Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging
Abstract
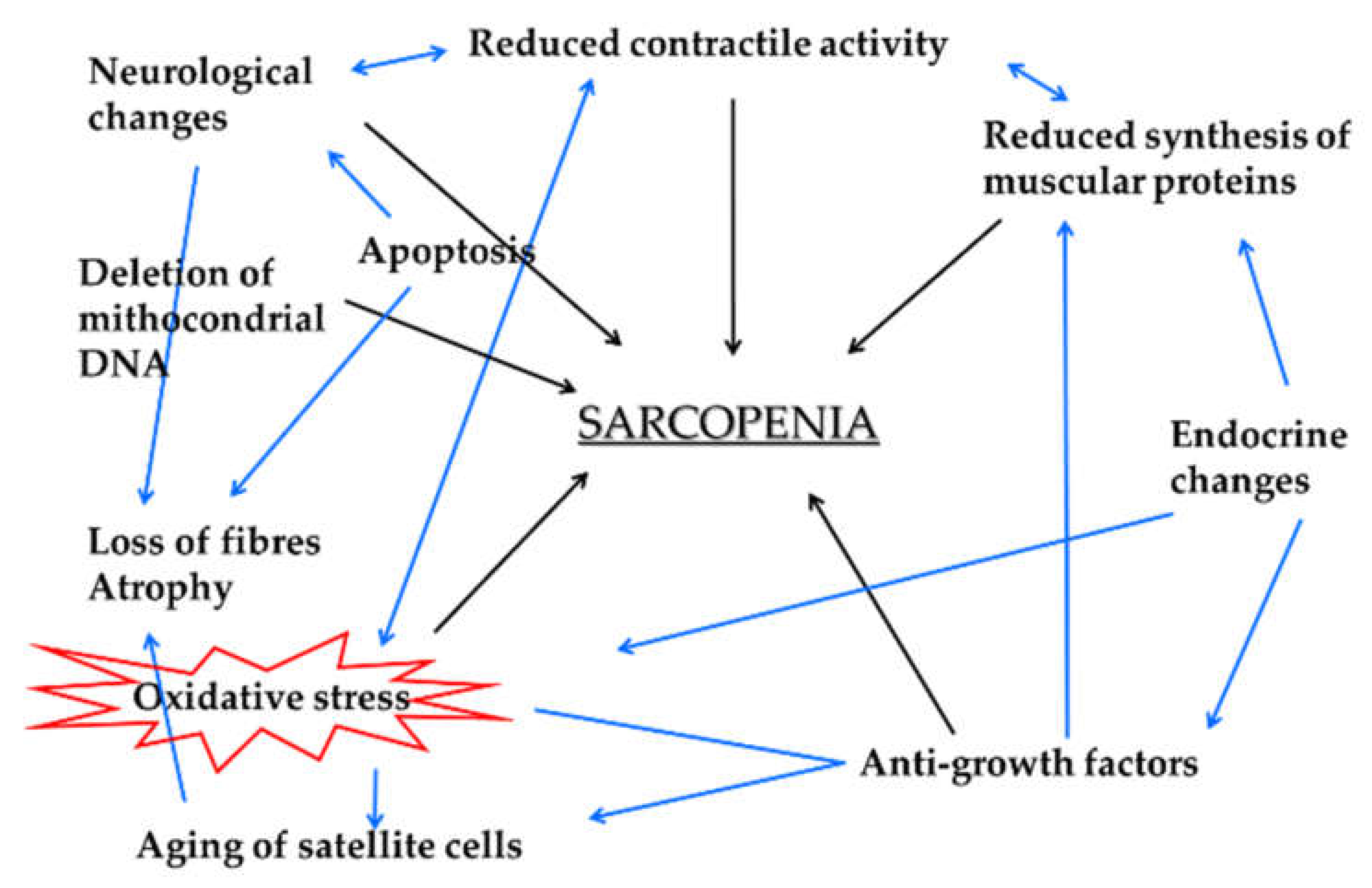
1. Background
2. Features of Selected Myokines
2.1. Myostatin
2.2. NGF
2.3. IGF-1
2.4. S-100
2.5. Irisin
3. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The Age-Related Loss of Skeletal Muscle Mass and Function: Measurement and Physiology of Muscle Fibre Atrophy and Muscle Fibre Loss in Humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Pelosi, L.; Musarò, A. An Overview About the Biology of Skeletal Muscle Satellite Cells. Curr. Genom. 2019, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult Stem Cells at Work: Regenerating Skeletal Muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Q.; Ding, L.-N.; Zeng, N.-X.; Liu, H.-M.; Zheng, S.-H.; Xu, J.-W.; Li, R.-M. Icariin Induces irisin/FNDC5 Expression in C2C12 Cells via the AMPK Pathway. Biomed. Pharmacother. 2019, 115, 108930. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, D.; Berardi, E.; Ceccarelli, G.; Sampaolesi, M. Adult Stem Cells and Skeletal Muscle Regeneration. Curr. Gene Ther. 2015, 15, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M. Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth. Int. J. Mol. Sci. 2020, 21, 7940. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, J.; Rigbolt, K.T.G.; Blagoev, B.; Pedersen, B.K.; Kratchmarova, I. Dynamics of the Skeletal Muscle Secretome during Myoblast Differentiation. Mol. Cell. Proteom. 2010, 9, 2482–2496. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Febbraio, M.A. The Ever-Expanding Myokinome: Discovery Challenges and Therapeutic Implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.-Y.; Formolo, D.A.; Kong, T.; Lau, S.W.-Y.; Ho, C.S.-L.; Leung, R.Y.H.; Hung, F.H.-Y.; Yau, S.-Y. Potential exerkines for physical exercise-elicited pro-cognitive effects: Insight from clinical and animal research. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 147, pp. 361–395. ISBN 978-0-12-816967-4. [Google Scholar]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Huang-Doran, I.; Zhang, C.-Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication in Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Vechetti, I.J.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The Role of Extracellular Vesicles in Skeletal Muscle and Systematic Adaptation to Exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Ardeljan, A.D.; Hurezeanu, R. Sarcopenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Christian, C.J.; Benian, G.M. Animal Models of Sarcopenia. Aging Cell 2020, 19, e13223. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular Mechanisms and Open Questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.P.S.; Chan, W.S.; Chan, C.B. Mitochondria Homeostasis and Oxidant/Antioxidant Balance in Skeletal Muscle-Do Myokines Play a Role? Antioxid. Basel Switz. 2021, 10, 179. [Google Scholar] [CrossRef]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxid. Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The Contribution of Reactive Oxygen Species to Sarcopenia and Muscle Ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Pelosi, L.; Sica, G.; Musarò, A. The Physiopathologic Role of Oxidative Stress in Skeletal Muscle. Mech. Ageing Dev. 2018, 170, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Duranti, G.; Di Filippo, E.S.; Bondi, D.; Verratti, V.; Doria, C.; Caporossi, D.; Sabatini, S.; Dimauro, I.; Pietrangelo, T. Endurance Training Improves Plasma Superoxide Dismutase Activity in Healthy Elderly. Mech. Ageing Dev. 2020, 185, 111190. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Bugera, E.M.; Duhamel, T.A.; Peeler, J.D.; Anderson, J.E. A Focused Review of Myokines as a Potential Contributor to Muscle Hypertrophy from Resistance-Based Exercise. Eur. J. Appl. Physiol. 2020, 120, 941–959. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Puglielli, C.; Mancinelli, R.; Beccafico, S.; Fanò, G.; Fulle, S. Molecular Basis of the Myogenic Profile of Aged Human Skeletal Muscle Satellite Cells during Differentiation. Exp. Gerontol. 2009, 44, 523–531. [Google Scholar] [CrossRef]
- Musarò, A. Muscle Homeostasis and Regeneration: From Molecular Mechanisms to Therapeutic Opportunities. Cells 2020, 9, 2033. [Google Scholar] [CrossRef]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Mariano Cezar, M.D.; Ruiz Lima, A.R.; Okoshi, K.; Okoshi, M.P. Skeletal Muscle Aging: Influence of Oxidative Stress and Physical Exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-P Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in Skeletal Muscle Physiology and Metabolism: Recent Advances and Future Perspectives. Acta Physiol. 2020, 228. [Google Scholar] [CrossRef]
- Nielsen, T.L.; Vissing, J.; Krag, T.O. Antimyostatin Treatment in Health and Disease: The Story of Great Expectations and Limited Success. Cells 2021, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 Transcription Factors Control Muscle Mass in Adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; Milan, G.; Masiero, E.; Carnio, S.; Lee, D.; Lanfranchi, G.; Goldberg, A.L.; Sandri, M. JunB Transcription Factor Maintains Skeletal Muscle Mass and Promotes Hypertrophy. J. Cell Biol. 2010, 191, 101–113. [Google Scholar] [CrossRef]
- Vainshtein, A.; Sandri, M. Signaling Pathways That Control Muscle Mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP Signaling Controls Muscle Mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Walton, K.L.; Hagg, A.; Colgan, T.D.; Johnson, K.; Qian, H.; Gregorevic, P.; Harrison, C.A. Specific Targeting of TGF-β Family Ligands Demonstrates Distinct Roles in the Regulation of Muscle Mass in Health and Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E5266–E5275. [Google Scholar] [CrossRef]
- Hoogaars, W.M.H.; Jaspers, R.T. Past, Present, and Future Perspective of Targeting Myostatin and Related Signaling Pathways to Counteract Muscle Atrophy. In Muscle Atrophy; Xiao, J., Ed.; Springer: Singapore, 2018; Volume 1088, pp. 153–206. ISBN 9789811314346. [Google Scholar]
- Sriram, S.; Subramanian, S.; Sathiakumar, D.; Venkatesh, R.; Salerno, M.S.; McFarlane, C.D.; Kambadur, R.; Sharma, M. Modulation of Reactive Oxygen Species in Skeletal Muscle by Myostatin Is Mediated through NF-κB: Myostatin Induces Reactive Oxygen Species. Aging Cell 2011, 10, 931–948. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and Other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Suh, J.; Lee, Y.-S. Similar Sequences but Dissimilar Biological Functions of GDF11 and Myostatin. Exp. Mol. Med. 2020, 52, 1673–1693. [Google Scholar] [CrossRef]
- Fan, X.; Gaur, U.; Sun, L.; Yang, D.; Yang, M. The Growth Differentiation Factor 11 (GDF11) and Myostatin (MSTN) in Tissue Specific Aging. Mech. Ageing Dev. 2017, 164, 108–112. [Google Scholar] [CrossRef]
- Walker, R.G.; Poggioli, T.; Katsimpardi, L.; Buchanan, S.M.; Oh, J.; Wattrus, S.; Heidecker, B.; Fong, Y.W.; Rubin, L.L.; Ganz, P.; et al. Biochemistry and Biology of GDF11 and Myostatin: Similarities, Differences, and Questions for Future Investigation. Circ. Res. 2016, 118, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.-H.; Na, J.; Im, S.I.; Oh, C.T.; Kim, J.-Y.; Park, S.-K.; Han, H.J.; Seok, J.; Choi, S.Y.; Ko, E.J.; et al. Antioxidant Effect of Human Placenta Hydrolysate against Oxidative Stress on Muscle Atrophy. J. Cell. Physiol. 2019, 234, 1643–1658. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal Muscle Wasting in Cachexia and Sarcopenia: Molecular Pathophysiology and Impact of Exercise Training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Neurotrophic Factors: An Overview. In Neurotrophic Factors; Skaper, S.D., Ed.; Springer: New York, NY, USA, 2018; Volume 1727, pp. 1–17. ISBN 978-1-4939-7570-9. [Google Scholar]
- Davies, A.M. The Survival and Growth of Embryonic Proprioceptive Neurons Is Promoted by a Factor Present in Skeletal Muscle. Dev. Biol. 1986, 115, 56–67. [Google Scholar] [CrossRef]
- Aloe, L.; Chaldakov, G.N. Homage to Rita Levi-Montalcini, the Queen of Modern Neuroscience: Homage to Rita Levi-Montalcini. Cell Biol. Int. 2013, 37, 761–765. [Google Scholar] [CrossRef]
- Stuerenburg, H.J.; Kunze, K. Tissue Concentrations of Nerve Growth Factor in Aging Rat Heart and Skeletal Muscle. Muscle Nerve 1998, 21, 404–406. [Google Scholar] [CrossRef]
- Lavasani, M.; Lu, A.; Peng, H.; Cummins, J.; Huard, J. Nerve Growth Factor Improves the Muscle Regeneration Capacity of Muscle Stem Cells in Dystrophic Muscle. Hum. Gene Ther. 2006, 17, 180–192. [Google Scholar] [CrossRef]
- Amano, T.; Yamakuni, T.; Okabe, N.; Sakimura, K.; Takahashi, Y. Production of Nerve Growth Factor in Rat Skeletal Muscle. Neurosci. Lett. 1991, 132, 5–7. [Google Scholar] [CrossRef]
- Erck, C.; Meisinger, C.; Grothe, C.; Seidl, K. Regulation of Nerve Growth Factor and Its Low-Affinity Receptor (p75NTR) during Myogenic Differentiation. J. Cell. Physiol. 1998, 176, 22–31. [Google Scholar] [CrossRef]
- Ettinger, K.; Lecht, S.; Arien-Zakay, H.; Cohen, G.; Aga-Mizrachi, S.; Yanay, N.; Saragovi, H.U.; Nedev, H.; Marcinkiewicz, C.; Nevo, Y.; et al. Nerve Growth Factor Stimulation of ERK1/2 Phosphorylation Requires Both p75NTR and α9β1 Integrin and Confers Myoprotection towards Ischemia in C2C12 Skeletal Muscle Cell Model. Cell. Signal. 2012, 24, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Garry, D.J. Muscle Stem Cells in Development, Regeneration, and Disease. Genes Dev. 2006, 20, 1692–1708. [Google Scholar] [CrossRef]
- Reddypalli, S.; Roll, K.; Lee, H.-K.; Lundell, M.; Barea-Rodriguez, E.; Wheeler, E.F. p75NTR-Mediated Signaling Promotes the Survival of Myoblasts and Influences Muscle Strength. J. Cell. Physiol. 2005, 204, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, Y.; Liu, G.; Yin, S.; Ma, J.; Liu, J.; Zhang, M.; Wang, Y. p75 Neurotrophin Receptor Regulates NGF-Induced Myofibroblast Differentiation and Collagen Synthesis through MRTF-A. Exp. Cell Res. 2019, 383, 111504. [Google Scholar] [CrossRef] [PubMed]
- de Perini, A.; Dimauro, I.; Duranti, G.; Fantini, C.; Mercatelli, N.; Ceci, R.; Di Luigi, L.; Sabatini, S.; Caporossi, D. The p75NTR-Mediated Effect of Nerve Growth Factor in L6C5 Myogenic Cells. BMC Res. Notes 2017, 10. [Google Scholar] [CrossRef]
- Pallottini, V.; Colardo, M.; Tonini, C.; Martella, N.; Strimpakos, G.; Colella, B.; Tirassa, P.; Bartolomeo, S.D.; Segatto, M. ProNGF/p75NTR Axis Drives Fiber Type Specification by Inducing the Fast-Glycolytic Phenotype in Mouse Skeletal Muscle Cells. Cells 2020, 9, 2232. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Carratù, M.R.; Fonzino, A.; Lograno, M.D.; Tricarico, D. Oxytocin/Osteocalcin/IL-6 and NGF/BDNF mRNA Levels in Response to Cold Stress Challenge in Mice: Possible Oxytonic Brain-Bone-Muscle-Interaction. Front. Physiol. 2019, 10, 1437. [Google Scholar] [CrossRef]
- Patel, D.I.; White, L.J.; Lira, V.A.; Criswell, D.S. Forced Exercise Increases Muscle Mass in EAE Despite Early Onset of Disability. Physiol. Res. 2016, 1013–1017. [Google Scholar] [CrossRef]
- La Rovere, R.M.L.; Quattrocelli, M.; Pietrangelo, T.; Di Filippo, E.S.; Maccatrozzo, L.; Cassano, M.; Mascarello, F.; Barthélémy, I.; Blot, S.; Sampaolesi, M.; et al. Myogenic Potential of Canine Craniofacial Satellite Cells. Front. Aging Neurosci. 2014, 6, 90. [Google Scholar] [CrossRef]
- Carrero-Rojas, G.; Benítez-Temiño, B.; Pastor, A.M.; Davis López de Carrizosa, M.A. Muscle Progenitors Derived from Extraocular Muscles Express Higher Levels of Neurotrophins and Their Receptors than Other Cranial and Limb Muscles. Cells 2020, 9, 747. [Google Scholar] [CrossRef]
- Diao, Y.-P.; Cui, F.-K.; Yan, S.; Chen, Z.-G.; Lian, L.-S.; Guo, L.-L.; Li, Y.-J. Nerve Growth Factor Promotes Angiogenesis and Skeletal Muscle Fiber Remodeling in a Murine Model of Hindlimb Ischemia. Chin. Med. J. 2016, 129, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and Their Receptors: A Convergence Point for Many Signalling Pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Gaglio, D.; Bianco, M.R.; Aprea, F.; Virtuoso, A.; Bonanomi, M.; Alberghina, L.; Papa, M.; Colangelo, A.M. Differentiation by Nerve Growth Factor (NGF) Involves Mechanisms of Crosstalk between Energy Homeostasis and Mitochondrial Remodeling. Cell Death Dis. 2018, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Poreba, E.; Durzynska, J. Nuclear Localization and Actions of the Insulin-like Growth Factor 1 (IGF-1) System Components: Transcriptional Regulation and DNA Damage Response. Mutat. Res. 2020, 784, 108307. [Google Scholar] [CrossRef]
- Werner, H.; Weinstein, D.; Bentov, I. Similarities and Differences between Insulin and IGF-I: Structures, Receptors, and Signalling Pathways. Arch. Physiol. Biochem. 2008, 114, 17–22. [Google Scholar] [CrossRef]
- Werner, H. Insulin-Like Growth Factors in Development, Cancers and Aging. Cells 2020, 9, 2309. [Google Scholar] [CrossRef]
- Werner, H.; Sarfstein, R.; Laron, Z. The Role of Nuclear Insulin and IGF1 Receptors in Metabolism and Cancer. Biomolecules 2021, 11, 531. [Google Scholar] [CrossRef]
- Papaconstantinou, J. Insulin/IGF-1 and ROS Signaling Pathway Cross-Talk in Aging and Longevity Determination. Mol. Cell. Endocrinol. 2009, 299, 89–100. [Google Scholar] [CrossRef]
- Martins, A.R.; Nachbar, R.T.; Gorjao, R.; Vinolo, M.A.; Festuccia, W.T.; Lambertucci, R.H.; Cury-Boaventura, M.F.; Silveira, L.R.; Curi, R.; Hirabara, S.M. Mechanisms Underlying Skeletal Muscle Insulin Resistance Induced by Fatty Acids: Importance of the Mitochondrial Function. Lipids Health Dis. 2012, 11, 30. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef]
- Philippou, A.; Maridaki, M.; Halapas, A.; Koutsilieris, M. The Role of the Insulin-like Growth Factor 1 (IGF-1) in Skeletal Muscle Physiology. Vivo Athens Greece 2007, 21, 45–54. [Google Scholar]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Miano, C.; Scicchitano, B.; Musarò, A. Signals from the Niche: Insights into the Role of IGF-1 and IL-6 in Modulating Skeletal Muscle Fibrosis. Cells 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between Sarcopenia and Levels of Growth Hormone and Insulin-like Growth Factor-1 in the Elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Mofarrahi, M.; Guo, Y.; Haspel, J.A.; Choi, A.M.K.; Davis, E.C.; Gouspillou, G.; Hepple, R.T.; Godin, R.; Burelle, Y.; Hussain, S.N.A. Autophagic Flux and Oxidative Capacity of Skeletal Muscles during Acute Starvation. Autophagy 2013, 9, 1604–1620. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Aucello, M.; Rizzuto, E.; Beccafico, S.; Mammucari, C.; Boncompagni, S.; Bonconpagni, S.; Belia, S.; Wannenes, F.; Nicoletti, C.; et al. Skeletal Muscle Is a Primary Target of SOD1G93A-Mediated Toxicity. Cell Metab. 2008, 8, 425–436. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 146. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines Can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef]
- Hou, X.; Li, Z.; Higashi, Y.; Delafontaine, P.; Sukhanov, S. Insulin-Like Growth Factor I Prevents Cellular Aging via Activation of Mitophagy. J. Aging Res. 2020, 2020, 4939310. [Google Scholar] [CrossRef]
- Riis, S.; Murray, J.B.; O’Connor, R. IGF-1 Signalling Regulates Mitochondria Dynamics and Turnover through a Conserved GSK-3β-Nrf2-BNIP3 Pathway. Cells 2020, 9, 147. [Google Scholar] [CrossRef]
- Sádaba, M.C.; Martín-Estal, I.; Puche, J.E.; Castilla-Cortázar, I. Insulin-like Growth Factor 1 (IGF-1) Therapy: Mitochondrial Dysfunction and Diseases. Biochim. Biophys. Acta 2016, 1862, 1267–1278. [Google Scholar] [CrossRef]
- Donato, R. S100: A Multigenic Family of Calcium-Modulated Proteins of the EF-Hand Type with Intracellular and Extracellular Functional Roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Fanò, G.; Biocca, S.; Fulle, S.; Mariggiò, M.A.; Belia, S.; Calissano, P. The S-100: A Protein Family in Search of a Function. Prog. Neurobiol. 1995, 46, 71–82. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 Proteins in Health and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. RAGE: A Single Receptor for Several Ligands and Different Cellular Responses: The Case of Certain S100 Proteins. Curr. Mol. Med. 2007, 7, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Champaiboon, C.; Guenther, B.D.; Sorenson, B.S.; Khammanivong, A.; Ross, K.F.; Geczy, C.L.; Herzberg, M.C. Anti-Infective Protective Properties of S100 Calgranulins. Anti Inflamm. Anti Allergy Agents Med. Chem. 2009, 8, 290–305. [Google Scholar] [CrossRef]
- Zimmer, D.B. Examination of the Calcium-Modulated Protein S100 Alpha and Its Target Proteins in Adult and Developing Skeletal Muscle. Cell Motil. Cytoskeleton 1991, 20, 325–337. [Google Scholar] [CrossRef]
- Most, P.; Remppis, A.; Weber, C.; Bernotat, J.; Ehlermann, P.; Pleger, S.T.; Kirsch, W.; Weber, M.; Uttenweiler, D.; Smith, G.L.; et al. The C Terminus (amino Acids 75-94) and the Linker Region (amino Acids 42-54) of the Ca2+-Binding Protein S100A1 Differentially Enhance Sarcoplasmic Ca2+ Release in Murine Skinned Skeletal Muscle Fibers. J. Biol. Chem. 2003, 278, 26356–26364. [Google Scholar] [CrossRef] [PubMed]
- Franzini-Armstrong, C.; Protasi, F. Ryanodine Receptors of Striated Muscles: A Complex Channel Capable of Multiple Interactions. Physiol. Rev. 1997, 77, 699–729. [Google Scholar] [CrossRef]
- Sun, Q.-A.; Wang, B.; Miyagi, M.; Hess, D.T.; Stamler, J.S. Oxygen-Coupled Redox Regulation of the Skeletal Muscle Ryanodine receptor/Ca2+ Release Channel (RyR1): Sites and Nature of Oxidative Modification. J. Biol. Chem. 2013, 288, 22961–22971. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Prosser, B.L.; Ghassemi, F.; Xu, L.; Pasek, D.A.; Eu, J.P.; Hernández-Ochoa, E.O.; Cannon, B.R.; Wilder, P.T.; Lovering, R.M.; et al. Modulation of Sarcoplasmic Reticulum Ca2+ Release in Skeletal Muscle Expressing Ryanodine Receptor Impaired in Regulation by Calmodulin and S100A1. Am. J. Physiol. Cell Physiol. 2011, 300, C998–C1012. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Belia, S.; Fanò Illic, G. The Arianna Thread: The Matching of S-100 Family with the RyR’s Muscle Receptor. Eur. J. Transl. Myol. 2020, 30, 8839. [Google Scholar] [CrossRef]
- Fulle, S.; Mariggiò, M.A.; Belia, S.; Petrelli, C.; Ballarini, P.; Guarnieri, S.; Fanò, G. Rapid Desensitization of PC12 Cells Stimulated with High Concentrations of Extracellular S100. Neuroscience 1999, 89, 991–997. [Google Scholar] [CrossRef]
- Chiappalupi, S.; Sorci, G.; Vukasinovic, A.; Salvadori, L.; Sagheddu, R.; Coletti, D.; Renga, G.; Romani, L.; Donato, R.; Riuzzi, F. Targeting RAGE Prevents Muscle Wasting and Prolongs Survival in Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 929–946. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the Pathophysiology of Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef]
- Spratt, D.E.; Barber, K.R.; Marlatt, N.M.; Ngo, V.; Macklin, J.A.; Xiao, Y.; Konermann, L.; Duennwald, M.L.; Shaw, G.S. A Subset of Calcium-Binding S100 Proteins Show Preferential Heterodimerization. FEBS J. 2019, 286, 1859–1876. [Google Scholar] [CrossRef]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/S100A9): A Key Protein between Inflammation and Cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Udeh, R.; Advani, S.; de Guadiana Romualdo, L.G.; Dolja-Gore, X. Calprotectin, an Emerging Biomarker of Interest in COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 775. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Andersen, K.; Fischer, C.; Nielsen, A.R.; Nielsen, S.; Akerström, T.; Aastrøm, M.; Borup, R.; Pedersen, B.K. Calprotectin Is Released from Human Skeletal Muscle Tissue during Exercise. J. Physiol. 2008, 586, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s Double Life: Intracellular Regulator and Extracellular Signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Steinbrenner, H. Cellular Adaptation to Xenobiotics: Interplay between Xenosensors, Reactive Oxygen Species and FOXO Transcription Factors. Redox Biol. 2017, 13, 646–654. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy Maintains Stemness by Preventing Senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Tubaro, C.; Arcuri, C.; Giambanco, I.; Donato, R. S100B Protein in Myoblasts Modulates Myogenic Differentiation via NF-kappaB-Dependent Inhibition of MyoD Expression. J. Cell. Physiol. 2010, 223, 270–282. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Izhar, S.; Proteau, G.; Slaughter, G.; Parker, T.G. S100B-RAGE Dependent VEGF Secretion by Cardiac Myocytes Induces Myofibroblast Proliferation. J. Mol. Cell. Cardiol. 2012, 52, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Schulte, S.; Podlog, L.W.; Hamson-Utley, J.J.; Strathmann, F.G.; Strüder, H.K. A Systematic Review of the Biomarker S100B: Implications for Sport-Related Concussion Management. J. Athl. Train. 2014, 49, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Morozzi, G.; Beccafico, S.; Bianchi, R.; Riuzzi, F.; Bellezza, I.; Giambanco, I.; Arcuri, C.; Minelli, A.; Donato, R. Oxidative Stress-Induced S100B Accumulation Converts Myoblasts into Brown Adipocytes via an NF-κB/YY1/miR-133 Axis and NF-κB/YY1/BMP-7 Axis. Cell Death Differ. 2017, 24, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Eshragi, M.; Ande, S.R.; Chazin, W.J.; Klonisch, T.; Halayko, A.J.; McNeill, K.D.; Hashemi, M.; Kerkhoff, C.; Los, M. S100A8/A9 Induces Autophagy and Apoptosis via ROS-Mediated Cross-Talk between Mitochondria and Lysosomes That Involves BNIP3. Cell Res. 2010, 20, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, Y.; Fei, C.; Guo, J.; Jia, Y.; Wu, D.; Wu, L.; Chang, C. Cellular Senescence Induced by S100A9 in Mesenchymal Stromal Cells through NLRP3 Inflammasome Activation. Aging 2019, 11, 9626–9642. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Tse, M.C.L.; Hu, X.; Jia, W.-H.; Du, G.-H.; Chan, C.B. Interaction of CREB and PGC-1α Induces Fibronectin Type III Domain-Containing Protein 5 Expression in C2C12 Myotubes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 1574–1584. [Google Scholar] [CrossRef]
- Flori, L.; Testai, L.; Calderone, V. The “Irisin System”: From Biological Roles to Pharmacological and Nutraceutical Perspectives. Life Sci. 2021, 267, 118954. [Google Scholar] [CrossRef] [PubMed]
- Panati, K.; Narala, V.R.; Narasimha, V.R.; Derangula, M.; Arva Tatireddigari, V.R.R.; Yeguvapalli, S. Expression, Purification and Biological Characterisation of Recombinant Human Irisin (12.5 kDa). J. Genet. Eng. Biotechnol. 2018, 16, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jeong, Y.J.; Song, I.-S.; Noh, Y.H.; Seo, K.W.; Kim, M.; Han, J. Glucocorticoid Receptor Positively Regulates Transcription of FNDC5 in the Liver. Sci. Rep. 2017, 7, 43296. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef]
- Lee, D.E.; Bareja, A.; Bartlett, D.B.; White, J.P. Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration. Cells 2019, 8, 183. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Puszczewicz, M. A Review on Irisin, a New Protagonist That Mediates Muscle-Adipose-Bone-Neuron Connectivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4687–4693. [Google Scholar]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell’Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, X.; Tang, N.; Huang, F.; Chen, Z.; Shi, G. Review of Research on the Role of Irisin in Tumors. OncoTargets Ther. 2020, 13, 4423–4430. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. mRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, S.; Kuloglu, T.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Cicek, D. Alterations of Irisin Concentrations in Saliva and Serum of Obese and Normal-Weight Subjects, before and after 45 Min of a Turkish Bath or Running. Peptides 2013, 50, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhang, L.; Ruan, J.; Zhang, X.; Chen, J.; Ma, C.; Yu, Z. Detection and Quantitation of Irisin in Human Cerebrospinal Fluid by Tandem Mass Spectrometry. Peptides 2018, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating Irisin Levels as a Predictive Biomarker for Sarcopenia: A Cross-Sectional Community-Based Study: Irisin as a Biomarker for Sarcopenia. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.-K. The Novel Myokine Irisin: Clinical Implications and Potential Role as a Biomarker for Sarcopenia in Postmenopausal Women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Wen, M.-S.; Yeh, J.-K.; Hsieh, I.-C.; Chen, C.-C.; Hsieh, M.-J.; Tsai, M.-L.; Yang, C.-H.; Wu, V.C.-C.; Hung, K.-C.; et al. Excessive Irisin Increases Oxidative Stress and Apoptosis in Murine Heart. Biochem. Biophys. Res. Commun. 2018, 503, 2493–2498. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.T.; Zhang, S.; Dubielecka, P.M.; Du, J.; Yano, N.; Chin, Y.E.; Zhuang, S.; Qin, G.; Zhao, T.C. Irisin Plays a Pivotal Role to Protect the Heart against Ischemia and Reperfusion Injury. J. Cell. Physiol. 2017, 232, 3775–3785. [Google Scholar] [CrossRef]
- Lu, L.; Ma, J.; Tang, J.; Liu, Y.; Zheng, Q.; Chen, S.; Gao, E.; Ren, J.; Yang, L.; Yang, J. Irisin Attenuates Myocardial Ischemia/reperfusion-Induced Cardiac Dysfunction by Regulating ER-Mitochondria Interaction through a Mitochondrial Ubiquitin Ligase-Dependent Mechanism. Clin. Transl. Med. 2020, 10, e166. [Google Scholar] [CrossRef]
- Xin, T.; Lu, C. Irisin Activates Opa1-Induced Mitophagy to Protect Cardiomyocytes against Apoptosis Following Myocardial Infarction. Aging 2020, 12, 4474–4488. [Google Scholar] [CrossRef]
- Ma, C.; Ding, H.; Deng, Y.; Liu, H.; Xiong, X.; Yang, Y. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front. Physiol. 2021, 12, 620608. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Miura, K.; Arima, H.; Fujiyoshi, A.; Kadota, A.; Kadowaki, S.; Zaid, M.; Miyagawa, N.; Satoh, A.; Kunimura, A.; et al. Relationship of Serum Irisin Levels to Prevalence and Progression of Coronary Artery Calcification: A Prospective, Population-Based Study. Int. J. Cardiol. 2018, 267, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-A.; Zhang, H.; Yu, Q.; Zhang, J.-F.; Wang, C.-Q.; Gu, J.; Chen, K. Association of Circulating Irisin Levels and the Characteristics and Prognosis of Coronary Artery Disease. Am. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin Levels in Genetic and Essential Obesity: Clues for a Potential Dual Role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef]
- Deng, W. Association of Serum Irisin Concentrations with Presence and Severity of Coronary Artery Disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 4193–4197. [Google Scholar] [CrossRef] [PubMed]
- Shimba, Y.; Togawa, H.; Senoo, N.; Ikeda, M.; Miyoshi, N.; Morita, A.; Miura, S. Skeletal Muscle-Specific PGC-1α Overexpression Suppresses Atherosclerosis in Apolipoprotein E-Knockout Mice. Sci. Rep. 2019, 9, 4077. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the Metabolic Effects of Irisin on Skeletal Muscle in Vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a Unique Non-Inflammatory Myokine in Stimulating Skeletal Muscle Metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef]
- Popov, D.V.; Lysenko, E.A.; Makhnovskii, P.A.; Kurochkina, N.S.; Vinogradova, O.L. Regulation of PPARGC1A Gene Expression in Trained and Untrained Human Skeletal Muscle. Physiol. Rep. 2017, 5, e13543. [Google Scholar] [CrossRef][Green Version]
- Kurdiova, T.; Balaz, M.; Mayer, A.; Maderova, D.; Belan, V.; Wolfrum, C.; Ukropec, J.; Ukropcova, B. Exercise-Mimicking Treatment Fails to Increase Fndc5 mRNA & Irisin Secretion in Primary Human Myotubes. Peptides 2014, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Albrecht, E.; Komolka, K.; Schering, L.; Langhammer, M.; Hoeflich, A.; Maak, S. Irisin Is Elevated in Skeletal Muscle and Serum of Mice Immediately after Acute Exercise. Int. J. Biol. Sci. 2014, 10, 338–349. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and Mitochondrial Metabolism--Emerging Concepts and Relevance in Ageing and Neurodegenerative Disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Arcuri, C.; Giambanco, I.; Bellezza, I.; Minelli, A.; Donato, R. Cellular and Molecular Mechanisms of Sarcopenia: The S100B Perspective. J. Cachexia Sarcopenia Muscle 2018, 9, 1255–1268. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine Irisin-Induced Protection against Oxidative Stress in Vitro. Involvement of Heme Oxygenase-1 and Antioxidazing Enzymes Superoxide Dismutase-2 and Glutathione Peroxidase. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 117–125. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxid. Basel Switz. 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Yano, N.; Zhang, L.; Wei, D.; Dubielecka, P.M.; Wei, L.; Zhuang, S.; Zhu, P.; Qin, G.; Liu, P.Y.; Chin, Y.E.; et al. Irisin Counteracts High Glucose and Fatty Acid-Induced Cytotoxicity by Preserving the AMPK-Insulin Receptor Signaling Axis in C2C12 Myoblasts. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E791–E805. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, X.; Wu, K.; Liu, K.; Wang, S.; Chen, X. Irisin Attenuates H2O2-Induced Apoptosis in Cardiomyocytes via microRNA-19b/AKT/mTOR Signaling Pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 7707–7717. [Google Scholar] [PubMed]

| Myokine | Principal Targets | Specific Membrane Partners | Intracellular Effect on Muscle | Regulation by Physical Exercise | Modulation by Muscle Aging | Possible Effects on Muscle Aging |
|---|---|---|---|---|---|---|
| Myostatin | Muscle (skeletal and cardiac), adipose tissue, brain | ActRII/B and TGFβRII receptors | Inhibition of protein synthesis and regenerative processes | inhibited | increased | inflammation and oxidative stress |
| NGF | Muscle (skeletal and cardiac), brain | TrkA and p75NTR receptors | Stimulation of regenerative processes | increased | increased/decreased | increase in the presence of type I fibers |
| IGF-1 | Muscle (skeletal and cardiac), bone, adipose tissue | tyrosine kinase receptors (IGF-1 and IGF-2) | Stimulation of protein synthesis and regenerative processes, inhibition of catabolic pathways | increased | increased/decreased | alteration of IGF/IGFR system |
| S100 | Muscle (skeletal and cardiac), brain | RAGE, G-protein-coupled receptors, N-glycans | Regulation of Ca2+-dependent mechanisms and regenerative processes | increased | decreased in myoblasts | limitation of regenerative processes |
| Irisin | Muscle, bone, adipose tissue, cardiovascular system | αV/β5 integrins (bone, adipose tissue) | Thermogenesis, glucose homeostasis, mitogenesis | increased | decreased | decreases stimulation of mitochondrial biogenesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. https://doi.org/10.3390/ijms22168520
Mancinelli R, Checcaglini F, Coscia F, Gigliotti P, Fulle S, Fanò-Illic G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. International Journal of Molecular Sciences. 2021; 22(16):8520. https://doi.org/10.3390/ijms22168520
Chicago/Turabian StyleMancinelli, Rosa, Franco Checcaglini, Francesco Coscia, Paola Gigliotti, Stefania Fulle, and Giorgio Fanò-Illic. 2021. "Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging" International Journal of Molecular Sciences 22, no. 16: 8520. https://doi.org/10.3390/ijms22168520
APA StyleMancinelli, R., Checcaglini, F., Coscia, F., Gigliotti, P., Fulle, S., & Fanò-Illic, G. (2021). Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. International Journal of Molecular Sciences, 22(16), 8520. https://doi.org/10.3390/ijms22168520









