Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System
Abstract
:1. Introduction
2. Classification of Nanoparticles and MONP Synthesis
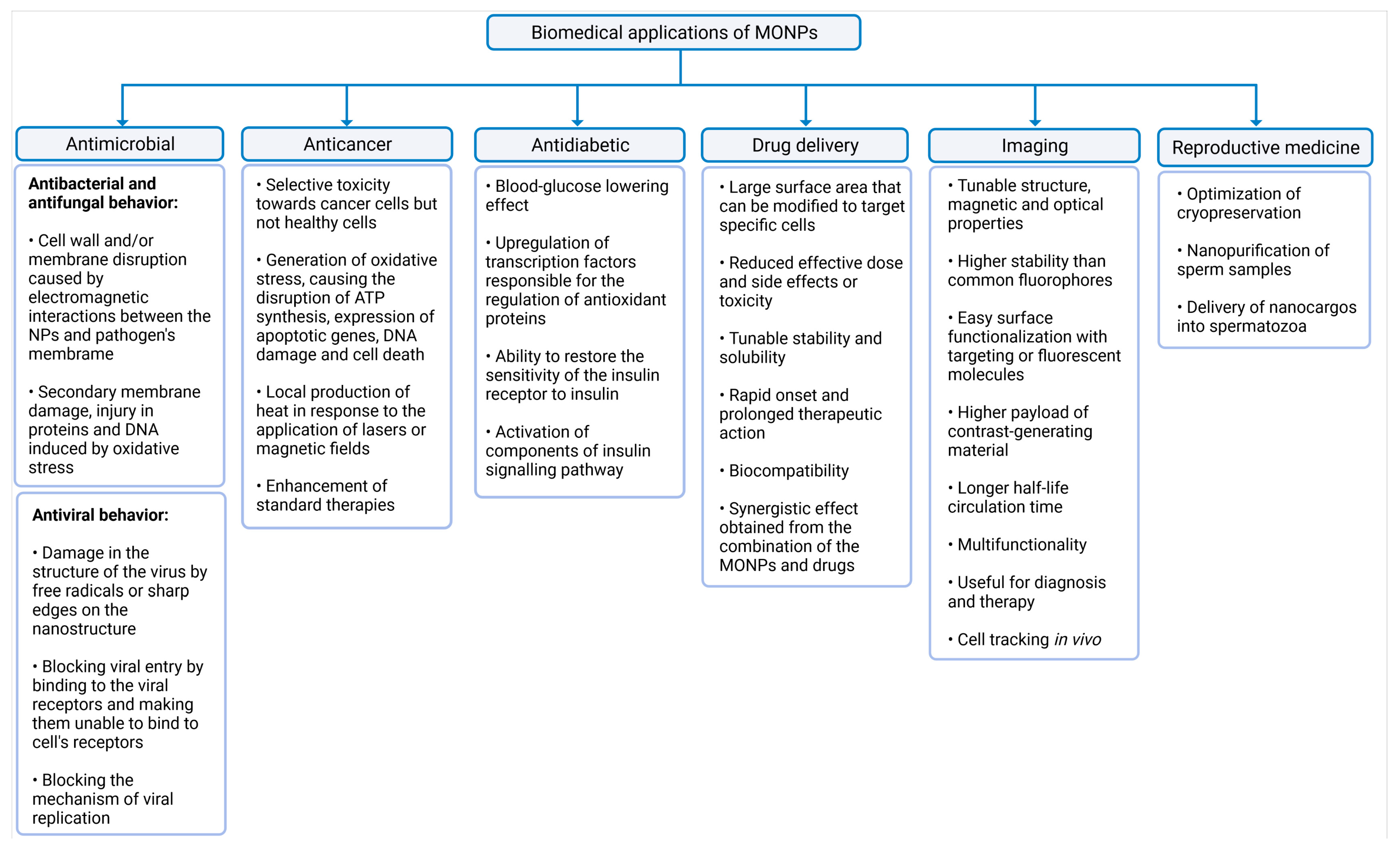
3. Biomedical Applications of MONPs
3.1. Antimicrobial, Anticancer, and Antidiabetic Activity
3.2. Drug Delivery Platforms and Imaging
3.3. An Asset for Reproductive Medicine
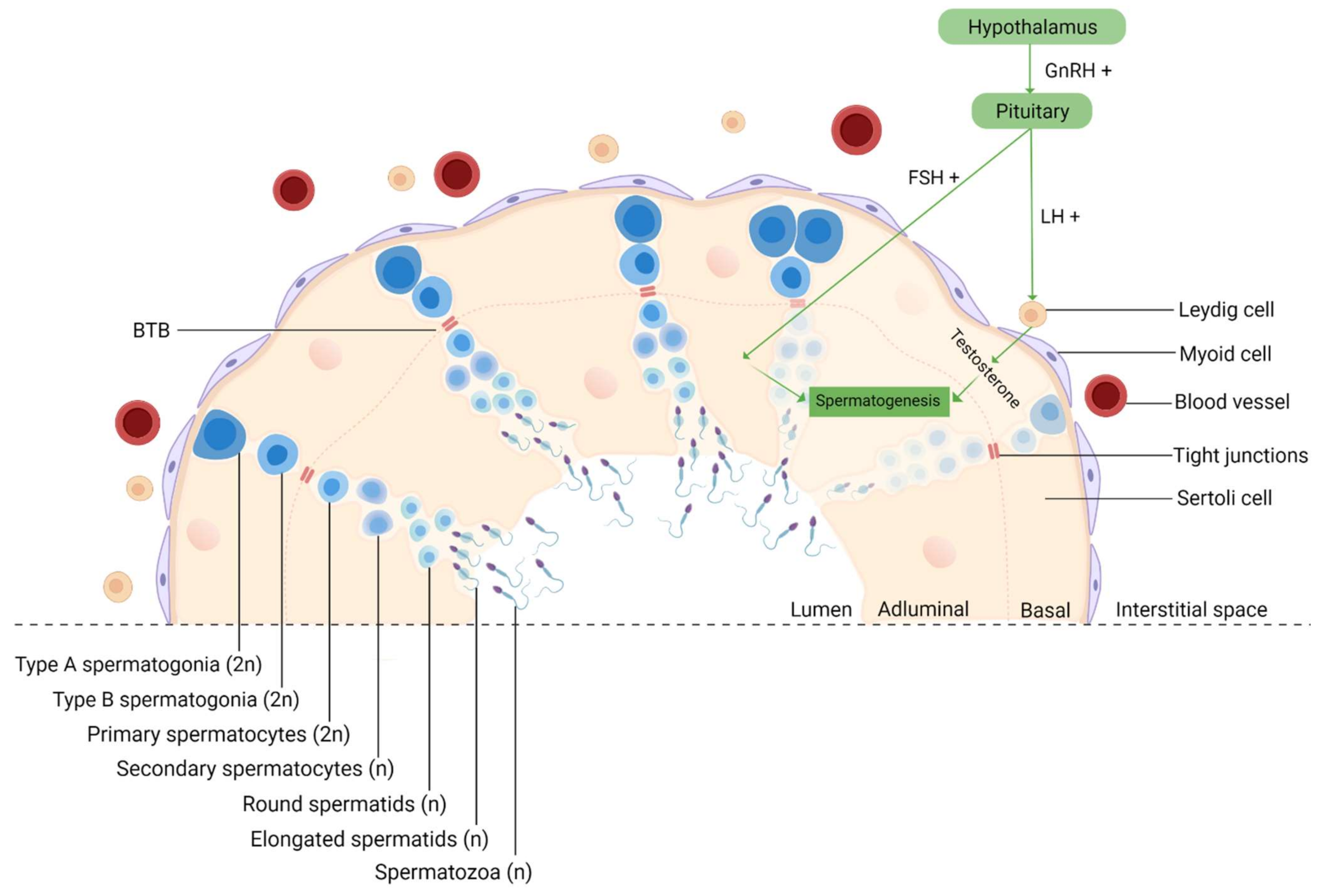
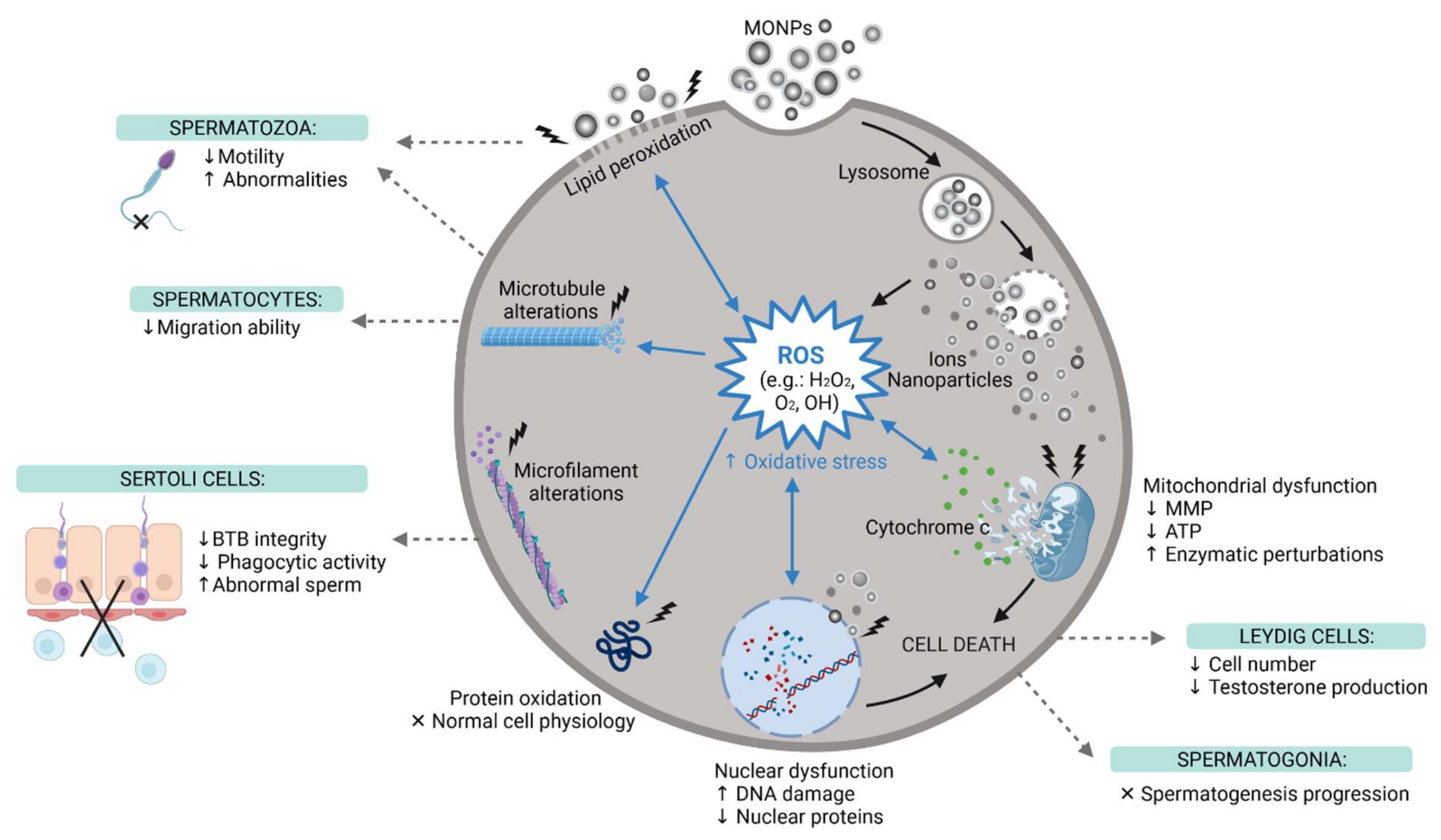
4. The Impact of MONPs on Male Fertility
4.1. In Vitro Studies
4.2. In Vivo Studies
4.3. MONPs in Human Reproductive Medicine
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–594. [Google Scholar] [CrossRef]
- Lövestam, G.; Rauscher, H.; Roebben, G.; Klüttgen, B.; Gibson, N.; Putaud, J.-P.; Stamm, H. Considerations on a Definition of Nanomaterial for Regulatory Purposes; JRC Reference Reports; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Singla, R.; Guliani, A.; Kumari, A.; Yadav, S.K. Metallic Nanoparticles, Toxicity Issues and Applications in Medicine; Springer: Singapore, 2016; ISBN 9789811008184. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, J.; Ghosh, S.; Datta, P.; Gomes, A.; Gomes, A. Physiologically important metal nanoparticles and their toxicity. J. Nanosci. Nanotechnol. 2014, 14, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. Checking the Biocompatibility of Plant-Derived Metallic Nanoparticles: Molecular Perspectives. Trends Biotechnol. 2016, 34, 440–449. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar] [PubMed]
- Rezaeian, Z.; Yazdekhasti, H.; Nasri, S.; Rajabi, Z.; Fallahi, P.; Amidi, F. Effect of selenium on human sperm parameters after freezing and thawing procedures. Asian Pac. J. Reprod. 2016, 5, 462–466. [Google Scholar] [CrossRef]
- Herman, S.; Lipinski, P.; Ogórek, M.; Starzynski, R.; Grzmil, P.; Bednarz, A.; Lenartowicz, M. Molecular Regulation of Copper Homeostasis in the Male Gonad during the Process of Spermatogenesis. Int. J. Mol. Sci. 2020, 21, 9053. [Google Scholar] [CrossRef] [PubMed]
- Taylor, U.; Barchanski, A.; Kues, W.; Barcikowski, S.; Rath, D. Impact of Metal Nanoparticles on Germ Cell Viability and Functionality Production of Metal Nanoparticles. Reprod. Domest. Anim. 2012, 47, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umrani, R.D.; Paknikar, K.M. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin- induced Type 1 and 2 diabetic rats. Nanomedicine 2014, 9, 89–104. [Google Scholar] [CrossRef]
- Falchi, L.; Khalil, W.A.; Hassan, M.; Marei, W.F.A. Perspectives of nanotechnology in male fertility and sperm function. Int. J. Vet. Sci. Med. 2018, 6, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, A.H.; Possebon, L.; Costa, S.D.S.; Souza, H.; Girol, A.; Pereira, M.d.L. Adverse effects of Metal-based Nanoparticles on Male Reproductive Cells. In Top 10 Contributions on Environmental Healt; Avid Science: Berlin, Germany, 2018; pp. 1–19. [Google Scholar]
- Matsumoto, A.M.; Bremner, W.J. Testicular Disorders, 13th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhou, Q.; Yue, Z.; Li, Q.; Zhou, R.; Liu, L. Exposure to PbSe Nanoparticles and Male Reproductive Damage in a Rat Model. Environ. Sci. Technol. 2019, 53, 13408–13416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Hahn, Y. ZnO Nanoparticles: Growth, Properties, and Applications; American Scientific Publishers: Los Angeles, CA, USA, 2010; Volume 5, ISBN 1588831701. [Google Scholar]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Cormode, D.; Naha, P.; Fayad, Z. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast Media Mol. Imaging 2014, 9, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Vega, A.I.; Gómez-Quintero, T.; Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef] [Green Version]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Ashraf, N.; Ashraf, T.; Zhou, R.B.; Yin, D.C. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: Their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 2019, 103, 2913–2935. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Laxman, V.; Linson, P. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnology 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Rajput, J.; Kaith, B.S.; Kaur, M.; Sharma, S. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010, 519, 1224–1229. [Google Scholar] [CrossRef]
- Pinho, A.; Rebelo, S.; Pereira, M. The Impact of Zinc Oxide Nanoparticles on Male (In)Fertility. Materials 2020, 13, 849. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Mathew, A.P.; Sosnik, A. Metal Oxide Nanoparticles as Versatile Therapeutic Agents Modulating Cell Signaling Pathways: Linking Nanotechnology with Molecular Medicine. Appl. Mater. Today 2017, 7, 91–103. [Google Scholar] [CrossRef]
- Al-fartusie, F.S.; Mohssan, S.N. Trace Elements and Their Vital Roles in Human Body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Raven, E.; Le Brun, N.E.; McMaster, J.; Reedijk, J.; Robinson, N.J. Bioinorganic Chemistry. Dalton Trans. 2013, 42, 3027–3028. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Govindaraju, K.; Mohamed Sadiq, A.; Tamilselvan, S.; Ganesh Kumar, V.; Singaravelu, G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2013, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, S.; Zafar, N.; Sharif, R. Antibacterial Activity of Metallic Nanoparticles. In Bacterial Pathogenesis and Antibacterial Control; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The-antimicrobial-activity-of-nanoparticles—Present-situati. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [Green Version]
- Navale, G.R.; Thripuranthaka, M.; Late, D.J.; Shinde, S.S. Antimicrobial Activity of ZnO Nanoparticles against Pathogenic Bacteria and Fungi. JSM Nanotechnol. Nanomed. 2015, 3, 1033. [Google Scholar]
- Mageshwari, K.; Sathyamoorthy, R. Flower-shaped CuO Nanostructures: Synthesis, Characterization andAntimicrobial Activity. J. Mater. Sci. Technol. 2013, 29, 909–914. [Google Scholar] [CrossRef]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef]
- Farias, I.; Santos, C.; Sampaio, F. Antimicrobial Activity of Cerium Oxide Nanoparticles on Opportunistic Microorganisms: A Systematic Review. BioMed Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef] [Green Version]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnologies Russ. 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Brandelli, A.; Ritter, A.C.; Veras, F. Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319637907. [Google Scholar]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 2019, 11, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutfy, S.A.; Al-Ansary, N.A.; Abdel-Ghani, N.T.; Hamed, A.R.; Mohamed, M.B.; Craik, J.D.; Salah Eldin, T.A.; Abdellah, A.M.; Hussein, Y.; Hasanin, M.T.M.; et al. Anti-proliferative activities of metallic nanoparticles in an in vitro breast cancer model. Asian Pac. J. Cancer Prev. 2015, 16, 6039–6046. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Mitjans, M. Antitumor activities of metal oxide nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E. Natural metallic nanoparticles for application in nano-oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef]
- Bai Aswathanarayan, J.; Rai Vittal, R.; Muddegowda, U. Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Anwar, H.; Mahmood, A.; Hussain, G.; Rasul, A.; Umar, M.; Naeem, M.; Ibrahim, M.; Ali, A. Synthesis cum characterization of MgO and MnO nanoparticles and their assessment as antidiabetic and antioxidative agents in diabetic rat model. Phys. B Phys. Condens. Matter 2020, 602, 412570. [Google Scholar] [CrossRef]
- Artimani, T.; Asl, S.; Saidijam, M.; Hasanvand, D.; Afshar, S. Amelioration of diabetes--induced testicular and sperm damage in rats by cerium oxide nanoparticle treatment. Andrologia 2018, 50, e13089. [Google Scholar] [CrossRef]
- El-gharbawy, R.M.; Mahmoud, A.; Abu-risha, S.E. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharmacother. 2016, 84, 810–820. [Google Scholar] [CrossRef]
- Alkaladi, A.; Abdelazim, A.M.; Afifi, M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef] [Green Version]
- Ali, L.; Shaker, S.; Pinol, R.; Millan, A.; Hanafy, M.; Helmy, M.; Kamel, M.; Mahmoud, S. Effect of superparamagnetic iron oxide nanoparticles on glucose homeostasis on type 2 diabetes experimental model. Life Sci. 2020, 245, 117361. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Vinodhini, S.; Elanchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2018, 10, 28–42. [Google Scholar] [CrossRef]
- Anderson, D.; Anderson, T.; Fahmi, F. Advances in Applications of Metal Oxide Nanomaterials as Imaging Contrast Agents. Phys. Status Solidi 2019, 216, 1801008. [Google Scholar] [CrossRef]
- Thurn, K.T.; Brown, E.M.B.; Wu, A.; Vogt, S.; Lai, B.; Maser, J.; Paunesku, T.; Woloschak, G.E. Nanoparticles for applications in cellular imaging. Nanoscale Res. Lett. 2007, 2, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Naseri, N.; Ajorlou, E.; Asghari, F.; Pilehvar-Soltanahmadi, Y. An update on nanoparticle-based contrast agents in medical imaging. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 1111–1121. [Google Scholar] [CrossRef]
- Arifin, D.; Long, C.; Gilad, A.; Alric, C.; Roux, S.; Tillement, O.; Link, T.; Arepally, A.; Bulte, J. Trimodal Gadolinium-Gold Pancreatic Islet Cells Restore Normoglycemia in Diabetic Mice and Can Be Tracked by Using US, Purpose: Methods: Results. Radiology 2011, 260, 790–798. [Google Scholar] [CrossRef] [Green Version]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2020, 33, 1906539. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, F.; Ding, D.; Song, C.; Guo, C.; Liu, S. TiO 2-x Based Nano-platform for Bimodal Cancer Imaging and NIR-Triggered Chem/Photodynamic/Photothermal Combination Therapy. Chem. Mater. 2017, 29, 9262–9274. [Google Scholar] [CrossRef]
- Zhan, Y.; Shi, S.; Ehlerding, E.B.; Graves, S.A.; Goel, S.; Engle, J.W.; Liang, J.; Tian, J.; Cai, W. Radiolabeled, Antibody-Conjugated Manganese Oxide Nanoparticles for Tumor Vasculature Targeted Positron Emission Tomography and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 38304–38312. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Wang, M.; Du, X.; Gu, H.; Zhang, C. Iodinated oil-loaded, fluorescent mesoporous silica-coated iron oxide nanoparticles for magnetic resonance imaging/computed tomography/fluorescence trimodal imaging. Int. J. Nanomed. 2014, 9, 2527–2538. [Google Scholar]
- Guo, Z.; Zhang, P.; Luo, Y.; Xie, H.Q.; Chakraborty, S.; Monikh, F.A.; Bu, L.; Liu, Y.; Ma, Y.; Zhang, Z.; et al. Intranasal exposure to ZnO nanoparticles induces alterations in cholinergic neurotransmission in rat brain. Nano Today 2020, 35, 100977. [Google Scholar] [CrossRef]
- Falchi, L.; Galleri, G.; Dore, G.M.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Pinna, A.; Nieddu, S.; Innocenzi, P.; et al. Effect of exposure to CeO 2 nanoparticles on ram spermatozoa during storage at 4 ° C for 96 hours. Reprod. Biol. Endocrinol. 2018, 16, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odhiambo, J.; Dejarnette, J.; Geary, T.; Kennedy, C.; Susan, S.; Sutovsky, M.; Sutovsky, P. Increased Conception Rates in Beef Cattle Inseminated with Nanopurified Bull Semen. Biol. Reprod. 2014, 91, 97–101. [Google Scholar] [CrossRef]
- Makhluf, S.; Qasem, R.; Rubinstein, S.; Gedanken, A. Loading Magnetic Nanoparticles into Sperm Cells Does Not Affect Their Functionality. Langmuir 2006, 22, 9480–9482. [Google Scholar] [CrossRef]
- Stern, S.T.; McNeil, S.E. Nanotechnology safety concerns revisited. Toxicol. Sci. 2008, 101, 4–21. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.L.; Liu, J.; Feng, X.L.; Zhou, T.; Shao, L.Q. Is Neurotoxicity of Metallic Nanoparticles the Cascades of Oxidative Stress? Nanoscale Res. Lett. 2016, 11, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, N.; Asklund, C.; Carlsen, E.; Skakkebæk, N.E. Coordinated European investigations of semen quality: Results from studies of Scandinavian young men is a matter of concern. Int. J. Androl. 2006, 29, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-, A.; Mendiola, J.; Weksler-derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, M.; Jain, G. Toxic effects of transition metals on male reproductive system: A review. J. Environ. Occup. Sci. 2014, 3, 204. [Google Scholar] [CrossRef] [Green Version]
- Benatta, M.; Kettache, R.; Buchholz, N.; Trinchieri, A. The impact of nutrition and lifestyle on male fertility. Arch. Ital. Urol. Androl. 2020, 92, 121–131. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, B.; Hong, W.; Chen, L.; Yao, L.; Zhao, Y.; Aguilar, Z.P.; Xu, H. ZnO nanoparticles induced male reproductive toxicity based on the effects on the endoplasmic reticulum stress signaling pathway. Int. J. Nanomed. 2019, 14, 9563–9576. [Google Scholar] [CrossRef] [Green Version]
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative Effect of Zinc Oxide Nanoparticles on Antioxidants and Sperm Characteristics in Streptozotocin-Induced Diabetic Rat Testes. BioMed Res. Int. 2015, 2015, 153573. [Google Scholar] [CrossRef]
- Pinho, A.R.; Martins, F.; Costa, M.E.; Senos, A.M.; da Cruz e Silva, O.A.; Pereira, M.D.; Rebelo, S. In Vitro Cytotoxicity Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells. Cells 2020, 9, 1081. [Google Scholar] [CrossRef]
- Mäkelä, J.-A.; Toppari, J. Spermatogenesis. In Endocrinology; Simoni, M., Huhtaniemi, I.T., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 417–455. ISBN 9783319444413. [Google Scholar]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In Sertoli Cell Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124170476. [Google Scholar]
- Gao, G.; Ze, Y.; Zhao, X.; Sang, X.; Zheng, L.; Ze, X.; Gui, S.; Sheng, L.; Sun, Q.; Hong, J.; et al. Titanium dioxide nanoparticle-induced testicular damage, spermatogenesis suppression, and gene expression alterations in male mice. J. Hazard. Mater. 2013, 258–259, 133–143. [Google Scholar] [CrossRef]
- Sundarraj, K.; Manickam, V.; Raghunath, A.; Periyasamy, M.; Viswanathan, M.; Perumal, E. Repeated Exposure to Iron Oxide Nanoparticles Causes Testicular Toxicity in Mice. Environ. Toxicol. 2016, 32, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, K.I.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Heal. Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef] [Green Version]
- McAuliffe, M.E.; Perry, M.J. Are nanoparticles potential male reproductive toxicants? A literature review. Nanotoxicology 2007, 1, 204–210. [Google Scholar] [CrossRef]
- Gallo, A.; Boni, R.; Buttino, I.; Tosti, E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicology 2016, 10, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.; Xiao, S.; Liu, X.; Chen, X.; Xia, Q.; Lei, S.; Li, H.; Zhong, Z.; Xiao, K. The Effects of Gold Nanoparticles on Leydig Cells and Male Reproductive Function in Mice. Int. J. Nanomed. 2020, 15, 9499–9514. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A. Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction, 1st ed.; Zini, A., Agarwal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9781441968579. [Google Scholar]
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157. [Google Scholar] [CrossRef] [Green Version]
- Hess, R.A.; De Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef]
- Pereira, C.; Serrano, J.; Martins, F.; Silva, O.; Rebelo, S. Nuclear envelope dynamics during mammalian spermatogenesis: New insights on male fertility. Biol. Rev. 2019, 94, 1195–1219. [Google Scholar] [CrossRef]
- Donnell, L.; Nicholls, P.; Bryan, M.; Mclachlan, R.; Stanton, P. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef]
- Tapia, J.; Peña, F. Apoptotic Events in Male Germ Cells and in Mature Mammalian Spermatozoa; Salido, G.M., Rosado, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2009; ISBN 9781402098734. [Google Scholar]
- Préaubert, L.; Tassistro, V.; Au, M.; Sari-minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol. Vitr. 2018, 50, 236–241. [Google Scholar] [CrossRef]
- Basioura, A.; Michos, I.; Ntemka, A.; Karagiannis, I.; Boscos, M. Effect of iron oxide and silver nanoparticles on boar semen CASA motility and kinetics. J. Hell. Vet. Med. Soc. 2020, 71, 2331–2338. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Z.; Zhang, H.; Liu, L.; Zhou, X. Repeated administrations of Mn3O4 nanoparticles cause testis damage and fertility decrease through PPAR-signaling pathway. Nanotoxicology 2020, 14, 326–340. [Google Scholar] [CrossRef]
- Pawar, K.; Kaul, G. Toxicity of titanium oxide nanoparticles causes functionality and DNA damage in buffalo (Bubalus bubalis) sperm in vitro. Toxicol. Ind. Health 2014, 30, 520–533. [Google Scholar] [CrossRef]
- Mao, Z.; Yao, M.; Xu, B.; Ji, X.; Jiang, H.; Han, X.; Tang, Q.; Zhou, Z.; Chen, R.; Li, X.; et al. Cytoskeletons of two reproductive germ cell lines response differently to titanium dioxide nanoparticles mediating vary reproductive toxicity. J. Biomed. Nanotechnol. 2017, 13, 409–416. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef]
- Barkhoradi, A.; Hekmatimoghaddam, S.; Jebali, A.; Khalili, M.; Talebi, A.; Noorani, M. Effect of zinc oxide nanoparticles on viability of human spermatozoa. Iran. J. Reprod. Med. 2013, 11, 767–771. [Google Scholar]
- Han, Z.; Yan, Q.; Ge, W.; Liu, Z.-G.; Gurunathan, S.; Felici, M.; Shen, W.; Zang, X.-F. Cytotoxic effects of ZnO nanoparticles on mouse testicular cells. Int. J. Nanomed. 2016, 11, 5187–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Xu, C.; Ji, G.; Liu, H.; Mo, Y.; Tollerud, D.J.; Gu, A.; Zhang, Q. Sublethal effects of zinc oxide nanoparticles on male reproductive cells. Toxicol. Vitr. 2016, 35, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bara, N.; Kaul, G. Enhanced steroidogenic and altered antioxidant response by ZnO nanoparticles in mouse testis Leydig cells. Toxicol. Ind. Health 2018, 34, 571–588. [Google Scholar] [CrossRef]
- Shen, J.; Yang, D.; Zhou, X.; Wang, Y.; Tang, S.; Yin, H. Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells. Int. J. Mol. 2019, 20, 4042. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Gromadzka-ostrowska, J.; Dziendzikowska, K.; Lankoff, A.; Radzikowska, J.; Wojewódzka, M.; Kruszewski, M. Silver nanoparticles effects on epididymal sperm in rats. Toxicol. Lett. 2012, 214, 251–258. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Castellini, C.; Ruggeri, S.; Mattioli, S.; Bernardini, G.; Macchioni, L.; Moretti, E.; Collodel, G. Long-term effects of silver nanoparticles on reproductive activity of rabbit buck. Syst. Biol. Reprod. Med. 2014, 60, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Yousef, M.; Al-hamadani, M.; Kamel, M. Reproductive Toxicity of Aluminum Oxide Nanoparticles and Zinc Oxide Nanoparticles in Male Rats. Nanoparticle 2019, 1, 3. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Li, J.; Qian, J.; Zhang, J.; Zhou, G.; Tong, J. SF-1 mediates reproductive toxicity induced by Cerium oxide nanoparticles in male mice. J. Nanobiotechnology 2019, 17, 41. [Google Scholar] [CrossRef]
- Nasri, S.; Rezai-zarchi, S.; Kerishchi, P.; Sadeghi, S. The Effect of Iron Oxide Nanoparticles on Sperm Numbers and Mobility in Male Mice. Zahedan J. Res. Med. Sci. 2015, 17, 10–12. [Google Scholar] [CrossRef]
- Varzeghani, S.M.; Parivar, K.; Abdollahifar, M.-A.; Karamian, A. Effects of Iron Oxide Nanoparticles on Mouse Sperm Parameters and Testicular Tissue. Iran. J. Toxicol. 2018, 12, 39–44. [Google Scholar] [CrossRef]
- Younus, A.I.; Yousef, M.I.; Abdel-NabiKamel, M.; Alrawi, R.; Abdulrahman, J.M. Changes in semen characteristics and sex hormones of rats treated with iron oxide nanoparticles, silver nanoparticles and their mixture. GSC Biol. Pharm. Sci. 2020, 12, 229–237. [Google Scholar] [CrossRef]
- Negahdary, M.; Arefian, Z.; Dastjerdi, H.A.; Ajdary, M. Toxic effects of Mn2O3 nanoparticles on rat testis and sex hormone. J. Nat. Sci. Biol. Med. 2015, 6, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Yousefalizadegan, N.; Mousavi, Z.; Rastegar, T.; Razavi, Y.; Najafizadeh, P. Reproductive toxicity of manganese dioxide in forms of micro-and nanoparticles in male rats. Int. J. Reprod. Biomed. 2019, 17, 361–370. [Google Scholar] [CrossRef]
- Hong, F.; Si, W.; Zhao, X.; Wang, L.; Zhou, Y.; Chen, M.; Ge, Y.; Zhang, Q.; Wang, Y.; Zhang, J. TiO2 Nanoparticle Exposure Decreases Spermatogenesis via Biochemical Dysfunctions in the Testis of Male Mice. J. Agric. Food Chem. 2015, 63, 7084–7092. [Google Scholar] [CrossRef]
- Hong, F.; Zhao, X.; Si, W.; Ze, Y.; Wang, L. Decreased spermatogenesis led to alterations of testis-specific gene expression in male mice following nano-TiO 2 exposure. J. Hazard. Mater. 2015, 300, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kajal, K.; Paulraj, R. Cytotoxic and Genotoxic Effects of Titanium Dioxide Nanoparticles in Testicular Cells of Male Wistar Rat. Appl. Biochem. Biotechnol. 2015, 175, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; El-hamid, M.; Noshy, P. Reproductive Toxicity Investigation of Titanium Dioxide Nanoparticles in Male Albino Rats. World J. Pharm. Pharm. Sci. 2015, 4, 34–49. [Google Scholar]
- Miura, N.; Ohtani, K.; Hasegawa, T.; Yoshioka, H.; Hwang, G.W. High sensitivity of testicular function to titanium nanoparticles. J. Toxicol. Sci. 2017, 42, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.M.; Ibrahim, M.A.; Noshy, P.A. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem. Biophys. Res. Commun. 2017, 486, 595–600. [Google Scholar] [CrossRef]
- Song, G.; Lin, L.; Liu, L.; Wang, K.; Ding, Y.; Niu, Q.; Mu, L.; Wang, H.; Shen, H.; Guo, S. Toxic effects of anatase titanium dioxide nanoparticles on spermatogenesis and testicles in male mice. Polish J. Environ. Stud. 2017, 26, 2739–2746. [Google Scholar] [CrossRef]
- Lauvås, A.J.; Skovmand, A.; Poulsen, M.S.; Kyjovska, Z.O.; Roursgaard, M.; Goericke-Pesch, S.; Vogel, U.; Hougaard, K.S. Airway exposure to TiO2 nanoparticles and quartz and effects on sperm counts and testosterone levels in male mice. Reprod. Toxicol. 2019, 90, 134–140. [Google Scholar] [CrossRef]
- Miura, N.; Ohtani, K.; Hasegawa, T.; Yoshioka, H.; Hwang, G.W. Biphasic adverse effect of titanium nanoparticles on testicular function in mice. Sci. Rep. 2019, 9, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Karimipour, M.; Khaksar, M.R.; Ghasemnejad-Berenji, M. Protective effects of orally administered thymol against titanium dioxide nanoparticle–induced testicular damage. Environ. Sci. Pollut. Res. 2020, 27, 2353–2360. [Google Scholar] [CrossRef]
- Ogunsuyi, O.M.; Ogunsuyi, O.I.; Akanni, O.; Alabi, O.A.; Alimba, C.G.; Adaramoye, O.A.; Cambier, S.; Eswara, S.; Gutleb, A.C.; Bakare, A.A. Alteration of sperm parameters and reproductive hormones in Swiss mice via oxidative stress after co-exposure to titanium dioxide and zinc oxide nanoparticles. Andrologia 2020, 52, e13758. [Google Scholar] [CrossRef]
- Talebi, A.R.; Khorsandi, L.; Moridian, M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J. Assist. Reprod. Genet. 2013, 30, 1203–1209. [Google Scholar] [CrossRef] [Green Version]
- Abbasalipourkabir, R.; Moradi, H.; Zarei, S.; Asadi, S.; Salehzadeh, A.; Ghafourikhosroshahi, A.; Mortazavi, M.; Ziamajidi, N. Toxicity of zinc oxide nanoparticles on adult male Wistar rats. Food Chem. Toxicol. 2015, 84, 154–160. [Google Scholar] [CrossRef]
- Mozaffari, Z.; Parivar, K.; Roodbari, N.H.; Irani, S. Histopathological Evaluation of the Toxic Effects of Zinc Oxide (ZnO) Nanoparticles on Testicular Tissue of NMRI Adult Mice. Adv. Stud. Biol. 2015, 7, 275–291. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.; Ali, H.; Saadeldin, I.; Ahmed, M. Querectin Alleviates Zinc Oxide Nanoreprotoxicity in Male Albino Rats. J. Biochem. Mol. Toxicol. 2016, 30, 489–496. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Kumar, A.; Prakash, J.; Singh, D.; Jagdale, P.; Shankar, J.; Kumar, M. Genotoxicity evaluation of zinc oxide nanoparticles in Swiss mice after oral administration using chromosomal aberration, micronuclei, semen analysis, and RAPD profile. Toxicol. Ind. Health 2017, 33, 821–834. [Google Scholar] [CrossRef]
- Mesallam, D.; Deraz, R.; Abdel Aal, S.; Ahmed, S. Toxicity of Subacute Oral Zinc Oxide Nanoparticles on Testes and Prostate of Adult Albino Rats and Role of Recovery. J. Histol. Histopathol. 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Radhi, M.J.; Adnan, G.; Latef, A. Effect of Zinc oxide nanoparticles (ZnO-NPs) on weights of some reproductive organs and sperm abnormalities in the tail of epididymis of albino mice. J. Pharm. Sci. Res. 2019, 11, 243–246. [Google Scholar]
- Salman, R.A. The Influence of ZnO NPs on Reproductive System Tissues of Albino Male Mice. Histopathological Study. Int. J. Sci. Res. 2021, 6, 2021–2025. [Google Scholar] [CrossRef]
- Hess, R.; Chen, P. Computer tracking of germ cells in the cycle of the seminiferous epithelium and prediction of changes in cycle duration in animals commonly used in reproductive biology and toxicology. J. Androl. 1992, 13, 185–190. [Google Scholar]
- Millaku, L.; Imeri, R.; Trebicka, A. Histopathological changes in testes of house sparrow (Passer domesticus). J. Mater. Environ. Sci. 2015, 6, 1292–1296. [Google Scholar]
- Saleh, R.; Agarwal, A. Oxidative Stress and Male Infertility: From Research Bench to Clinical Practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Shandilya, R.; Mishra, P.K.; Pathak, N.; Lohiya, N.K.; Sharma, R.S. Nanotechnology in reproductive medicine: Opportunities for clinical translation. Clin. Exp. Reprod. Med. 2020, 47, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Durfey, C.L.; Swistek, S.E.; Liao, S.F.; Crenshaw, M.A.; Clemente, H.J.; Thirumalai, R.V.K.G.; Steadman, C.S.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Nanotechnology-based approach for safer enrichment of semen with best spermatozoa. J. Anim. Sci. Biotechnol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Chang, M.; Chang, Y.-J.; Wang, T.-Y.; Yu, Q. Sperm Movement Control Utilizing Surface Charged Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5713–5722. [Google Scholar] [CrossRef] [PubMed]
- Moridi, H.; Hosseini, S.A.; Shateri, H.; Kheiripour, N.; Kaki, A.; Hatami, M.; Ranjbar, A. Protective effect of cerium oxide nanoparticle on sperm quality and oxidative damage in malathioninduced testicular toxicity in rats: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 261–266. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.J.; Doo, S.H.; Yang, W.J.; Choi, D.; Kim, J.H.; Won, J.H.; Song, Y.S. Use of nanoparticles to monitor human mesenchymal stem cells transplanted into penile cavernosum of rats with erectile dysfunction. Korean J. Urol. 2015, 56, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobyliak, N.M.; Falalyeyeva, T.M.; Kuryk, O.G.; Beregova, T.V.; Bodnar, P.M.; Zholobak, N.M.; Shcherbakov, O.B.; Bubnov, R.V.; Spivak, Y.M. Antioxidative effects of cerium dioxide nanoparticles ameliorate age-related male infertility: Optimistic results in rats and the review of clinical clues for integrative concept of men health and fertility. EPMA J. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitounis, D.; Klein, J.P.; Mery, L.; El-Merhie, A.; Forest, V.; Boudard, D.; Pourchez, J.; Cottier, M. Ex vivo detection and quantification of gold nanoparticles in human seminal and follicular fluids. Analyst 2018, 143, 475–486. [Google Scholar] [CrossRef]

| MONPs | Characteristics | Concentration and Exposure Time | Cell Type | Parameters | Main Findings | Reference |
|---|---|---|---|---|---|---|
| Cerium oxide | Formula: CeO2 Size: ~7 nm SA: 400 m2/g Shape: Ellipsoidal crystallites | 0.01, 0.1, 1, 10 µg/mL 1 h | Spermatozoa (Human) | - Sperm vitality; - DNA damage; - Uptake of NPs | - Sperm viability higher than the normality threshold—58% - Increased DNA damage (≥0.01 µg/mL); - Accumulation of NPs at the plasma membrane, particularly along the flagellum, without internalization | [108] |
| Iron oxide | Formula: Fe3O4 Size: 40 nm Shape: spherical | 0.192 mg/mL 30, 45, and 60 min | Spermatozoa (Boar) | - Motility and kinetics | - No effects on sperm motility | [109] |
| Manganese oxide | Formula: Mn3O4 Size: ~ 20 ± 4.1 nm Shape: irregular sphere-like morphology | 0, 5, 10, 20 µg/mL 6 and 24 h | Sertoli Cells (Rats) | - ROS production; - MMP and apoptosis; | - Increase in ROS (5 µg/mL, 24 h); - Alterations in the mitochondrial membrane integrity and increase in the apoptotic rates (≥5 µg/mL, 24 h) | [110] |
| Titanium oxide | Formula: TiO2 Size: ~30–90 nm Zeta potential: −27.3 mV | 1, 10, 100 µg/mL 0, 3, 6 h | Spermatozoa (Bufallo) | - Viability; - Acrosomal and plasma membrane integrity; - Capacitation; - Acrosome-reaction; - DNA fragmentation; - Uptake of NPs | - Viability decrease (100 µg/mL, 3 and 6 h); - Decrease in the integrity of the plasma membrane (≥1 µg/mL, 6 h) and acrosomal membrane (100 µg/mL, 6 h); - Increase in capacitation (≥10 µg/mL, 6 h); - Increase in acrosomal reaction (≥1 µg/mL, 3 and 6 h); - Increased DNA fragmentation (≥10 µg/mL, 6 h); - Uptake of NPs mainly in the plasma membrane and sperms’ head | [111] |
| Formula: TiO2 Size: ~21 nm Shape: spherical Zeta potential: −124.55 ± 13.20 mV HS: 115.2 ± 11.3 nm Purity: >99.5% PDI: 0.19 | 0.1, 1, 10, 100 µg/mL 24 h | Spermatocytes and Sertoli cells (Mouse) | - Viability; - Apoptosis; - Uptake of NPs - Cytoskeleton; - Migration ability; - Phagocytic activity | - Cell viability was not affected; - Increase in the early apoptosis ratio for both cells and in the late apoptosis ratio for Sertoli cells (100 µg/mL); - Dose-dependent uptake of the nanoparticles, mainly in the cytoplasm; - Disordered microtubules (spermatocytes) and microfilaments (Sertoli cells); - Decreased migration ability of spermatocytes (100 µg/mL); - Weakened phagocytic capacity of Sertoli cells (100 µg/mL) | [112] | |
| Formula: TiO2 Size: ~21 nm Shape: partly irregular and semispherical | 1, 10 µg/L 15, 30, 45, 90 min | Spermatozoa (Human) | - Viability; - Motility characteristics; - DNA damage; - ROS production | - Cell viability was not affected; - Increase in progressive and nonprogressive sperm (1, 10 µg/L for ≥ 45 min); - Increase in DNA damage (1, 10 µg/L for ≥ 30 min); - Increase in ROS production (1, 10 µg/L for ≥ 15 min) | [113] | |
| Zinc oxide | Formula: ZnO Size: ~50 nm Shape: amorphous | 10, 100, 500, 1000 µg/mL 45, 90, and 180 min | Spermatozoa (Human) | - Viability | - Increase in cell death (≥100 µg/mL, 180 min and ≥ 500 µg/mL, ≥ 45 min) | [114] |
| Formula: ZnO Size: ~70 nm Shape: spherical Dispersion: polydisperse Surface roughness: high (22.9 nm) | 0, 5, 10, 15, 20 µg/mL 3, 6, 12, and 24 h | Leydig and Sertoli cells (Mouse) | - Viability; - ROS production; - Uptake of NPs; - MMP and apoptosis; - DNA damage | - Decreased viability in both cell types (≥15 µg/mL, ≥6 h); - Increase in ROS production (≥10 µg/mL, ≥6 h) - Accumulation and uptake of nanoparticles’ aggregates in the cytoplasm and nucleus; - Loss of MMP and apoptosis increase (≥15 µg/mL, 6–12 h); - DNA leakage with an increase in chromosome breaks or loss (≥15 µg/mL, ≥12 h) | [115] | |
| Formula: ZnO Size: 177 nm Shape: spheroid or ellipsoid Zeta potential: −27.4 ± 1.0 mV Purity: >97% | 0, 0.04, 0.08, 0.4, 0.8, 4, 8, 16 µg/mL 24 h | Spermatocytes and Sertoli cells (Mouse) | - Viability; - Oxidative stress indexes (ROS, GSH, MDA) of both cell types; - Membrane permeability, MMP and cytochrome c of Sertoli cells; - TNF-α and Erk1/2 levels of Sertoli cells; - Connexin-43, occludin, claudin-5, ZO-1 expression of Sertoli cells; - DNA damage of spermatocytes; - Cell cycle analysis (cyclin E2, cyclin A2, CDK2) of spermatocytes | - Decrease in cell viability (≥8 µg/mL); - Increase in ROS and MDA levels and decrease in GSH (8 µg/mL); - Increase in membrane permeability with decrease in MMP (8 µg/mL), but no significant changes in cytochrome c (8 µg/mL); - Increase in TNF-α and phosphorylation of Erk1/2 (8 µg/mL);- Decrease in claudin-5, occludin, ZO-1 and connexin-43 expression (8 µg/mL); - Increase in p-Chk1, p-Chk2 and ϒ-H2AX expression but decrease in APE1 (8 µg/mL) but DNA damage can be partly rescued by antioxidants; - Increase in cyclin E2, cyclin A2, CDK2 expression with an increase of cell numbers in the S phase (8 µg/mL) | [116] | |
| Formula: ZnO Size: 20–40 nm Shape: spherical HS: 75 nm | 0–200 µg/mL 1, 4, and 12 h | Leydig cells (Mouse) | - Viability; - Cell morphology; - Uptake of NPs; - Apoptosis; - Oxidative stress indexes (SOD, CAT); - Steroidogenesis-related genes expression (StAR, P450scc); - Antioxidant enzyme related gene (SOD); - Testosterone levels in cells’ supernatant | - Decrease in cell viability (≥2 µg/mL, ≥1 h); - Loss of normal morphology (≥5 µg/mL, 4 h); - Randomly dispersed agglomerates of NPs in the cytoplasm, autophagosomes, autolysosomes, mitochondria and in nuclear membranes (50 µg/mL, 4 h); - Apoptosis increase (5 or 20 µg/mL, 4 h); - Increase in SOD (1, 5 µg/mL, 4 h and 5, 20, 50 µg/mL, 12 h), CAT (1, 5, 20 µg/mL, 4 h and 5, 20 µg/mL, 12 h) activity; - Increase in StAR (1, 5 µg/mL, 4 h and 1 µg/mL, 12 h) and P450scc expression (1, 5 µg/mL, 4 h); - Decrease in SOD mRNA (1, 5 µg/mL, 4 h); - Increase in testosterone production (2 µg/mL, 12 h) | [117] | |
| Formula: ZnO Size: 30 nm Zeta potential: 38.25 ± 1.06 mV HS: 66.36 ± 0.93 nm | 0, 2, 3, 4, 8 µg/mL 24 h | Leydig cells (Mouse) | - Viability; - Oxidative stress indexes (GPx, GSH, SOD, MDA); - Apoptosis-related proteins (cleaved Casp-8 and Casp-3, Bcl-2, Bax); - Autophagy-related proteins (Atg-5, Beclin-1) and LC3-II/LC3-I ratio | - Decrease in cell viability (≥3 µg/mL); - Increase in MDA levels (≥3 µg/mL) and decrease in SOD, GSH (≥3 µg/mL) and GPx (≥2 µg/mL) levels; - Increase in the expression of cleaved Casp-8, Casp-3 and Bax and decrease in Bcl-2 expression; - Increase in LC3-II to LC3-I ratio and Atg-5 and Beclin-1 expression (4 µg/mL) | [118] | |
| Formula: ZnO Size: 88 nm SA: 12 m2/g Shape: spherical Crystal structure: hexagonal wurtzite Zeta potential: −15 mV (pH = 6) and −55 mV (pH = 12) | 1, 5, 8, 10, 20 µg/mL 6 and 12 h | Spermatogonia(Mouse) | - Viability; - Apoptosis and necrosis; - ROS production; - DNA damage; - Cytoskeleton dynamics; - Nucleoskeleton dynamics; - Nuclei morphological changes | - Decrease in cell viability (20 µg/mL, 12 h); - Cell death by necrosis (20 µg/mL, 12 h); - Increase in ROS levels (20 µg/mL, 6 h and ≥5 µg/mL, 12 h); - Increase in DNA damage (20 µg/mL, ≥6 h); - Interference with microtubule and microfilament protein levels (20 µg/mL for 6 h and 12 h); - Alterations of the basal levels and distribution of the nuclear lamina and nuclear envelope proteins (20 µg/mL, 12 h); - Visible morphological deformities in the cells’ nuclei. | [92] |
| MONPs | Characteristics | Dosage and Exposure Duration | Route of Administration | Animal Model/Tissue/Organ/Fluid | Parameters | Main Findings | Reference |
|---|---|---|---|---|---|---|---|
| Aluminum oxide | Formula: Al2O3 Size: 50 nm | 70 mg/kg/day 75 days | Oral | Wistar Rats Testis Prostate Epididymis Sperm Plasma | - Reproductive organs weight; - mtTFA, UCP2 testis levels; - DNA fragmentation; - p53, TNF-α, IL-6 testis levels; - Oxidative stress indexes (GPx, GST, CAT, SOD, GSH, TAC, TBARS, NO); - Steroidogenic enzymes levels (17-KSR, 17β-HSD); - Sperm quality; - Reproductive and thyroid hormones levels (testosterone, FSH, LH, TSH, T3, T4); - Testis histopathology | - Decline in testis and epididymis weight but increase in prostate weight; - Suppression and increase of MtTFA and UCP2 expression, respectively; - Massive DNA fragmentation; - Increase in p53, TNF-α and IL-6 levels; - Decrease in GPx, GST, CAT, SOD, GSH, TAC levels and increase in TBARS and NO levels; - Increase and decrease in 17β-HSD and 17-KSD levels, respectively; - Reduction in sperm quality; - Decrease in testosterone and TSH levels, increase in FSH, LH, T3 and T4 levels; - Degenerative changes in testis | [123] |
| Cerium oxide | Formula: CeO2 Size: <25 nm Purity: >99% | 10, 20, 40 mg/kg/day 32 days | Oral | C57BL/6J Mice Testis Epididymis Epididymis Sperm Plasma | - Ce accumulation; - Testis weight; - Sperm quality; - Testis histopathology; - Testicular marker enzymes levels (ACP, G6PD, γ-GT, SDH); - Testosterone and transcription factors genes expression (StAR, P450scc, P450c17, 3β-HSD, 17β-HSD, SF-1) | - Increase of Ce content in testis and in denatured sperm DNA (≥20 mg/kg); - Decrease in testis weight (40 mg/kg); - Reduction in sperm quality (≥20 mg/kg); - Seminiferous tubules damage and apoptosis in interstitial tissue (≥20 mg/kg); - Decreased activities of G6PD, SDH, γ-GT (≥20 mg/kg) and ACP (40 mg/kg); - Decrease in testosterone levels and expression of SF-1, StAR, P450scc, P450c17, 3β-HSD (≥20 mg/kg) | [124] |
| Iron oxides | Formula: Fe2O3 Size: 20 ± 5 nm | 5, 10, 20, 40 mg/kg 2 weeks | Intraperitoneal | Mice Testis Epididymis Epididymis Sperm | - Sperm quality; - Testis histopathology | - Reduction in sperm quality (≥5 mg/kg); - Reduction of spermatids and spermatocytes in ST and detachment of spermatogonia and spermatocytes from ST wall | [125] |
| Formula: Fe2O3 Size: <50 nm | 25, 50 mg/kg/week 4 weeks | Intraperitoneal | Albino Mice Testis Epididymis Serum | - Total protein in the testis; - Sperm quality; - Testis and serum LDH and testosterone levels; - Testis histopathology; - Fe accumulation; - Oxidative stress indexes (ROS, MDA, SOD, NO, LPO, PC, CAT, GPx, GSH, vitamin C); - DNA damage and apoptosis (Bax, cleaved-Casp3 and -PARP) | - Decrease in total protein in the testis (≥25 mg/kg); - Reduction in sperm quality (≥25 mg/kg); - Increase in testosterone and LDH levels (≥25 mg/kg); - Detachment, sloughing and vacuolization of ST (≥25 mg/kg); - Increased Fe levels in the testis and in serum (≥25 mg/kg); - Increase in ROS, LPO, PC, SOD, NO, CAT, GPx (≥25 mg/kg), decrease in CAT, GSH (50 mg/kg) and vitamin C (≥25 mg/kg) levels; - Increase in the expression of Bax, cleaved-PARP and -Casp3, confirming DNA damage and apoptosis | [97] | |
| Formula: Fe3O4 Size: 20–30 nm | 50, 150, 300 mg/kg/day 4 days | Intraperitoneal | NMRI Mice Epididymis Testis Semen | - Sperm quality; - Testis cell number (spermatogonia, primary spermatocytes, spermatids, Sertoli and Leydig cells); - ST morphometry; - Volume of testis and interstitial tissue | - No significant changes in sperm number, decrease in VCL, VSL, VAP and rapid progressive motility values and increase in the percentage of immotile sperm (300 mg/kg/day); - Reductions in the total number of testicular cells; - Reduction in ST length, volume of the testis and interstitial tissue (300 mg/kg/day) | [126] | |
| Formula: Fe3O4 Size: <50 nm | 5 mg/kg/day 79 days | Oral | Wistar Rats Epididymis Sperm Plasma Testis | - Sperm quality; - Reproductive and thyroid hormones levels (testosterone, TSH, FSH, LH, T3, T4); - Activity enzymes related to testosterone production (17β-HSD and 17-KSD activity) | - Reduction in sperm count, motility and increase in abnormal sperm; - Decrease in testosterone and TSH levels, increase in FSH, LH, T3 and T4 levels; - Reduction in 17β-HSD and 17-KSD activity | [127] | |
| Manganese oxides | Formula: Mn2O3 Size: ~70 nm | 100, 200, 400 mg/kg/day 14 days | Oral | Wistar Rats Testis Epididymis Blood | - Reproductive hormones levels (testosterone, LH and FSH); - Testis cell number (spermatogonia, primary spermatocytes, spermatids, Leydig cells); - Testis histopathology | - Decrease in testosterone, LH and FSH levels (400 mg/kg); - Reduction in testicular cell number (400 mg/kg); - Cellular disruption of ST (≥200 mg/kg), interstitial edema of ST, appearance of vacuoles in epithelium and reduction in cell regulation (400 mg/kg) | [128] |
| Formula: MnO2 Size: 25–85 nm | 100 mg/kg/week 4 weeks | Subcutaneous | Wistar Rats Testis Epididymis Seminal vesicle Prostate Serum Epididymis Sperm | - Testis cell number (sperm, spermatozoa, spermatogonia and spermatocytes); - Reproductive organs weight; - Reproductive hormones levels (testosterone, E2, FSH); - Sperm quality; - Testis histopathology; - ST morphometry; | - Reduction in testicular cell number; - No difference in the prostate, epididymis and left testicle’s weight; - No significant difference in FSH, E2 and testosterone levels (4th week); - Decrease in sperm number and motility (100% immotile sperm, 4th week); - Fluid accumulation in the interstitial space of germline cells; - Decrease in ST mean diameter | [129] | |
| Formula: Mn3O4 Size: ~20 ± 4.1 nm Shape: irregular sphere-like morphology | 10 mg/kg/week0, 60, 120 days | Intravenous | Sprague–Dawley Rats Testis Epididymis Sperm Serum | - Mn biodistribution in testis and serum; - Testis morphometry and histopathology; - Reproductive hormones levels (testosterone, LH, FSH); - Oxidative stress indexes (MDA, SOD); - Sperm quality; - Fertility evaluation; - Transcription profiling in the testis | - Increase in Mn content in serum and testis (≥60 days); - Reduction of the thickness of germinative layer (≥60 days) and ST degeneration (120 days); - Decline in testosterone and FSH but increase in LH levels (120 days); - Increase in SOD and MDA levels (120 days); - Increase in sperm abnormalities, decrease in sperm concentration and motility (120 days); - Decrease in fertility and fetal survival rate (120 days); - Upregulation of PPAR-signaling pathway and increased expression of cytochrome P450 | [110] | |
| Titanium oxide | Formula: TiO2 Size: 5–6 nm SA: 174.8 m2/g HS: 294 nm Zeta potential: 9.28 mV | 2.5, 5, 10 mg/kg/day 60 days | Intragastric | ICR Mice Testis Epididymis Epididymis Sperm | - Testis weight; - Sperm quality; - LDH, SODH, SDH, G-6PD, ACP, AKP, TNOS, Ca2+-ATPase, Ca2+/Mg2+-ATPase, and Na+ /K+ -ATPase levels; - Oxidative stress indexes (ROS, MDA, PC, 8-OHdG); - Testis and epididymis histopathology | - Reduction in testis weight (≥5 mg/kg); - Decline in sperm concentration, motility (≥5 mg/kg) and increase in morphological abnormalities (≥2.5 mg/kg); - Decreased activity of LDH, SODH (≥5 mg/kg), SDH, G6PD, ATPases (≥2.5 mg/kg), and elevated activity of ACP (≥5 mg/kg), AKP and NOS (≥2.5 mg/kg); - Increase in ROS (≥2.5 mg/kg), MDA, PC and 8-OHdG (≥5 mg/kg) levels; - ST degeneration, reduced number of Leydig cells and mature sperm within the lumen, sperm breakages, spermatolysis, androgone fusion and/or pycnosis (≥2.5 mg/kg); | [130] |
| Formula: TiO2 Size: ~ 5.5 nm SA: 174.8 m2/g HS: 208–330 nm Zeta potential: 9.28 mV | 1.25, 2.5, 5 mg/kg/day 6 months | Intragastric | CD-1 Mice Testis Epididymis Sperm | - Reproductive organs weight; - Ti accumulation; - Sperm quality; - Testis and epididymis histopathology; - Cdc2, Cyclin B1, Gsk3-β, TERT, Tesmin, TESP-1, XPD, XRCCI, PGAM1/4 and DMC1 expression | - Decrease in testis (≥2.5 mg/kg) and epididymis weight (≥1.25 mg/kg); - Increase in Ti content in testis and epididymis (≥2.5 mg/kg); - Decrease in sperm number, motility rate and increase in abnormalities (≥1.25 mg/kg); - Pathological changes in the testis and epididymis with NPs agglomerates in the ST and few spermatozoa in epididymis lumen (≥1.25 mg/kg); - Decreased expression of Cdc2, DMC1, TERT, Tesmin, Cyclin B1, XRCC1 and XPD and increased expression of Gsk3-β and PGAM4 (≥1.25 mg/kg) | [131] | |
| Formula: TiO2 Size: 21 nm | 5, 25, 50 mg/kg/week 4 weeks | Intravenous | Wistar Rats Testis Serum | - Ti accumulation; - Oxidative stress indexes (CAT, SOD, GPx, LPO); - CK, testosterone and Casp-3 levels; - Sperm number; - DNA damage and apoptosis; - Testis histopathology | - Accumulation of Ti in the testis (≥5 mg/kg); - Decrease in SOD and GPx and increase in CAT and LPO levels (≥25 mg/kg); - Increase in CK levels and in Casp3 activity (50 mg/kg) but decrease in testosterone levels (≥25 mg/kg); - Decline in sperm count; - DNA damage and apoptosis (≥25 mg/kg); - Disorganized and disrupted ST with NPs aggregates in spermatids, Sertoli and Leydig cells (50 mg/kg) | [132] | |
| Formula: TiO2 Size: 10 nm | 100 mg/kg/day 4 and 8 weeks | Oral intubation | Albino Rats Testis Epididymis Seminal vesicle Prostate gland Epididymis Sperm Serum | - Reproductive organs weight; - Testosterone levels; - Sperm quality; - Testis, epididymis, prostate gland and seminal vesicle histopathology | - Decrease in testis, epididymis (8th week), and seminal vesicle weight (4th week); - Decrease in testosterone levels (≥4th week); - Decrease in sperm motility, concentration and viability with increase of sperm abnormalities (≥4th week); - Interstitial edema and sloughing of SE, pyknosis, karyolysis and karyoschisis in testis; congestion, vacuolation and inflammatory cells infiltration with spermatid coagulum in epididymis; congestion, hyperplasia and desquamation of prostate’s epithelial lining; congestion in seminal vesicle | [133] | |
| Formula: TiO2 Z-average size: 150 d.nm | 0.1, 1, 2, 10 mg/kg/week 4 weeks | Intravenous | C57BL/6J Mice Testis Epididymis Sperm Epididymis Plasma | - Reproductive organs weight; - Sperm quality; - Reproductive hormones levels (testosterone, LH, FSH, GnRH); - Ti accumulation | - No significant changes in the testis and epididymis weight; - Decrease in sperm number (10 mg/kg) and in motile and progressive sperm (≥0.1 mg/kg); - Only testosterone levels were decreased (0.1 mg/kg); - No significant accumulation of Ti in the testis | [134] | |
| Formula: TiO2 N/A | 100 mg/kg/day 8 weeks | Oral intubation | Albino Rats Epididymis Sperm Serum Blood Testis | - Sperm quality; - Oxidative stress indexes (CAT, GSH, MDA); - Testosterone, Casp-3 and Testin levels; - Testis histopathology | - Decrease in sperm quality; - Decline in the levels of testosterone and GSH and increase in MDA levels, with non-significant effect on CAT; - Activation of Casp3, indicating apoptosis and upregulation of Testin gene; - Interstitial edema and sloughing of the germinal epithelium with apoptotic changes | [135] | |
| Formula: TiO2 Size: ~10 nm SA: 120 m2/g Purity: >99.8% Shape: rhabditiform Zeta potential: −20.7 to −3.77 mV | 0, 10, 50, 100 mg/kg/day 28 days | Intragastrical | ICR Mice Epididymis Sperm Epididymis Testis | - Reproductive organs weight; - Sperm quality; - Oxidative stress indexes (SOD, MDA); - Testis histopathology | - No significant changes in testis and epididymis weight; - No significant changes in sperm density and increase in sperm malformation (≥50 mg/kg); - Decrease in SOD (100 mg/kg) and increase in MDA (≥50 mg/kg) content; - Disordered and vacuolized spermatogenic cells with reduced number (≥50 mg/kg) | [136] | |
| Formula: TiO2 Size: 17 nm SA: 107.7 m2/g Z-average size: 218 nm PDI: 0.24 | 63 µg/week 7 weeks | Intratracheal | C57BL/6J Testis Epididymis | - Reproductive organs weight; - Sperm count; - Testosterone levels; | - No significant changes in testis and epididymis weight; - No significant changes in sperm count; - No significant effect on testosterone levels; | [137] | |
| Formula: TiO2 Z-average size: 150 d.nm | 0, 2, 10 mg/kg/week 4 weeks | Intravenous | C57BL/6J Mice Testis Epididymis Sperm Epididymis | - Reproductive organs weight; - Sperm quality; - Ti accumulation | - No significant changes in testis and epididymis weight; - Decrease in sperm number and in motile and progressive sperm (≥2 mg/kg); - No significant accumulation of Ti in the testis | [138] | |
| Formula: TiO2 Size: ~40 nm | 100 mg/kg/day 60 days | Oral gavage | Wistar Rats Testis Epididymis Sperm | - Sperm quality; - Oxidative stress indexes (CAT, SOD, GPx, MDA, GSH, FRAP values); - SE and ST morphometry; - Testis histopathology | - Decline in sperm quality; - Increase in MDA levels, decrease in CAT, SOD, GPx, GSH and FRAP values; - Decline in the diameter of ST and height of SE; - ST with irregular shape, wide interstitial space with reduced number of Leydig cells | [139] | |
| Formula: TiO2 Size: < 25 nm Shape: spherical Zeta potential: +2.8 to +5.8 mV PDI: 0.822 HS: 1492 nm | 9.38, 18.75, 37.5, 75 mg/kg/day 35 days | Intraperitoneal | Swiss Mice Testis Serum Epididymis Sperm | - Testis weight; - Sperm quality; - Reproductive hormone levels (testosterone, LH, FSH); - Oxidative stress indexes (SOD, CAT, GSH, MDA); - Testis tissue morphometry; - Testis histopathology | - No significant changes in testicular weight; - Decrease in motile sperm (≥9.38 mg/kg) and in sperm count with an increase in sperm abnormalities (≥18.75 mg/kg); - Decrease in LH (≥9.38 mg/kg) and FSH (75 mg/kg) levels, with no significant changes in testosterone levels; - Reduced activity of SOD (≤37.5 mg/kg), CAT (≥9.38 mg/kg) and GSH (9.38 mg/kg) and increased MDA levels (≥18.75 mg/kg); - Decrease in germinal height (9.38, 37.5, 75 mg/kg) and increase of luminal width (≥9.38 mg/kg); - Increased number of damaged ST, Leydig cell degeneration and necrosis of spermatogenic cells (75 mg/kg) | [140] | |
| Zinc Oxide | Formula: ZnO N/A | 0, 5, 50, 300 mg/kg/day 35 days | Oral | NMRI Mice Epididymis Testis Epididymis Sperm | - Testis weight; - Sperm quality; - ST histopathology; - SE maturity; - ST and SE morphometry | - Decrease in testis weight (300 mg/kg); - Decrease in sperm number and motility, increase in abnormalities (≥50 mg/kg); - Increase in detached, sloughed (≥50 mg/kg), vacuolized (≥5 mg/kg) and multinucleated ST (300 mg/kg); - SE maturation arrest with abnormal spermatogenesis (≥50 mg/kg); - Decrease in ST diameter and SE height (≥50 mg/kg) | [141] |
| Formula: ZnO Size: 10–30 nm SA: 20/30 m2/g Crystal phase: single Crystal morphology: nearly spherical Density: 5.606 g/cm3 Purity: ≥99% | 0, 50, 100, 150, 200 mg/kg/day 10 days | Intraperitoneal | Wistar Rats Liver Kidneys Epididymis Sperm Serum | - SOD, GPx, MDA, TAC, TOS levels; - Sperm quality; | - No difference in the levels of SOD and GPx, increase in MDA (≥100 mg/kg) and TOS (200 mg/kg) and decrease in TAC (200 mg/kg) levels; - Decrease in sperm count, viability, normal morphology (≥50 mg/kg) and motility (≥100 mg/kg); | [142] | |
| Formula: ZnO Size: 20 nm SA: >90 m2/g Color: white Crystal morphology: nearly spherical Purity: ≥99% | 0, 250, 500, 700 mg/kg/day 7 days | Intraperitoneal | NMRI Mice Testis | - Testis weight; - Testis histopathology | - No alterations in testis weight; - No alterations in the tunica albuginea thickness and no increase in degenerated ST. Decrease in ST and SE diameter (250 and 500 mg/kg). Decrease in the number of A type spermatogonia (≥500 mg/kg), primary spermatocytes (500 mg/kg) and fibroblasts (≥250 mg/kg). Higher number of degenerated cells, and multinucleated spermatids (≥250 mg/kg). No alterations in the number of Sertoli, spermatids, spermatozoa, and B type spermatogonia cells | [143] | |
| Formula: ZnO Size: ~ 70 nm Shape: spherical Nature: crystalline Dispersion: polydisperse Surface roughness: high (22.9 nm) | 0, 1, 5 mg/kg single dose at PND21 | Intravenous | CD-1 Mice Epididymis Testis Epididymis Sperm | - SE and ST morphometry; - Sperm morphology | - Reduction in SE thickness (5 mg/kg, PND28 and PND42) but no differences in ST diameter; - Increase in sperm abnormalities (≥1 mg/kg, 49 days after injection) | [115] | |
| Formula: ZnO Size: <50 nm SA: >10.8 m2/g Purity: >97% | 0, 100, 400 mg/kg/day 12 weeks | Intragastric | Albino Rats Epididymis Testis Epididymis Sperm Serum | - Sperm quality; - Oxidative stress indexes (MDA, CAT, SOD, GPx, GSH); - Testosterone levels; - Expression of enzymes related to testosterone production (3β-HSD, 17β-HSD and Nr5A1); - Testis histopathology | - Decline in sperm motility, viability (≥100 mg/kg) and concentration and increase in sperm abnormalities (400 mg/kg); - Increase in MDA (400 mg/kg), decrease in GSH, GPx, SOD and CAT (≥100 mg/kg) levels; - Reduction in testosterone production (≥100 mg/kg); - Reduction in the expression of 3β-HSD, 17β-HSD and Nr5A1 (≥100 mg/kg); - Increased cell apoptosis, ST damage, sloughing of immature germ cells from ST (≥100 mg/kg) | [144] | |
| Formula: ZnO Size: 39.45 ± 19.88 nm HS: 447.5 nm Aggregation: large and irregular PDI: 0.13 nm Shape: hexagonal Zeta potential: −32.1 mV | 300, 2000 mg/kg twice at 24 h interval | Oral | Swiss Mice Liver Epididymis Sperm | - Sperm quality; - Liver ROS and 8-oxo-G levels | - Decline in sperm count (2000 mg/kg), motility, viability (≥300 mg/kg) and increase in aberrant sperm during the maturation phase (2000 mg/kg); - Increase in ROS levels and 8-oxo-G expression (2000 mg/kg) | [145] | |
| Formula: ZnO Size: <100 nm Purity: ≥99.5% Color: white | 0, 422 mg/kg/day 4 weeks | Oral gavage | Albino Rats Testis Prostate Serum | - Oxidative stress indexes (MDA, GSH, CAT, SOD); - Testis and prostatic cytokines content (TNF-α, IL-4); - Testis and prostate DNA fragmentation; - Testis and prostate histopathology; | - Elevation of MDA and reduction of GSH, CAT, SOD; - Increase in TNF-α and decrease in IL-4; - Confirmed DNA fragmentation; - Tunica albuginea with congested blood vessels, disorganized ST with cell loss and absence of spermatozoa, SE separated from basement membranes and some germ cells with dark pyknotic nuclei; | [146] | |
| Formula: ZnO Size: 50 nm Shape: cube Color: white Purity: 99.99% | 100, 200 mg/kg/day 7 and 14 days | Oral gavage | Albino Mice Testis Epididymis Seminal vesicle Prostate Epididymis Sperm | - Reproductive organs weight; - Sperm abnormalities | - Decline in testis and epididymis weight but hypertrophy of seminal vesicle and prostate (≥100 mg/kg, ≥7 days); - Increase in sperm abnormalities (≥100 mg/kg, ≥7 days) | [147] | |
| Formula: ZnO Size: 30 nm Zeta potential: 38.25 ± 1.06 mV HS: 66.36 ± 0.93 nm | 0, 100, 200, 400 mg/kg/day 28 days | Intragastric | Kunming Mice Testis Epididymis Serum | - Testosterone levels; - Testis and epididymis histopathology; - Gene expression related to apoptosis (cleaved Casp-3 and -8, Bax, Bcl-2) and autophagy (Atg-5, Beclin-1, ratio LC3-II/LC3-I) | - Decrease in testosterone levels (≥200 mg/kg); - Mildly disorganized ST (200 mg/kg), disintegration of SE, germ cell depletion and reduction in round sperm in the ST (400 mg/kg); - Upregulation of cleaved Casp-8 (≥100 mg/kg), Casp-3 and Bax (400 mg/kg) and downregulation in Bcl-2 (≥100 mg/kg) expression in the testis. Increase in Atg-5, Beclin-1 expression, and LC3-II/LC3-I ratio in the testis (≥100 mg/kg); | [118] | |
| Formula: ZnO Size: 30 nm Shape: spherical | 0, 50, 150, 450 mg/kg/day 14 days | Oral gavage | Kunming Mice Epididymis Testis Testis Sperm Serum | - Reproductive organs weight; - Sperm count; - Testis histopathology; - Zinc accumulation; - Gene expression related to apoptosis (Casp-3, -9 and -12, JNK, Bcl-2/Bax) ER stress (BIP, XBP1s, IRE1α, CHOP) and testosterone production (StAR, cytochrome P450scc); - Testosterone levels | - Increase in testis (150 mg/kg) and epididymis weight (50 and 450 mg/kg); - Low number of sperm in the ST lumen (50 mg/kg), ST degeneration and vacuolization of Sertoli cells (150 mg/kg), Leydig cells vacuolization, absent ST with degenerated and necrotic spermatogenic cells (450 mg/kg); - Zinc accumulation in the epididymis (50 and 450 mg/kg) but not in the testis; - Upregulation of BIP, XBP1s, Casp-12 (450 mg/kg), IRE1α, Casp-3 (≥50 mg/kg), CHOP (≥150 mg/kg) and Casp-9 (150 mg/kg). Downregulation of JNK at 50 mg/kg but upregulation at 150 mg/kg and down-regulation of Bax/Bcl-2; - Decrease in sperm number and testosterone levels (≥150 mg/kg), related to the downregulation of StAR | [90] | |
| Formula: ZnO Size: 100 nm | 100 mg/kg/day 75 days | Oral | Wistar Rats Testis Prostate Epididymis Sperm Plasma | - Reproductive organs weight; - mtTFA, UCP2 testis levels; - DNA fragmentation; - p53, TNF-α, IL-6 testis levels; - Oxidative stress indexes (GPx, GST, CAT, SOD, GSH, TAC, TBARS, NO); - Steroidogenic enzymes levels (17-KSR, 17β-HSD); - Sperm quality; - Reproductive and thyroid hormones levels (testosterone, FSH, LH, TSH, T3, T4); - Testis histopathology | - Decline in testis and epididymis weight but increase in prostate weight; - Suppression and induction of MtTFA and UCP2 expression, respectively; - Massive DNA fragmentation; - Increase in p53, TNF-α and IL-6 levels; - Decrease in GPx, GST, CAT, SOD, GSH, TAC levels and increase in TBARS and NO levels; - Increase and decrease in 17β-HSD and 17-KSD levels, respectively;- Reduction in sperm count, motility and increase in sperm abnormalities; - Decrease in testosterone and TSH levels, increase in FSH, LH, T3 and T4 levels; - ST with irregular shaped and empty lumina, spermatogenic cells with pyknotic nuclei, few Leydig cells | [123] | |
| Formula: ZnO Size: <100 nm Shape: rod-like Zeta potential: +17 to +20.6 mV PDI: 0.729 HS: 882.8 nm | 9.38, 18.75, 37.5, 75 mg/kg/day 35 days | Intraperitoneal | Swiss Mice Serum Epididymis Sperm Testis | - Testis weight; - Sperm quality; - Reproductive hormones levels (testosterone, LH, FSH); - Oxidative stress indexes (SOD, CAT, GSH, MDA); - Morphometric parameters; - Testis histopathology; | - No significant changes in testis weight; - Decrease in motile sperm, lower sperm number (≥9.38 mg/kg), increase in sperm abnormalities (18.75 and 37.5 mg/kg) and higher testosterone levels (≥9.38 mg/kg); - Decrease in LH (9.38, 18.75 and 75 mg/kg) and FSH (≥37.5 mg/kg) levels; - Reduced SOD and CAT activity but increased MDA activity (≥9.38 mg/kg) with no significant changes in GSH; - Decrease in germinal height (≥9.38 mg/kg) and increase of luminal width (9.38, 37.5, 75 mg/kg); - Increased number of damaged ST, Leydig cell degeneration and necrosis of spermatogenic cells (≥9.38 mg/kg) | [140] | |
| Formula: ZnO Size: 80 nm | 0, 150, 350 mg/kg 15 days | Oral | Albino Mice Testis Prostate Seminal Vesicle Epididymis | - Testis, prostate, seminal vesicle, and epididymis histopathology | - Mild damage in seminal vesicles and epididymis (150 mg/kg) and severe damage in all tissues of the reproductive system (350 mg/kg) | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassal, M.; Rebelo, S.; Pereira, M.d.L. Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System. Int. J. Mol. Sci. 2021, 22, 8061. https://doi.org/10.3390/ijms22158061
Vassal M, Rebelo S, Pereira MdL. Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System. International Journal of Molecular Sciences. 2021; 22(15):8061. https://doi.org/10.3390/ijms22158061
Chicago/Turabian StyleVassal, Mariana, Sandra Rebelo, and Maria de Lourdes Pereira. 2021. "Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System" International Journal of Molecular Sciences 22, no. 15: 8061. https://doi.org/10.3390/ijms22158061
APA StyleVassal, M., Rebelo, S., & Pereira, M. d. L. (2021). Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System. International Journal of Molecular Sciences, 22(15), 8061. https://doi.org/10.3390/ijms22158061








