A.; Bua, R.O.; Barone, R.; Rymen, D.; Régal, L.; Deconinck, N.; Dionisi-Vici, C.; Fung, C.W.; Garozzo, D.; Jaeken, J.; et al. MALDI-MS profiling of serum O-glycosylation and N-glycosylation in COG5-CDG. J. Mass Spectrom. 2017, 52, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.N.; Masri, K.A.; Dell, A.; Thonar, E.J.; Klier, G.; Lowenthal, R.M. Defective glycosylation of erythrocyte membrane glycoconjugates in a level of membrane-bound form of galactosyltransferase. Blood 1989, 73, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Dutta, D.; Ghoshal, A.; Mukhopadhyay, S.; Saha, B.; Sundar, S.; Jarmalavicius, S.; Forgber, M.; Mandal, C.; Walden, P.; et al. Glycosylation of erythrocyte spectrin and its modification in visceral leishmaniasis. PLoS ONE 2011, 6, e0028169. [Google Scholar] [CrossRef][Green Version]
- Gamblin, S.J.; Haire, L.F.; Russell, R.J.; Stevens, D.J.; Xiao, B.; Ha, Y.; Vasisht, N.; Steinhauer, D.A.; Daniels, R.S.; Elliot, A.; et al. The Structure and Receptor Binding Properties of the 1918 Influenza Hemagglutinin. Science 2004, 303, 1838–1842. [Google Scholar] [CrossRef]
- Aich, U.; Beckley, N.; Shriver, Z.; Raman, R.; Viswanathan, K. Glycomics-based analysis of chicken red blood cells provides insight into the selectivity of the viral agglutination assay. FEBS J. 2011, 278, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.; Balish, A.; Veguilla, V.; Sun, H.; Schiffer, J.; Lu, X.; Katz, J.M.; Hancock, K. Influenza Virus Titration, Antigenic Characterization, and Serological Methods for Antibody Detection. In Influenza Virus: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; Volume 1, pp. 25–51. ISBN 978-1-61779-620-3. [Google Scholar]
- Nobusawa, E.; Ishihara, H.; Morishita, T.; Sato, K.; Nakajima, K. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): A single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 2000, 278, 587–596. [Google Scholar] [CrossRef]
- Bull, P.C.; Lowe, B.S.; Kortok, M.; Molyneux, C.S.; Newbold, C.I.; Marsh, K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 1998, 4, 358–360. [Google Scholar] [CrossRef]
- Katz, J.M.; Hancock, K.; Xu, X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev. Anti. Infect. Ther. 2011, 9, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Srinivasan, A.; Raman, R.; Viswanathan, K.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nat. Biotechnol. 2008, 26, 107–113. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viswanathan, K.; Raman, R.; Chandrasekaran, A.; Raguram, S.; Tumpey, T.M.; Sasisekharan, V.; Sasisekharan, R. Quantitative biochemical rationale for differences in transmissibility of 1918 pandemic influenza A viruses. Proc. Natl. Acad. Sci. USA 2008, 15, 2800–2805. [Google Scholar] [CrossRef]
- Dodge, T.; Hanahan, J. The Preparation and Chemical Characteristics Erythrocytes of Hemoglobin-Free Ghosts of Human Erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Palmigiano, A.; Messina, A.; Bua, R.O.; Barone, R.; Sturiale, L.; Zappia, M.; Garozzo, D. CSF N-Glycomics Using MALDI MS Techniques in Alzheimer’s Disease. In Biomarkers for Alzheimer’s Disease Drug Development; Humana Press: New York, NY, USA, 2018; Volume 1750, pp. 75–91. [Google Scholar]
- Sturiale, L.; Barone, R.; Garozzo, D. The impact of mass spectrometry in the diagnosis of congenital disorders of glycosylation. J. Inherit. Metab. Dis. 2011, 34, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bua, R.O.; Messina, A.; Sturiale, L.; Barone, R.; Garozzo, D.; Palmigiano, A. N-Glycomics of Human Erythrocytes. Int. J. Mol. Sci. 2021, 22, 8063. https://doi.org/10.3390/ijms22158063
Bua RO, Messina A, Sturiale L, Barone R, Garozzo D, Palmigiano A. N-Glycomics of Human Erythrocytes. International Journal of Molecular Sciences. 2021; 22(15):8063. https://doi.org/10.3390/ijms22158063
Chicago/Turabian StyleBua, Rosaria Ornella, Angela Messina, Luisa Sturiale, Rita Barone, Domenico Garozzo, and Angelo Palmigiano. 2021. "N-Glycomics of Human Erythrocytes" International Journal of Molecular Sciences 22, no. 15: 8063. https://doi.org/10.3390/ijms22158063
APA StyleBua, R. O., Messina, A., Sturiale, L., Barone, R., Garozzo, D., & Palmigiano, A. (2021). N-Glycomics of Human Erythrocytes. International Journal of Molecular Sciences, 22(15), 8063. https://doi.org/10.3390/ijms22158063





