Chronological Age Affects MSC Senescence In Vitro—A Systematic Review
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Included Studies
2.2. Cell Surface Characterisation
2.3. Molecular Markers of Senescence
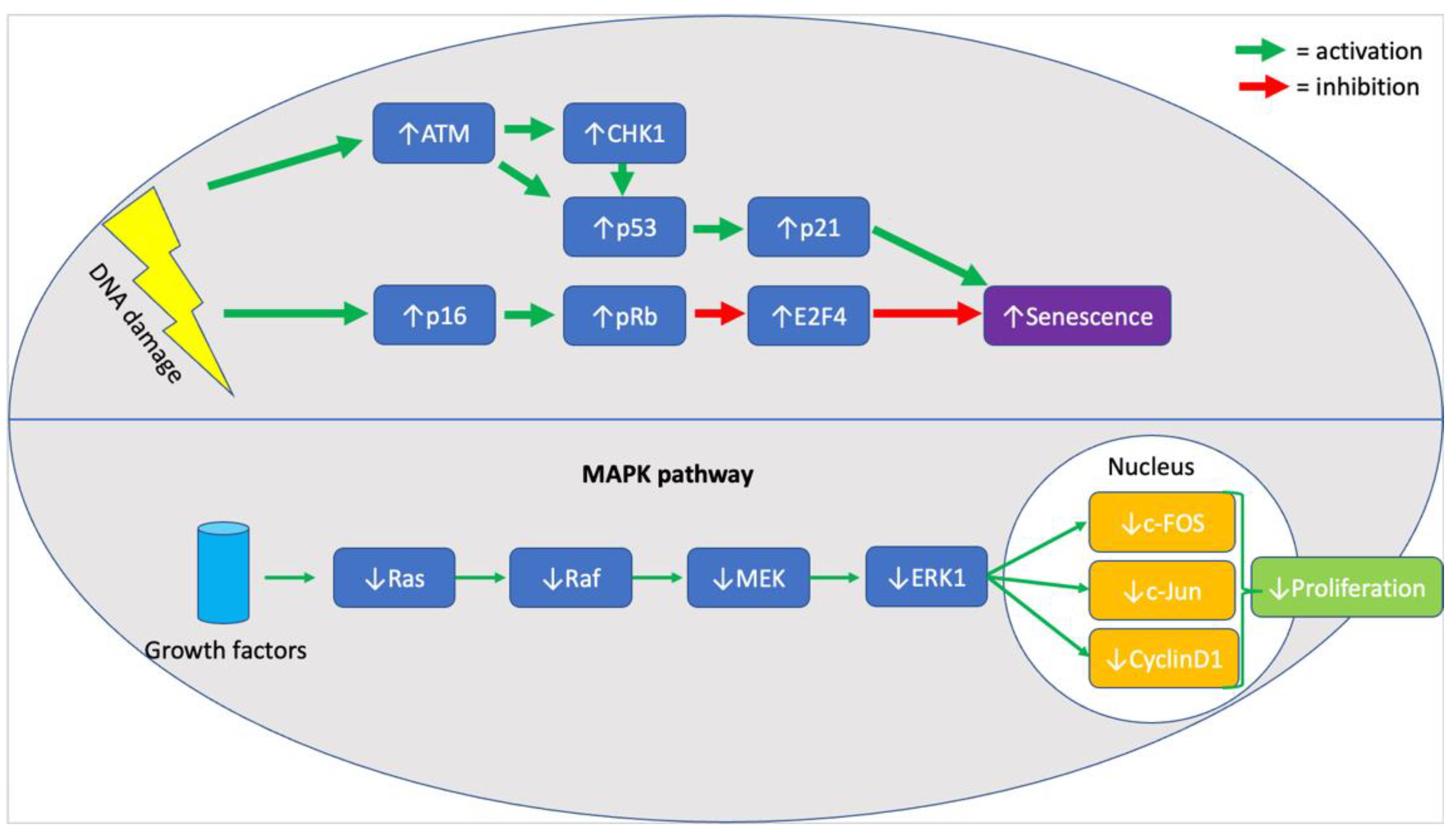
2.4. Increased Expression of p21, p53, p16, ROS, and NF-κB with Age
2.5. Decreased Expression of SOD with Age
3. Discussion
3.1. Senescence
3.2. Cell Cycle Arrest and Apoptosis
3.3. Stress and Inflammation
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Databases
4.2. Search Terms and Inclusion and Exclusion Criteria
4.3. Data Collection and Extraction
4.4. Quality Assessment of Included Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic transplants of bone marrow. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.; Huang, J.; Mizuno, H.; Alfonso, Z.; Fraser, J.; Benhaim, P.; Hedrick, M. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Schwab, K.E.; Hutchinson, P.; Gargett, C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum. Reprod. Oxf. Engl. 2008, 23, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Tondreau, T.; Meuleman, N.; Delforge, A.; Dejeneffe, M.; Leroy, R.; Massy, M.; Mortier, C.; Bron, D.; Lagneaux, L. Mesenchymal Stem Cells Derived from CD133-Positive Cells in Mobilized Peripheral Blood and Cord Blood: Proliferation, Oct4 Expression, and Plasticity. Stem Cells 2005, 23, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Kim, J.-M.; Jung, I.-H.; Kim, J.C.; Choi, S.-H.; Cho, K.S.; Kim, C.-S. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: In vitro and in vivo evaluations. J. Clin. Periodontol. 2011, 38, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, Y.; Nakajima, H.; Sugiyama, D.; Hirose, I.; Kitamura, T.; Tsuji, K. Human Placenta-Derived Cells Have Mesenchymal Stem/Progenitor Cell Potential. Stem Cells 2004, 22, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Gómez, T.H.; Boquete, I.M.F.; Gimeno-Longas, M.J.; Muiños-López, E.; Prado, S.D.; De Toro, F.J.; Blanco, F.J. Quantification of Cells Expressing Mesenchymal Stem Cell Markers in Healthy and Osteoarthritic Synovial Membranes. J. Rheumatol. 2010, 38, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, W.C.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef]
- Aronin, C.E.P.; Tuan, R.S. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 67–74. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Yang, X.; Han, Z.-P.; Qu, F.-F.; Shao, L.; Shi, Y.-F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bühring, H.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel Markers for the Prospective Isolation of Human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271. [Google Scholar] [CrossRef]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the Optimal Culture Conditions for Clinical Scale Production of Human Mesenchymal Stem Cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggal, S.; Brinchmann, J.E. Importance of serum source for the in vitro replicative senescence of human bone marrow derived mesenchymal stem cells. J. Cell. Physiol. 2011, 226, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarugaser, R.; Hanoun, L.; Keating, A.; Stanford, W.L.; Davies, J.E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [Green Version]
- Yannarelli, G.; Pacienza, N.; Cuniberti, L.; Medin, J.; Davies, J.; Keating, A. Brief Report: The Potential Role of Epigenetics on Multipotent Cell Differentiation Capacity of Mesenchymal Stromal Cells. Stem Cells 2012, 31, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Banfi, A.; Muraglia, A.; Dozin, B.; Mastrogiacomo, M.; Cancedda, R.; Quarto, R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000, 28, 707–715. [Google Scholar] [CrossRef]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef]
- Noer, A.; Boquest, A.C.; Collas, P. Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence. BMC Cell Biol. 2007, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells (Review). Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lei, H.; Dong, P.; Fu, X.; Yang, Z.; Yang, Y.; Ma, J.; Liu, X.; Cao, Y.; Xiao, R. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplant. 2017, 26, 1505–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Semon, J.; Kaushal, D.; O’Sullivan, R.; Glowacki, J.; Gimble, J.; Bunnell, B. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res. Ther. 2011, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Ferretti, C.; Lucarini, G.; Andreoni, C.; Salvolini, E.; Bianchi, N.; Vozzi, G.; Gigante, A.; Mattioli-Belmonte, M. Human Periosteal Derived Stem Cell Potential: The Impact of age. Stem Cell Rev. Rep. 2014, 11, 487–500. [Google Scholar] [CrossRef]
- Alt, E.U.; Senst, C.; Murthy, S.N.; Slakey, D.P.; Dupin, C.L.; Chaffin, A.E.; Kadowitz, P.J.; Izadpanah, R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012, 8, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.-X.; Eckstein, V.; et al. Aging and Replicative Senescence Have Related Effects on Human Stem and Progenitor Cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.N.Y.; Schmitt, C.A. Genotoxic Stress-Induced Senescence. Adv. Struct. Saf. Stud. 2019, 1896, 93–105. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.N.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.L.; Chaumeil, J.; Skok, J.A. Chromosome dynamics and the regulation of V(D)J recombination. Immunol. Rev. 2010, 237, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Jang, D.-J. Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res. 2009, 69, 8200–8207. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.K.; Ogando, C.R.; See, C.W.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Kurpinski, K.; Jang, D.-J.; Bhattacharya, S.; Rydberg, B.; Chu, J.; So, J.; Wyrobek, A.; Li, S.; Wang, D. Differential Effects of X-Rays and High-Energy 56Fe Ions on Human Mesenchymal Stem Cells. Int. J. Radiat. Oncol. 2009, 73, 869–877. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [Green Version]
- PRISMA. PRISMA Checklist. Available online: http://prisma-statement.org/PRISMAStatement/Checklist (accessed on 18 May 2021).
- PRISMA. PRISMA Flow Diagram. Available online: http://prisma-statement.org/prismastatement/flowdiagram.aspx (accessed on 18 May 2021).
- NIEHS. OHAT Risk of Bias Rating Tool for Human and Animal Studies. 2015. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 27 May 2021).

| Primary Author (Publication Year) | Brief Description | Source | Sample Size | Donor Ages (All Numbers in y.o.) | Characterisation |
|---|---|---|---|---|---|
| Stoltzing et al. (2008) | Assessed cellular “fitness” by means of proliferation rates and markers of cellular ageing | BM from posterior iliac crest and purchased (frozen) | 57 | Young (7–18) Adult (19–40) Aged (41–55) | CD13+ CD44+ CD90+ CD105+ Stro-1+ D7-Fib+ Irrespective of age Levels of CD44, CD90, CD105, and Stro-1 showed significant age-related changes |
| Wagner et al. (2009) | Compared the genetic profiles of younger vs. older donors and pinpointed 67 age-induced and 60 age-inhibited genes | BM aspirates for allogeneic transplantation or femoral head | 12 | Young (21–25) Adult (44–55) Elder (80–92) | CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD146+ CD166+ CD31- CD34- CD45- No age-related difference |
| Pandey et al. (2011) | Investigated changes in miRNA expression of donor ASCs and BMSCs | Subcutaneous white adipose tissue and BM from iliac crest and marrow discarded during orthopaedic procedures. | 16 | Young ASCs (31.5 ± 10.4) Old ASCs (63 ± 6) Young BMSC (31.5 ± 8.7) Old BMSC (56.3 ± 5) | CD29+ CD44+ CD90+ CD105+ CD166+ HLA-I+ CD3- CD34- CD45- CD11b- CD19- |
| Alt et al. (2012) | Investigated the repair and regeneration potential and molecular features of ASCs; found increase of CHEK1 and p16ink4a with age and abnormal levels of mir-27b, mir-106a, mir-199a, and let-7. | Abdominal Adipose Tissue | 40 | Young (<20, mean 16.75 ± 1.4) Middle (30–40, mean 34.4 ± 1.6) Old (>50, mean 61.33 ± 7.4) | CD44+ CD90+ CD105+ CD146+ CD3- CD4- CD11b- CD34- CD45- |
| Siegel et al. (2013) | Investigated effects of age on markers of senescence and compared their levels in BMSCs and pluripotent MSCs | BM during orthopaedic procedures, from patients without metabolic or neoplastic diseases | 53 | 13–80 | CD29+ CD44+ CD59+ CD73+ CD90+ CD105+ CD140b+ CD166+ HLA-ABC+ in >96% of cells ≤0.5% cells being CD14+ CD19+ CD34+ CD43+ CD45+ CD86+ CD93+ CD133+ CD243+ SSEA-1+ Another group of cells with big heterogeneity, ranging from 2.6% to 84.2% Negative correlation of CD71+ CD146+ CD274+ with increasing age and females to males |
| Choudhery et al. (2014) | Investigated the effect of age on population doublings, SOD activity, differentiation, and senescence | Adipose Tissue from liposuction procedures under local anaesthesia | 11 | <40 years of age > 50 years of age | CD44+ CD73+ CD90+ CD105+ CD3- CD14- CD19- CD34- CD45- |
| Ferretti et al. (2015) | Investigated the effect of age on markers of periosteum-derived precursor cells, including Ki67, p53, and NO | Periosteal tissue during surgery for orthopaedic trauma | 8 | 2 with mean age 16 2 with mean age 28 2 with mean age 63 2 with mean age 92 | N/A |
| Marędziak et al. (2016) | Investigated the age-dependent increase in hASC senescence | Subcutaneous adipose tissue during total hip/knee arthroplasty or other open procedures connected with fracture reduction and fixation | 28 | Group 1 > 20 Group 2 > 50 Group 3 > 60 Group 4 > 70 | CD90+ CD73b+ CD44+ CD29+ CD34- |
| Liu et al. (2017) | Investigated the effect of ageing on various biomarkers and genetic markers, including SA-β-galactosidase, ROS, proteasome activity, hTERT1, SIRT1, p53, p21, p16, RB1 & RB2 | Subcutaneous adipose tissue from right chest regions during various surgical procedures | 24 | Children (6 to 12) Young adult (22 to 27) Elderly (60 to 73) | CD44+ CD73+ CD90+ CD105+ CD34- CD11b- CD19- CD45- HLA-DR- No significant difference between groups |
| Primary Author (Publication Year) | Molecular Markers of Senescence Measured | Means of Measurement | Outcomes | Other Points |
|---|---|---|---|---|
| Stolzing et al. (2008) | p21 and p53 | Flow cytometry | Age-related increase (adult = 3x young, aged = 6x young) | Age-related reduction in cell numbers and differentiation potential were observed. |
| ROS | Significantly increased in aged | |||
| NO | Age-related increase (adult = 3x young, aged = 6x young) | |||
| Advanced glycation end-product (AGE), Receptors for AGEs (RAGE), Carbonyls | Age-related increase | |||
| SOD | Using specialised kit | Age-related decrease (aged = 0.6x young) | ||
| Heat Shock Proteins (HSP27, HSP70 and HSP90) | Immunohistochemistry | Age-related decrease | ||
| Rate of apoptosis | Age-related increase | |||
| Wagner et al. (2009) | Numerous genes assessed for upregulation or downregulation | PCR, upregulation, or downregulation with p-value < 0.01 | 67 genes upregulated, including MEOX2, SHOX2, HOXC6 60 genes downregulated, including HOXA5, HOXB3, HOXB7, PITX2 | Markers of chronological senescence showed similar changes to markers of replicative senescence. |
| Pandey et al. (2011) | NF-κB in adipose derived MSCs (ASCs) | Immunohistochemistry | Young: NF-κB localised mostly in nucleus Old: NF-κB localised in the nucleus too, but elevated levels were found in the cytoplasm. | Differential expression of miRNA is an integral component of biologic ageing in MSCs. |
| Proteomic profile | Western blot analysis | Levels of p-IκB, p-Iκk, iNOS, ERK1/2, phosphorylated c-fos, c-jun and JNK were significantly decreased in ASCs from older donors. NF-κB/p65 levels were significantly elevated in ASCs from older donors | ||
| miRNA profile | qPCR | BMSCs: 45 miRNA molecules were different in expression, of which 43 were downregulated and 2 were upregulated. ASCs: 14 miRNA molecules were different in expression, of which 12 were downregulated and 2 were upregulated. | BMSCs: Increased activity found in PTEN, mTOR, RAN pathways with age, and decreased activity in Wnt/β-cat, tight-junction, SAPK/JNK signalling, cleavage, and polyadenylation of pre-mRNA ASCs: Increased activity in ephrin receptor signalling and PPARα/RXRα with age, and decreased activity in RAN and AMPK signalling and cell-cycle regulation. | |
| RNA profile | (Real-time) RT-PCR | Increased NF-κB and non-canonical targets IL-4 receptor and myc in old. Also increased WNT/β-cat in old. Decreased MAPK elements, iNOS, VCAM1, and IKK, and NF-kB downstream canonical targets. Decreased cell-cycle regulators such as cyclin-dependent kinases. | ||
| Alt et al. (2012) | CHEK1 (G2 phase arrest) | RT-PCR | 4.4-fold increase (old compared to young) | Age-related decline of multi-lineage differentiation potential in MSCs Adipogenic differentiation was downregulated from about 33% in Group 1 to about 10% in Group 3 Osteogenic differentiation was downregulated from about 50% in Group 1 to about 22% in Group 3 |
| CDK inhibitor p16ink4a (G2 phase arrest) | 22.9-fold increase | |||
| ATM, E2F4 transcription factor, Retinoblastoma 1 (Rb 1), BRCA1, HMGA2 | RT-PCR | Marginally increased | ||
| ATR apoptotic regulator | RT-PCR | 2.6 ± 0.9-fold increase in group 3 vs. group 1 | ||
| NFkB apoptotic regulator | 4 ± 0.4-fold increase | |||
| TNFa apoptotic regulator | 5 ± 1.4-fold increase | |||
| hTERT, p53, Casp3, Casp8, Casp9 | Decreased | |||
| XRCC4 (cellular DNA repair) | 3 ± 1.1-fold increase | |||
| XRCC6 (cellular DNA repair) | 4.8 ± 0.4-fold increase | |||
| APEX1 (cellular DNA repair) | 1.15 ± 0.4-fold decrease | |||
| Let7g | qRT-PCR | 3.95 ± 0.2-fold decrease | ||
| mir-27B | 3.43 ± 0.7-fold decrease | |||
| mir-106a | 0.94 ± 0.3-fold decrease | |||
| mir-199a | 4.68 ± 0.6-fold decrease | |||
| Siegel et al. (2013) | Oct4, Nanog, Prdm14 and SOX2 as markers of pluripotency | qRT-PCR | No correlation with donor age | Prdm14 mRNA expression is positively correlated to the clonogenic potential of MSCs in vitro |
| Choudhery et al. (2014) | p16 | qRT-PCR | Significantly higher in >50 y.o. than in <40 y.o. | Adipogenic differentiation potential of AT-MSCs is independent of donor age, osteogenic and chondrogenic potential decrease AT-MSCs undergo a neuronal-like differentiation irrespective of donor age |
| p21 | ||||
| SA-β-gal | SA-β-gal staining kit | 12.2% ± 1.1% in >50 years vs. 5.2% ± 1.9% in <40 years | ||
| SOD | commercially available colorimetric assay kit | 26.0 ± 2.3 in <40 years of age vs. 11.7 ± 2.9 in >50 years of age | ||
| Ferretti et al. (2015) | Ki67 | Immunohistochemistry | Lowest in 92 y.o. and highest in 16 y.o. | N/A |
| p53 | Increase in 92 y.o. compared to 28 y.o. and 16 y.o. | |||
| NO | NO production | Increase in 92 y.o. compared to 28 y.o. and 16 y.o. | ||
| Bmp2 | qRT-PCR | No differences in 16 and 28 y.o. Increase in 63 and 92 y.o. with the highest value in 63 y.o. | ||
| Runx2 | Significantly increased in 16 and 92 y.o. compared to 28 and 63 y.o. | |||
| IL-6 | Significant increase only in 92 y.o. | |||
| OPG | Increased in both 16 and 92 y.o. compared to 28 and 63 y.o. | |||
| RANKL | ||||
| Oct4 | Significantly decreased in 16 y.o. compared to the rest | |||
| Nanog | Increased in both 28 and 92 y.o. cells | |||
| Sox2 | ||||
| Marędziak et al. (2016) | SA-β-galactosidase | Senescence Cells Histochemical Staining Kit | Statistically insignificant differences | N/A |
| ROS level | Measurement of H2DCF-DA | Significantly decreased in young patients (>20) No significant differences between older age groups | ||
| NO levels | Griess reagent kit | Increased with age | ||
| SOD | SOD Assay kit | Decreased with age | ||
| Dead cells | Cell stain Double Staining Kit | Increased with age, but not with statistical significance | ||
| P21 and P53 | N/A | Increased in older age groups | ||
| Liu et al. (2017) | SA-β-galactosidase | β-galactosidase Staining Kit | Significant increase in passage 5 cells in elderly group compared to child group | N/A |
| Mitochondrial superoxide content | MitoSOX Red reagent | Increased in elderly group | ||
| Total ROS | DCFH-DA | Increase with age | ||
| Proteasome activity | Proteasome activity assay kit | Decreasing trend in young adult group and elderly group compared to child group | ||
| hTERT1 and SIRT1 | qRT-PCR | Statistically insignificant differences | ||
| p53, p21 and p16 | Increase with age, but the only significant difference was between the child group and the elderly group with respect to p21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapetanos, K.; Asimakopoulos, D.; Christodoulou, N.; Vogt, A.; Khan, W. Chronological Age Affects MSC Senescence In Vitro—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7945. https://doi.org/10.3390/ijms22157945
Kapetanos K, Asimakopoulos D, Christodoulou N, Vogt A, Khan W. Chronological Age Affects MSC Senescence In Vitro—A Systematic Review. International Journal of Molecular Sciences. 2021; 22(15):7945. https://doi.org/10.3390/ijms22157945
Chicago/Turabian StyleKapetanos, Konstantinos, Dimitrios Asimakopoulos, Neophytos Christodoulou, Antonia Vogt, and Wasim Khan. 2021. "Chronological Age Affects MSC Senescence In Vitro—A Systematic Review" International Journal of Molecular Sciences 22, no. 15: 7945. https://doi.org/10.3390/ijms22157945
APA StyleKapetanos, K., Asimakopoulos, D., Christodoulou, N., Vogt, A., & Khan, W. (2021). Chronological Age Affects MSC Senescence In Vitro—A Systematic Review. International Journal of Molecular Sciences, 22(15), 7945. https://doi.org/10.3390/ijms22157945







