Occurrence of Calcium Oscillations in Human Spermatozoa Is Based on Spatial Signaling Enzymes Distribution
Abstract
1. Introduction
2. Results
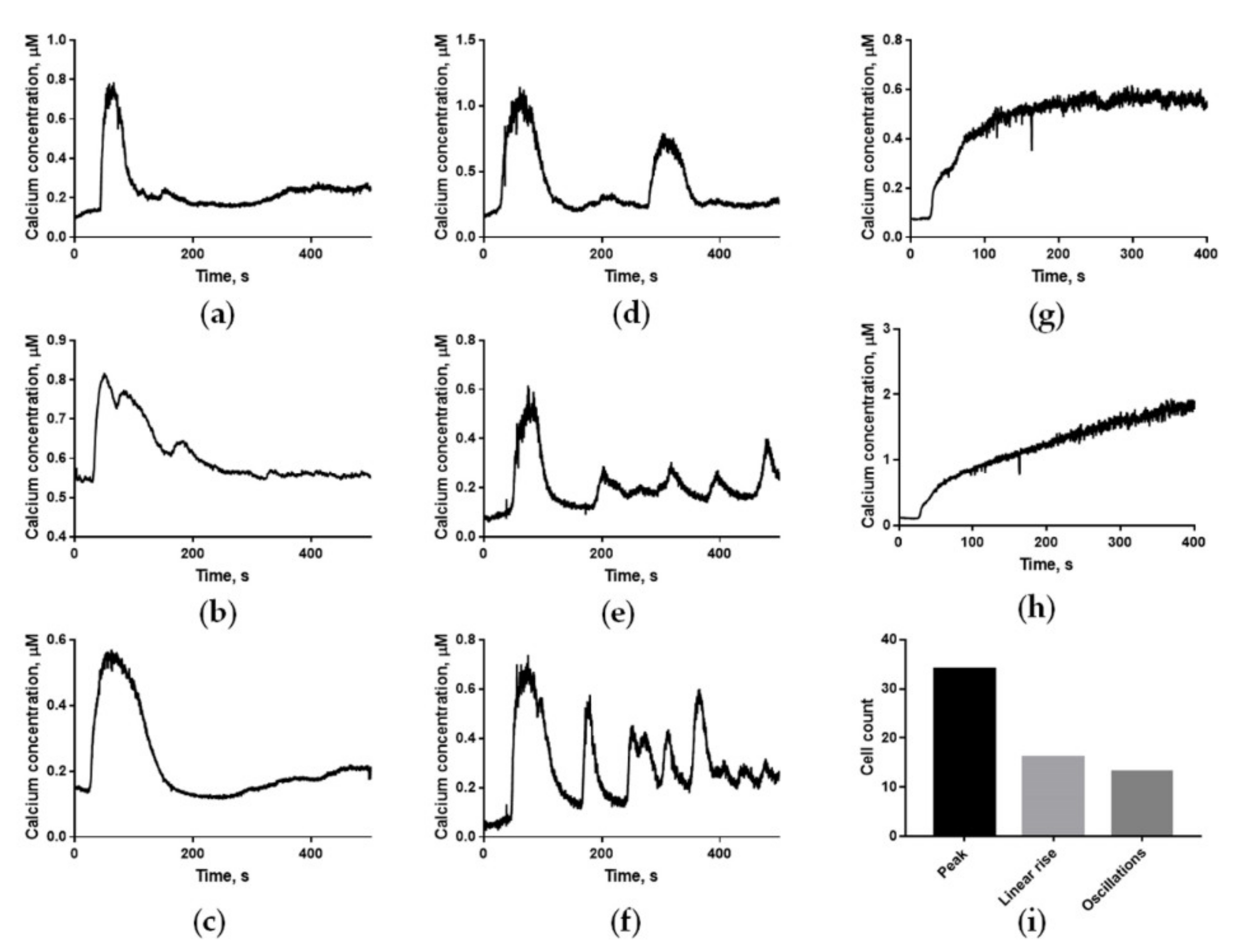
2.1. Human Sperm Cells Show Three Different Types of Calcium Responses to Progesterone
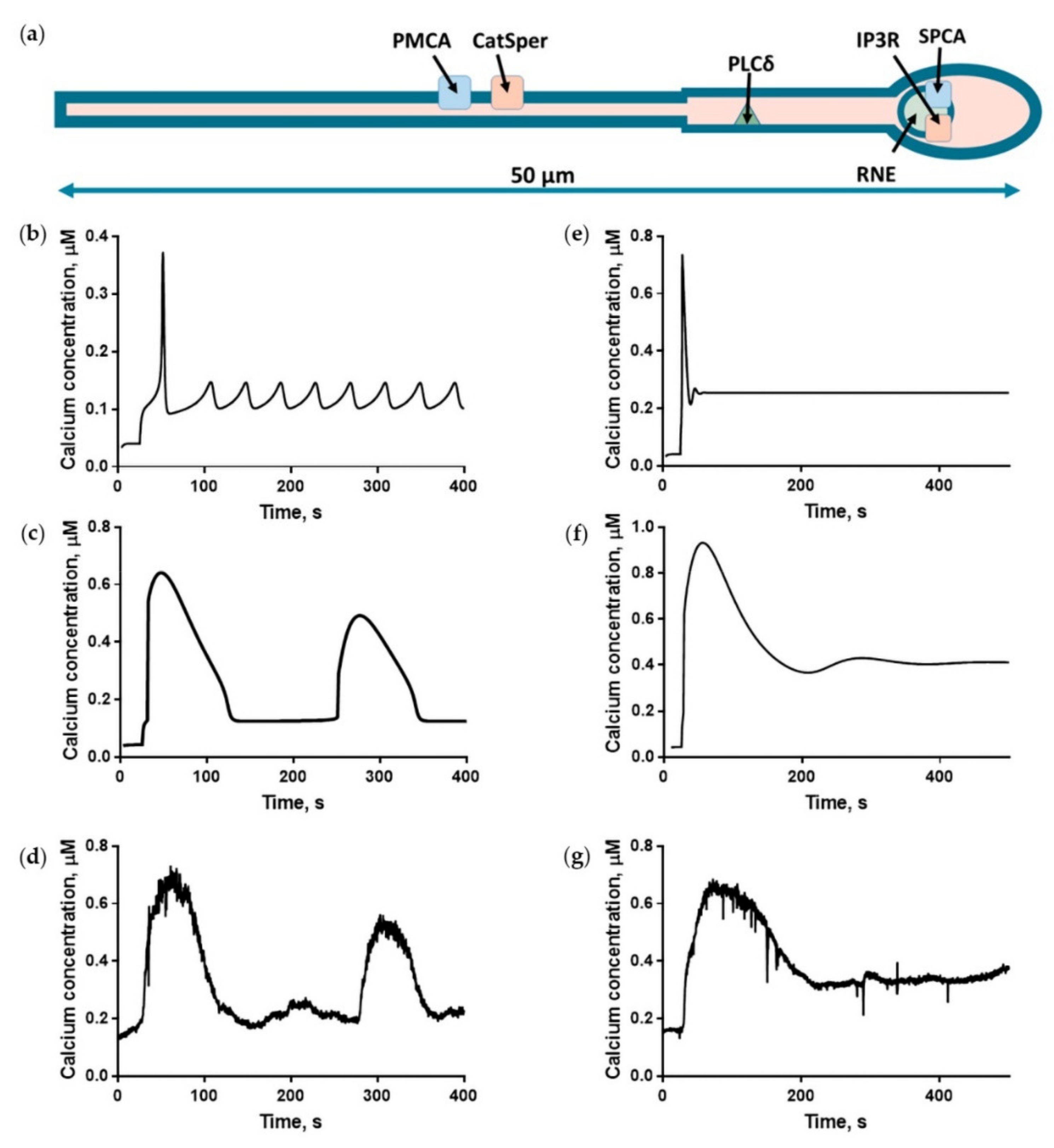
2.2. The Three-Dimensional but Not the Homogeneous Model Provided a Quantitative Description of the Progesterone-Induced Calcium Response
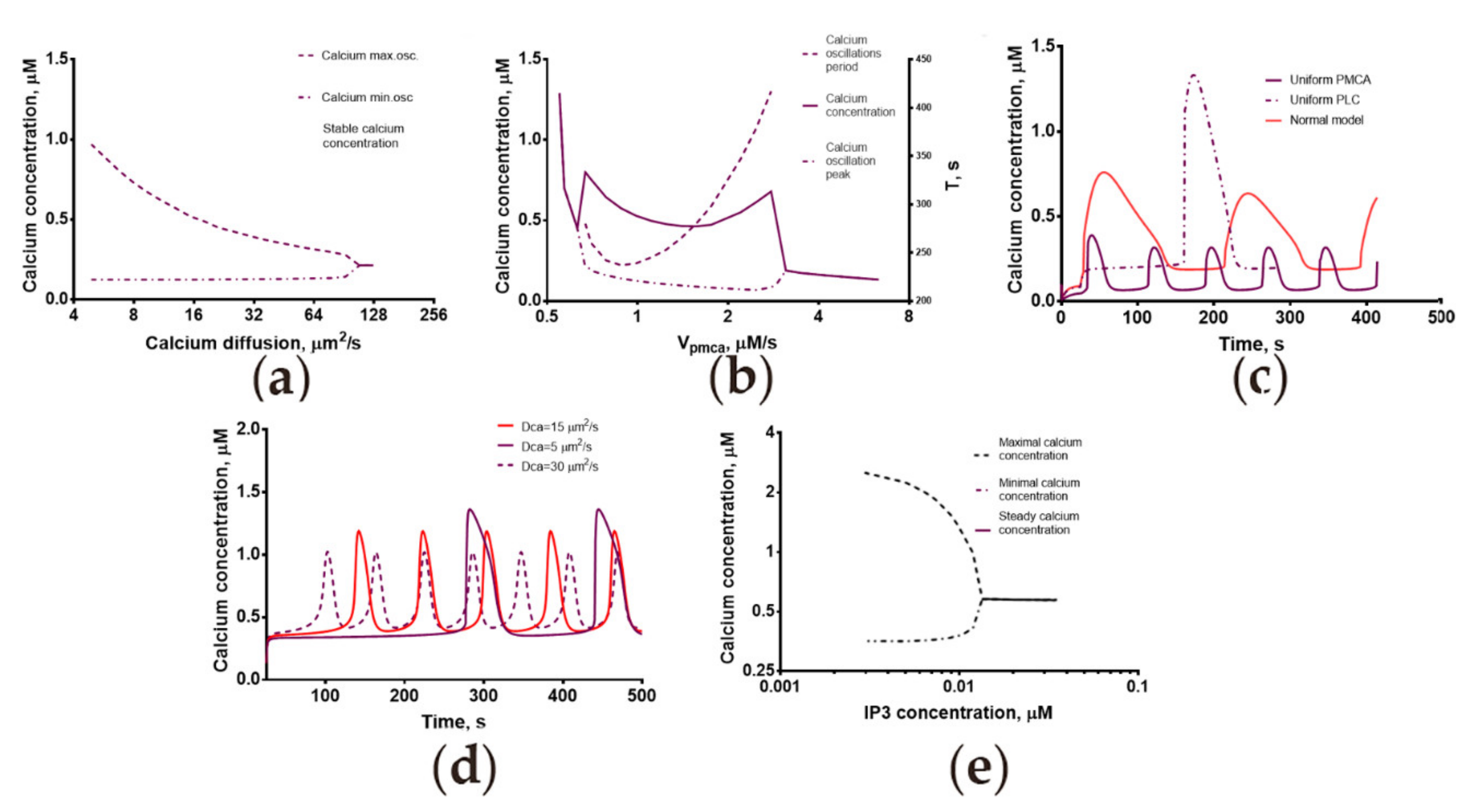
2.3. Calcium Dynamics in Human Spermatozoa Is Strongly Dependent on the Calcium Diffusion Coefficient
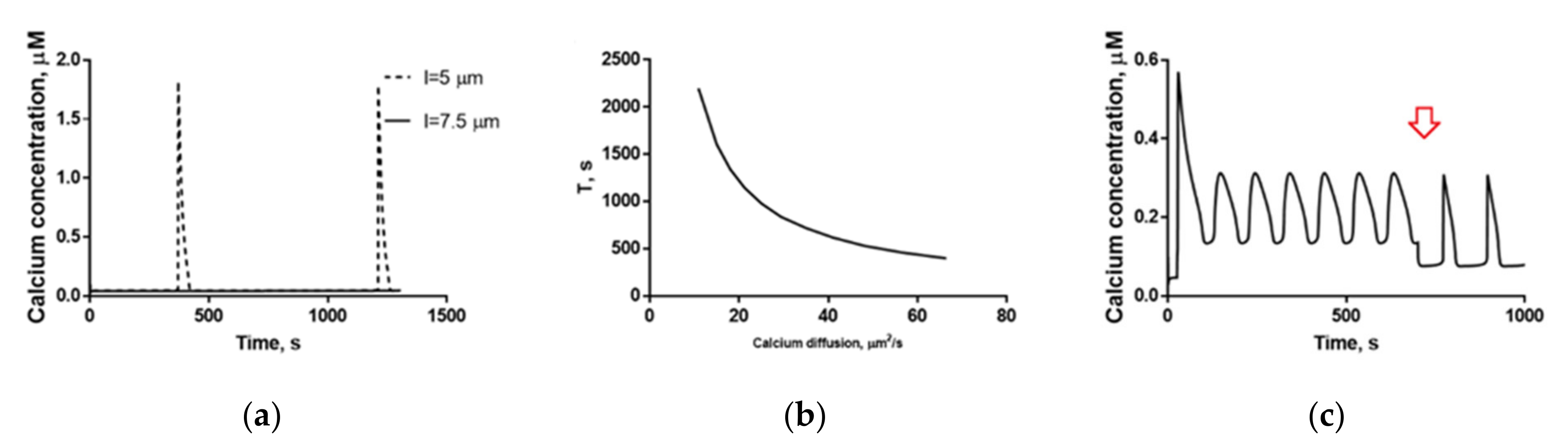
2.4. Mechanisms of Spontaneous Oscillations and Calcium Oscillations in Nominally Calcium-Free Medium Revealed by the Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Sperm Cells Collection
4.3. Microscopy
4.4. Intracellular Calcium Measurement
4.5. Computational Model Construction and Validation
4.5.1. The Heterogeneous Model of Progesterone-Induced Activation of Human Spermatozoa
4.5.2. The Homogeneous Model of Progesterone-Induced Activation of Human Spermatozoa
4.5.3. Considerations about the Inclusion of PLCδ into the Models
4.5.4. Integration of the Models
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okabe, M. The Acrosome Reaction: A Historical Perspective. In Sperm Acrosome Biogenesis and Function during Fertilization; Springer: Cham, Switzerland, 2016; pp. 1–13. [Google Scholar] [CrossRef]
- Armon, L.; Eisenbach, M. Behavioral Mechanism during Human Sperm Chemotaxis: Involvement of Hyperactivation. PLoS ONE 2011, 6, e28359. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Merritt, J.E.; Rink, T.J. Rapid increases in cytosolic free calcium in response to muscarinic stimulation of rat parotid acinar cells. J. Biol. Chem. 1987, 262, 4958–4960. [Google Scholar] [CrossRef]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Li, L.-F.; Xiang, C.; Zhu, Y.-B.; Qin, K.-R. Modeling of progesterone-induced intracellular calcium signaling in human spermatozoa. J. Theor. Biol. 2014, 351, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.; Michelangeli, F.; Publicover, S. Regulation and roles of Ca2+ stores in human sperm. Reproduction 2015, 150, R65–R76. [Google Scholar] [CrossRef]
- Sánchez-Tusie, A.A.; Vasudevan, S.R.; Churchill, G.C.; Nishigaki, T.; Treviño, C.L. Characterization of NAADP-Mediated Calcium Signaling in Human Spermatozoa. Biochem. Biophys. Res. Commun. 2014, 443, 531–536. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Beebe, S.J.; Danforth, D.R.; Alexander, N. Progesterone and 17 Alpha-Hydroxyprogesterone. Novel Stimulators of Calcium Influx in Human Sperm. J. Biol. Chem. 1990, 265, 1376–1380. [Google Scholar] [CrossRef]
- José, O.; Hernández-Hernández, O.; Chirinos, M.; González-González, M.E.; Larrea, F.; Almanza, A.; Felix, R.; Darszon, A.; Treviño, C.L. Recombinant human ZP3-induced sperm acrosome reaction: Evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem. Biophys. Res. Commun. 2010, 395, 530–534. [Google Scholar] [CrossRef]
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Pérez, J.; Álvaro, C.; Blanco, M.T.M.; Concha, I.I.; Ramírez, A.; et al. Intracellular calcium movements of boar spermatozoa during ‘in vitro’ capacitation and subsequent acrosome exocytosis follow a multiple-storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martínez, E.; Darszon, A.; Treviño, C.L. pH-dependent Ca+2 oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef]
- Magnus, G.; Keizer, J. Minimal Model of P-Cell Mitochondrial Ca2+ Handling. Model. Physiol. 1997, 273, C717–C733. [Google Scholar] [CrossRef]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted Ablation of Plasma Membrane Ca2+-ATPase (PMCA) 1 and 4 Indicates a Major Housekeeping Function for PMCA1 and a Critical Role in Hyperactivated Sperm Motility and Male Fertility for PMCA. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef]
- Gualtieri, R.; Talevi, R. Selection of highly fertilization-competent bovine spermatozoa through adhesion to the Fallopian tube epithelium in vitro. Reproduction 2003, 125, 251–258. [Google Scholar] [CrossRef]
- Harper, C.; Barratt, C.; Publicover, S.J. Stimulation of Human Spermatozoa with Progesterone Gradients to Simulate Approach to the Oocyte. J. Biol. Chem. 2004, 279, 46315–46325. [Google Scholar] [CrossRef]
- De Oliveira, G.M.; Lefièvre, L.; Ford, C.; Herrero, M.B.; Barratt, C.; Connolly, T.J.; Nash, K.; Morales-Garcia, A.; Kirkman-Brown, J.C.; Publicover, S. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: A role for NO synthesised in the female reproductive tract. Development 2008, 135, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Romarowski, A.; Sánchez-Cárdenas, C.; Gomez, H.V.R.; Molina, L.D.C.P.; Trevino, C.L.; Hernández-Cruz, A.; Darszon, A.; Buffone, M.G. A Specific Transitory Increase in Intracellular Calcium Induced by Progesterone Promotes Acrosomal Exocytosis in Mouse Sperm1. Biol. Reprod. 2016, 94, 63. [Google Scholar] [CrossRef]
- Achikanu, C.; Pendekanti, V.; Teague, R.; Publicover, S. Effects of pH manipulation, CatSper stimulation and Ca2+-store mobilization on [Ca2+]i and behaviour of human sperm. Hum. Reprod. 2018, 33, 1802–1811. [Google Scholar] [CrossRef]
- Servin-Vences, M.R.; Tatsu, Y.; Ando, H.; Guerrero, A.; Yumoto, N.; Darszon, A.; Nishigaki, T. A caged progesterone analog alters intracellular Ca2+ and flagellar bending in human sperm. Reproduction 2012, 144, 101–109. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C. Insights into the Function of a Sperm-Surface Progesterone Receptor: Evidence of Ligand-Induced Receptor Aggregation and the Implication of Proteolysis. Exp. Cell Res. 1993, 205, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Sagare-Patil, V.; Galvankar, M.; Satiya, M.; Bhandari, B.; Gupta, S.; Modi, D. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int. J. Androl. 2012, 35, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cárdenas, C.; Servin-Vences, R.; José, O.; Trevino, C.L.; Hernández-Cruz, A.; Darszon, A. Acrosome Reaction and Ca2+ Imaging in Single Human Spermatozoa: New Regulatory Roles of [Ca2+]i. Biol. Reprod. 2014, 91, 67. [Google Scholar] [CrossRef]
- Xia, J.; Reigada, D.; Mitchell, C.H.; Ren, D. CATSPER Channel-Mediated Ca2+ Entry into Mouse Sperm Triggers a Tail-to-Head Propagation. Biol. Reprod. 2007, 77, 551–559. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef]
- Vyklicka, L.; Lishko, P.V. Dissecting the signaling pathways involved in the function of sperm flagellum. Curr. Opin. Cell Biol. 2020, 63, 154–161. [Google Scholar] [CrossRef]
- Tomes, C.N. The proteins of exocytosis: Lessons from the sperm model. Biochem. J. 2015, 465, 359–370. [Google Scholar] [CrossRef]
- Allen, V.; Swigart, P.; Cheung, R.; Cockcroft, S.; Katan, M. Regulation of inositol lipid-specific phospholipase Cδ by changes in Ca2+ ion concentrations. Biochem. J. 1997, 327, 545–552. [Google Scholar] [CrossRef]
- Fukami, K.; Yoshida, M.; Inoue, T.; Kurokawa, M.; Fissore, R.A.; Yoshida, N.; Mikoshiba, K.; Takenawa, T. Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J. Cell Biol. 2003, 161, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Arenaza, I.U.; Osinalde, N.; Akimov, V.; Puglia, M.; Candenas, L.; Pinto, F.M.; Muñoa-Hoyos, I.; Gianzo, M.; Matorras, R.; Irazusta, J.; et al. Phosphoproteomic and Functional Analyses Reveal Sperm-specific Protein Changes Downstream of Kappa Opioid Receptor in Human Spermatozoa. Mol. Cell. Proteom. 2019, 18, S118–S131. [Google Scholar] [CrossRef]
- Ho, H.-C.; Suarez, S.S. Characterization of the Intracellular Calcium Store at the Base of the Sperm Flagellum That Regulates Hyperactivated Motility. Biol. Reprod. 2003, 68, 1590–1596. [Google Scholar] [CrossRef]
- Van Der Horst, G.; Maree, L.; Kotzé, S.H.; O’Riain, M.J. Sperm structure and motility in the eusocial naked mole-rat, Heterocephalus glaber: A case of degenerative orthogenesis in the absence of sperm competition? BMC Evol. Biol. 2011, 11, 351. [Google Scholar] [CrossRef]
- Priego-Espinosa, D.A.; Darszon, A.; Guerrero, A.; González-Cota, A.L.; Nishigaki, T.; Martínez-Mekler, G.; Carneiro, J. Modular analysis of the control of flagellar Ca2+-spike trains produced by CatSper and CaV channels in sea urchin sperm. PLoS Comput. Biol. 2020, 16, e1007605. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Enríquez, J.; Priego-Espinosa, D.A.; Darszon, A.; Beltran, C.; Martínez-Mekler, G. Network model predicts that CatSper is the main Ca2+ channel in the regulation of sea urchin sperm motility. Sci. Rep. 2017, 7, 4236. [Google Scholar] [CrossRef]
- Espinal-Enríquez, J.; Darszon, A.; Guerrero, A.; Martínez-Mekler, G. In Silico Determination of the Effect of Multi-Target Drugs on Calcium Dynamics Signaling Network Underlying Sea Urchin Spermatozoa Motility. PLoS ONE 2014, 9, e104451. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, L.U.; Galindo, B.E.; Sánchez, D.; Santillan, M. What Is the Core Oscillator in the Speract-Activated Pathway of the Strongylocentrotus purpuratus Sperm Flagellum? Biophys. J. 2012, 102, 2481–2488. [Google Scholar] [CrossRef]
- Olson, S.D.; Suarez, S.S.; Fauci, L.J. A Model of CatSper Channel Mediated Calcium Dynamics in Mammalian Spermatozoa. Bull. Math. Biol. 2010, 72, 1925–1946. [Google Scholar] [CrossRef]
- Simons, J.; Fauci, L. A Model for the Acrosome Reaction in Mammalian Sperm. Bull. Math. Biol. 2018, 80, 2481–2501. [Google Scholar] [CrossRef]
- Bedu-Addo, K.; Barratt, C.; Kirkman-Brown, J.C.; Publicover, S. Patterns of [Ca2+]i mobilization and cell response in human spermatozoa exposed to progesterone. Dev. Biol. 2007, 302, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Mediero, M.; Lozano, G.M.; Bejarano, I.; Ortiz, Á.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reprod. Biol. Endocrinol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Brewis, I.A.; Morton, I.E.; Mohammad, S.N.; Browes, C.E.; Moore, H.D. Measurement of intracellular calcium concentration and plasma membrane potential in human spermatozoa using flow cytometry. J. Androl. 2000, 21, 238–249. [Google Scholar] [PubMed]
- López-González, I.; Torres-Rodríguez, P.; Sánchez-Carranza, O.; Solís-López, A.; Santi, C.; Darszon, A.; Treviño, C. Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef]
- Matthews, E.A.; Edietrich, D. Buffer mobility and the regulation of neuronal calcium domains. Front. Cell. Neurosci. 2015, 9, 48. [Google Scholar] [CrossRef]
- Naaby-Hansen, S.; Diekman, A.; Shetty, J.; Flickinger, C.J.; Westbrook, A.; Herr, J.C. Identification of calcium-binding proteins associated with the human sperm plasma membrane. Reprod. Biol. Endocrinol. 2010, 8, 6. [Google Scholar] [CrossRef]
- Mossman, J.A.; Pearson, J.T.; Moore, H.D.; Pacey, A. Variation in mean human sperm length is linked with semen characteristics. Hum. Reprod. 2013, 28, 22–32. [Google Scholar] [CrossRef]
- Cummins, J.M.; Woodall, P.F. On mammalian sperm dimensions. Reproduction 1985, 75, 153–175. [Google Scholar] [CrossRef]
- Kirkman-Brown, J.C.; Barratt, C.L.R.; Publicover, S.J. Slow calcium oscillations in human spermatozoa. Biochem. J. 2004, 378, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Samples, J.T.; Abrams, R.M. Reliability of Urine Temperature as a Measurement of Basal Body Temperature. JOGN Nurs. 1984, 13, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Eid, J.-F. Elevation of Intratesticular and Scrotal Skin Surface Temperature in Men with Varicocele. J. Urol. 1989, 142, 743–745. [Google Scholar] [CrossRef]
- Vera-Sigüenza, E.; Pages, N.; Rugis, J.; Yule, D.I.; Sneyd, J. A Mathematical Model of Fluid Transport in an Accurate Reconstruction of Parotid Acinar Cells. Bull. Math. Biol. 2018, 81, 699–721. [Google Scholar] [CrossRef] [PubMed]
- Lechleiter, J.; Girard, S.; Clapham, D.; Peralta, E. Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature 1991, 350, 505–508. [Google Scholar] [CrossRef]
- Bell, M.; Bartol, T.; Sejnowski, T.; Rangamani, P. Dendritic spine geometry and spine apparatus organization govern the spatiotemporal dynamics of calcium. J. Gen. Physiol. 2019, 151, 1017–1034. [Google Scholar] [CrossRef]
- Marhl, M.; Schuster, S.; Brumen, M.; Heinrich, R. Modelling the interrelations between calcium oscillations and ER membrane potential oscillations. Biophys. Chem. 1997, 63, 221–239. [Google Scholar] [CrossRef]
- Zador, A.; Koch, C. Linearized models of calcium dynamics: Formal equivalence to the cable equation. J. Neurosci. 1994, 14, 4705–4715. [Google Scholar] [CrossRef]
- Morbeck, D.E.; Baumann, N.A.; Oglesbee, D. Composition of single-step media used for human embryo culture. Fertil. Steril. 2017, 107, 1055–1060.e1. [Google Scholar] [CrossRef]
- De Jonge, C.J.; Barratt, C. Methods for the Assessment of Sperm Capacitation and Acrosome Reaction Excluding the Sperm Penetration Assay. Adv. Struct. Saf. Stud. 2012, 927, 113–118. [Google Scholar] [CrossRef]
- Balabin, F.A.; Morozova, D.; Mayorov, A.S.; Martyanov, A.A.; Panteleev, M.A.; Sveshnikova, A. Clusterization of Inositol Trisphosphate Receptors Determines the Shape of the Calcium Oscillation Peak in Platelet Cytosol. Mosc. Univ. Phys. Bull. 2018, 73, 526–533. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Naraghi, M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium 1997, 22, 255–268. [Google Scholar] [CrossRef]
- Moraru, I.; Morgan, F.; Li, Y.; Loew, L.; Schaff, J.C.; Lakshminarayana, A.; Slepchenko, B.M.; Gao, F.; Blinov, M. Virtual Cell modelling and simulation software environment. IET Syst. Biol. 2008, 2, 352–362. [Google Scholar] [CrossRef]
- Wang, M.; Weiss, M.; Simonovic, M.; Haertinger, G.; Schrimpf, S.P.; Hengartner, M.; von Mering, C. PaxDb, a Database of Protein Abundance Averages Across All Three Domains of Life. Mol. Cell. Proteom. 2012, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Milo, R. What is the total number of protein molecules per cell volume? A call to rethink some published values. BioEssays 2013, 35, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Serres, C.; Escalier, D.; David, G. Ultrastructural morphometry of the human sperm flagellum with a stereological analysis of the lengths of the dense fibres. Biol. Cell 1984, 49, 153–161. [Google Scholar] [CrossRef]
- Sunanda, P.; Panda, B.; Dash, C.; Padhy, R.N.; Routray, P. An illustration of human sperm morphology and their functional ability among different group of subfertile males. Andrology 2018, 6, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Laufer, N.; Segal, S.; Yaffe, H.; Svartz, H.; Grover, N. Volume and Shape of Normal Human Spermatozoa. Fertil. Steril. 1977, 28, 456–458. [Google Scholar] [CrossRef]
- Thomas, P.; Meizel, S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem. J. 1989, 264, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, E.; Evans, J.P.; Bernard, D.J.; Jänne, P.A.; Nussbaum, R.L. Disrupted Sperm Function and Fertilin β Processing in Mice Deficient in the Inositol Polyphosphate 5-Phosphatase Inpp5b. Dev. Biol. 2001, 240, 641–653. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Qi, H.; Wei, F.; Chen, J.; Shuai, J. Comparison of gating dynamics of different IP3R channels with immune algorithm searching for channel parameter distributions. Phys. Biol. 2016, 13, 056005. [Google Scholar] [CrossRef]
- Kawai, T.; Miyata, H.; Nakanishi, H.; Sakata, S.; Morioka, S.; Sasaki, J.; Watanabe, M.; Sakimura, K.; Fujimoto, T.; Sasaki, T.; et al. Polarized PtdIns(4,5)P2 distribution mediated by a voltage-sensing phosphatase (VSP) regulates sperm motility. Proc. Natl. Acad. Sci. USA 2019, 116, 26020–26028. [Google Scholar] [CrossRef] [PubMed]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI—A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Saltelli, A.; Ratto, M.; Tarantola, A.S.; Campolongo, F. Sensitivity Analysis for Chemical Models. Chem. Rev. 2005, 105, 2811–2828. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobkin, J.; Balabin, F.A.; Yakovenko, S.A.; Simonenko, E.Y.; Sveshnikova, A.N. Occurrence of Calcium Oscillations in Human Spermatozoa Is Based on Spatial Signaling Enzymes Distribution. Int. J. Mol. Sci. 2021, 22, 8018. https://doi.org/10.3390/ijms22158018
Korobkin J, Balabin FA, Yakovenko SA, Simonenko EY, Sveshnikova AN. Occurrence of Calcium Oscillations in Human Spermatozoa Is Based on Spatial Signaling Enzymes Distribution. International Journal of Molecular Sciences. 2021; 22(15):8018. https://doi.org/10.3390/ijms22158018
Chicago/Turabian StyleKorobkin, Julia, Fedor A. Balabin, Sergey A. Yakovenko, Ekaterina Yu. Simonenko, and Anastasia N. Sveshnikova. 2021. "Occurrence of Calcium Oscillations in Human Spermatozoa Is Based on Spatial Signaling Enzymes Distribution" International Journal of Molecular Sciences 22, no. 15: 8018. https://doi.org/10.3390/ijms22158018
APA StyleKorobkin, J., Balabin, F. A., Yakovenko, S. A., Simonenko, E. Y., & Sveshnikova, A. N. (2021). Occurrence of Calcium Oscillations in Human Spermatozoa Is Based on Spatial Signaling Enzymes Distribution. International Journal of Molecular Sciences, 22(15), 8018. https://doi.org/10.3390/ijms22158018






