Genome-Wide Identification of Aquaporin Gene Family in Pitaya Reveals an HuNIP6;1 Involved in Flowering Process
Abstract
:1. Introduction
2. Results
2.1. Identification, Classification, Nomenclature, and Properties of HuAQPs
2.2. Gene Structure and Conserved Motif Analyses of HuAQPs
2.3. Gene Mapping on the Pitaya Chromosomes
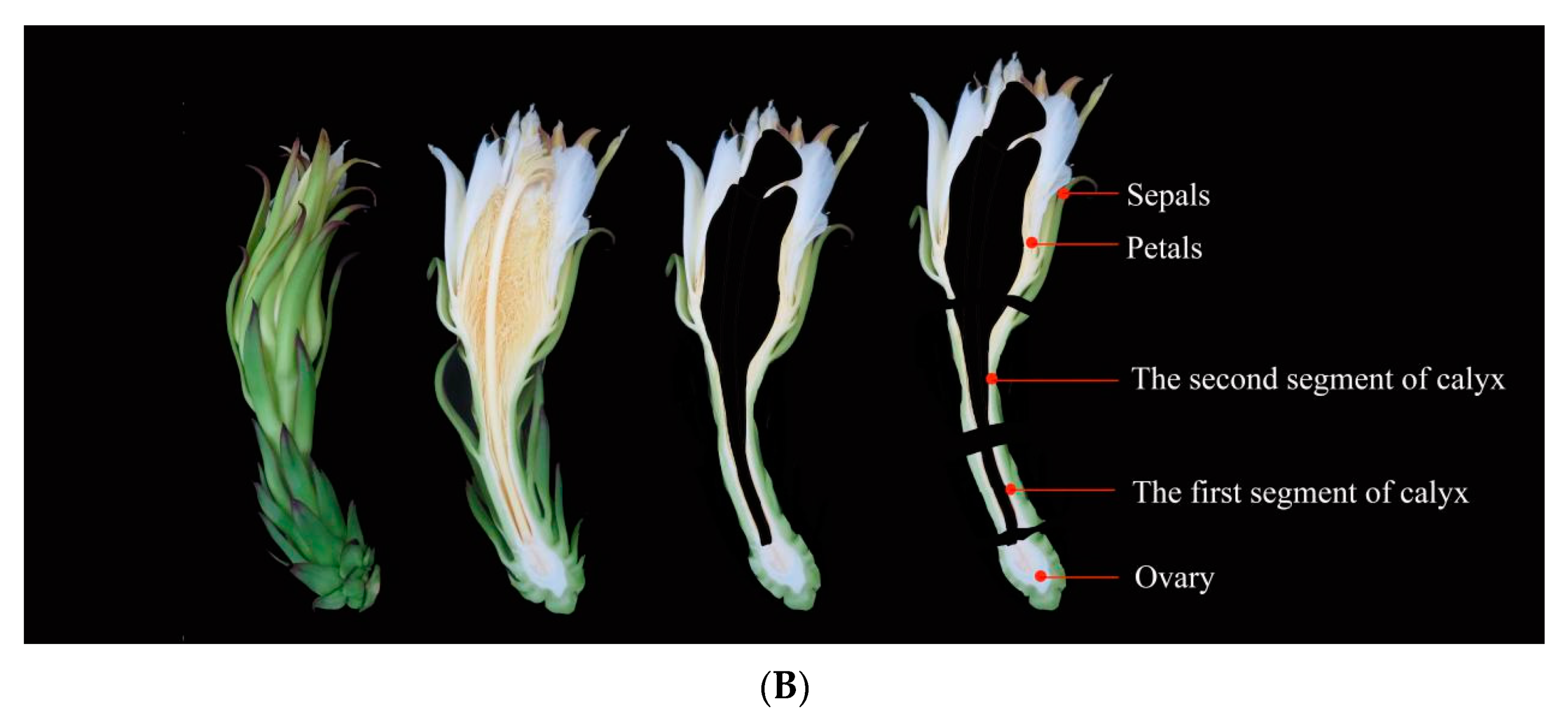
2.4. Expression Patterns of HuAQPs during the Flower-Opening Process in Pitaya
2.5. Expression Patterns of HuNIP6;1 during Flower Opening in Pitaya
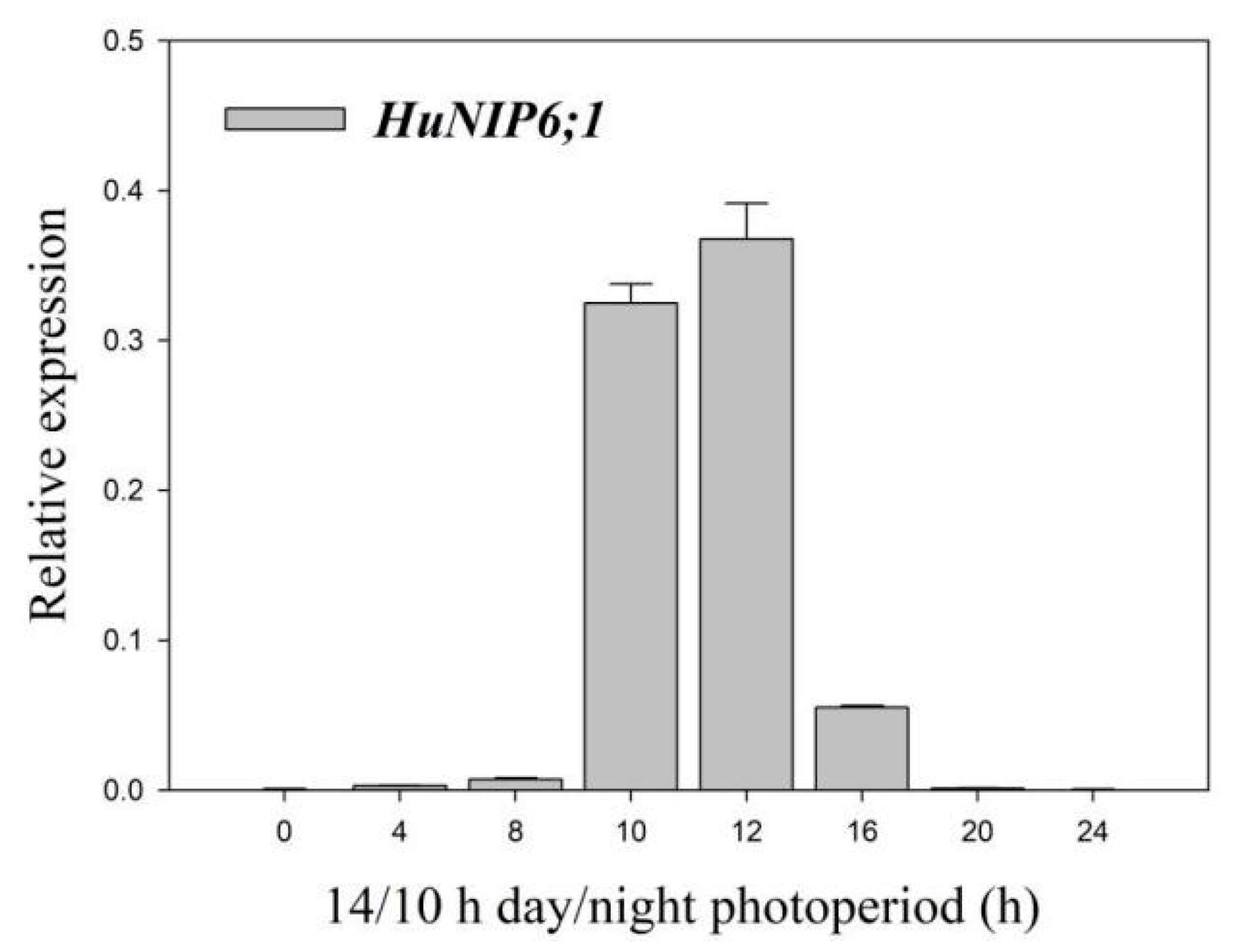
2.6. Expression Pattern of HuNIP6;1 in Diurnal Cycle in Pitaya under 14 h/10 h Day/Night
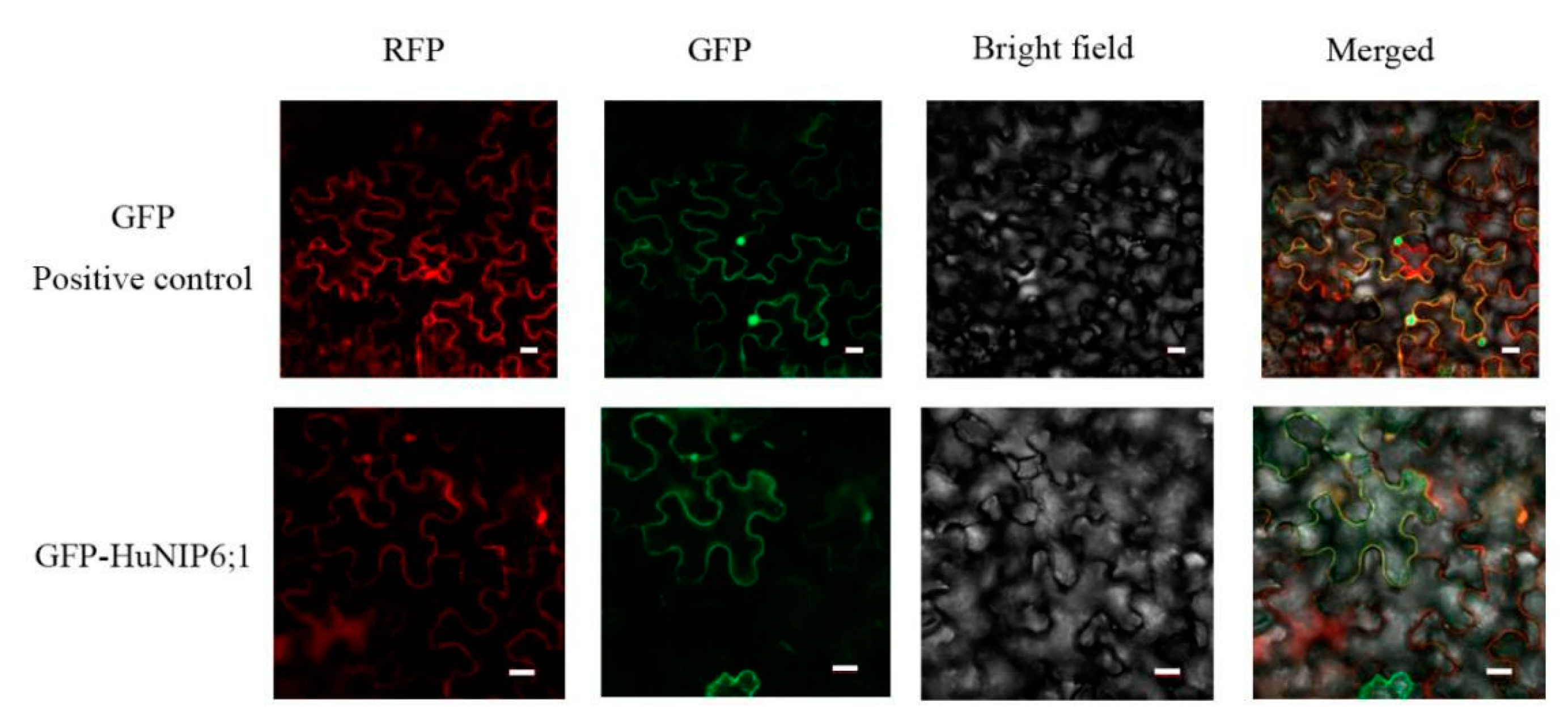
2.7. Subcellular Localization of HuNIP6;1
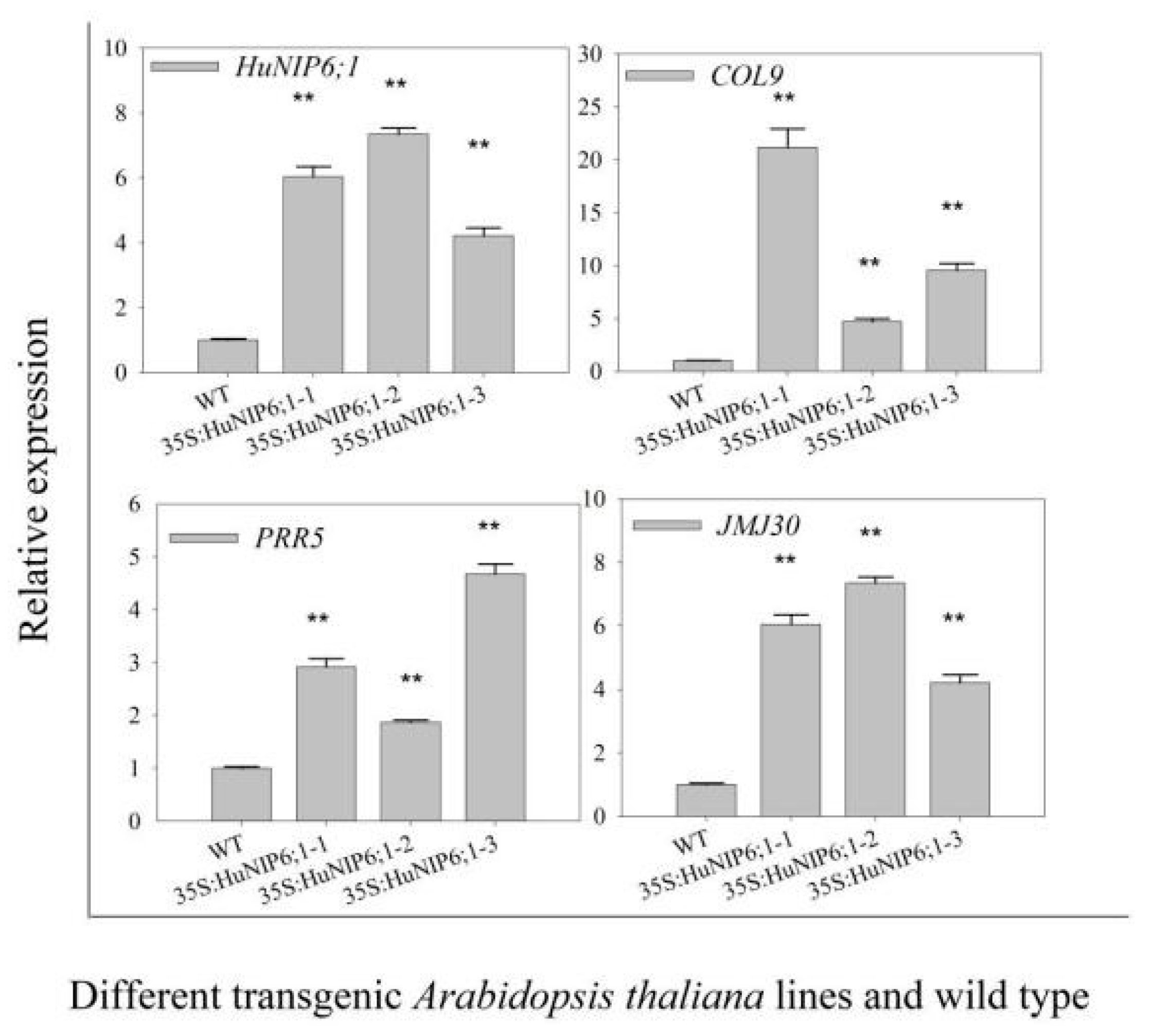
2.8. Over-Expression of HuNIP6;1 in Arabidopsis thaliana
3. Discussion and Conclusions
4. Materials and Methods
4.1. Sequence Analysis
4.2. Phylogenetic Analysis
4.3. Gene-Structure Analysis and Identification of Conserved Motifs
4.4. Chromosomal Distribution
4.5. Plant Materials
4.6. RNA Isolation, cDNA Synthesis and RT-qPCR Analyses
4.7. Cloning of HuNIP6;1 Gene
4.8. Subcellular Localization of HuNIP6;1
4.9. Arabidopsis thaliana Transformation and Phenotypic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| AQP | Aquaporin |
| CCA1 | Circadian clock associated 1 |
| cDNA | Complementary DNA |
| CO | Constans |
| COL9 | Constans-like 9 |
| FD | Flowering locus D |
| LFY | LEAFY |
| FT | Flowering locus T |
| GFP | Green fluorescent protein |
| eGFP | Enhance green fluorescent protein |
| kDa | Kilodaltons |
| LHY | Late elongated hypocotyl |
| MJM | Jumonji-C domain-containing protein |
| NCBI | National Center for Biotechnology Information |
| ORF | Open reading frame |
| pI | Theoretical isoelectric |
| PRR | Pseudo response regulator |
| RT-qPCR | Reverse transcription quantitative real-time polymerase chain reaction |
| SOC1 | Suppressor of overexpression of constans 1 |
| TOC1 | Timing of CAB expression 1 |
References
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 34–42. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, P.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 Protein. Science 1992, 358, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, C.; François, B.; Eva, W.; Maarten, J.; Chrispeels, R.J. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.L.; Yang, S.Z.; Wang, Y.L.; Mohammed, B.; Fu, X.P. Genome-wide identification and characterization of aquaporin gene family in Beta vulgaris. PeerJ 2017, 5, e3747. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tao, J.J.; Golam, J.A.; Li, J.W.; Yang, Y.X. Genome-wide identification and expression analysis of aquaporin gene family related to abiotic stress in watermelon. Genome 2019, 62, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Yang, L.; Liu, N.; Yang, J.; Zhou, X.K.; Xia, Y.C.; He, Y.; He, Y.Q.; Gong, H.J.; Dong, F.; et al. Genome-wide identification structure characterization, and expression pattern profiling of aquaporin gene family in cucumber. BMC Plant Biol. 2019, 1, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, H.; Han, B.G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the AQP1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.; Libson, A.; Miercke, L.J.; Weitzman, C.; Nollert, P.; Krucinski, J. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Gong, J.; Huang, Q.X.; Mo, Y.Y.; Yang, L.F.; Xie, G.S. Structure of a glycerol-conducting channel and the basis for its selectivity. PLoS ONE 2015, 10, e0141022. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Ma, J.F.; Kazunori, T.; Naoki, Y.; Namiki, M.; Saeko, K.; Maki, K.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Paul, R.H.; Christian, P.C.; Sara, D.B.; Lynne, M.R.; Lorenzo, F. Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol. 2007, 145, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Azeez, B.; Dominique, T.; Christophe, D.; Lisa, S.; Nathalie, L.C.; Francis, M.; Schoefs, B.; Bouhidel, K. Life with and without AtTIP1;1, an Arabidopsis aquaporin preferentially localized in the apposing tonoplasts of adjacent vacuoles. Plant Mol. Biol. 2009, 70, 193–209. [Google Scholar] [CrossRef]
- Christopher, E.; Gea, G.; Xabier, L. How is silicic acid transported in plants? Silicon 2020, 12, 2641–2645. [Google Scholar] [CrossRef] [Green Version]
- Sarda, X.; Tousch, D.; Ferrare, K.; Legrand, E.; Dupuis, J.M.; Casse-Delbart, F.; Lamaze, T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 1997, 12, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinen, R.B.; Qing, Y.; François, C. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [Green Version]
- Norbert, U.; Beate, O.; David, T.H.; Matthias, F.; Nate, M.; Ralf, K. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 2008, 20, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.X.; He, X.L.; Zhao, B.C.; Zhou, C.J.; Liang, Y.Z.; Ge, R.C.; Shen, Y.Z.; Huang, Z.J. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic Arabidopsis. Plant Cell Physiol. 2010, 51, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Gerd, P.B.; Robert, B.; Marie, C.; Berny, F.C.; Gerd, P.; Bienert, R.B.; Heinen, M.C.; Berny, F.C. Maize plasma membrane aquaporin ZmPIP2;5, but not ZmPIP1;2, facilitates transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1838, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Song, X.Q.; He, H.; Chu, L.W.; Zhou, H.J.; Zhao, Y.Q.; Xu, Y.K.; Zeng, W.; Lin, X.C.; Lu, M.Z. Genome-wide identification of plasma membrane aquaporin gene family in Populus and functional identification of PIP1;1 involved in osmotic stress. Environ. Exp. Bot. 2020, 179. [Google Scholar] [CrossRef]
- Mott, I.W.; Wang, R.R.C. Comparative transcriptome analysis of salt-tolerant wheat germplasm lines using wheat genome arrays. Plant Sci. 2007, 173, 327–339. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.B.; Charles, B.; Matton, D.P. Characterization of a fertilization-induced and developmentally regulated plasma-membrane aquaporin expressed in reproductive tissues, in the wild potato Solanum chacoense Bitt. Planta 2002, 3, 485–493. [Google Scholar] [CrossRef]
- Marc, B.; Frank, V.; Mieke, W.A.; Koen, W.; Henk, V.; Celestina, M. Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiol. 2005, 137, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Xue, J.Q.; Li, Y.H.; Liu, X.J.; Dai, F.W.; Jia, W.S.; Luo, Y.B.; Gao, J.P. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 2008, 148, 894–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajime, S.; Takehiro, S.; Ichiro, T. Expression analysis of genes encoding plasma membrane aquaporins during seed and fruit development in tomato. Plant Sci. 2006, 171, 277–285. [Google Scholar] [CrossRef]
- Paula, M.; Claudia, B.; Gustavo, M.; Karina, A.; Moira, S.; Marcos, C.; Gabriela, A. A fruit-specific plasma membrane aquaporin subtype PIP1;1 is regulated during strawberry (Fragaria×ananassa) fruit ripening. Physiol. Plant 2008, 132, 538–551. [Google Scholar] [CrossRef]
- Kong, W.L.; Mohammed, B.; Fu, X.P. Genome-wide identification and characterization of aquaporins and their role in the flower opening processes in Carnation (Dianthus caryophyllus). Molecules 2018, 23, 1895. [Google Scholar] [CrossRef] [Green Version]
- Lichtenzveig, J.S.; Abbo, A.; Nerd, N.; Tel-Zur Mizrahi, Y. Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus. Am. J. Bot. 2000, 87, 1058–1065. [Google Scholar] [CrossRef]
- Mizrahi, Y.; Mouyal, J.; Nerd, A.; Sitrit, Y. Metaxenia in the vine cacti Hylocereus polyrhizus and Selenicereus spp. Ann. Bot. 2004, 93, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, J.; Nerd, A.; Mizrahi, Y. Flowering behavior and pollination requirements in climbing Cacti with fruit crop potential. HortScience 1994, 29, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Pushpakumara, D.K.N.G.; Gunasena, H.P.M.; Karyawasam, M. Flowering and fruiting phenology, pollination vectors and breeding system of dragon fruit (Hylocereus spp.). Sri Lankan J. Agric. Sci. 2005, 42, 81–91. [Google Scholar]
- Chen, J.Y.; Xie, F.F.; Cui, Y.Z.; Chen, C.B.; Lu, W.J.; Hu, X.D.; Hua, Q.Z.; Zhao, J.; Wu, Z.J.; Gao, D.; et al. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis. Hortic. Res. 2021, 8, 164. [Google Scholar] [CrossRef]
- Till, A.D.; Benjamin, P.; Nadine, B.; Anja, H.; Gerd, P.B. Genome-wide identification of aquaporin encoding genes in Brassica oleracea and their phylogenetic sequence comparison to Brassica crops and Arabidopsis. Front. Plant Sci. 2015, 6, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Deokar, A.A.; Tar’an, B. Genome-wide analysis of the aquaporin gene family in Chickpea (Cicer arietinum L.). Front. Plant. Sci. 2016, 7, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, R.; Bélanger, R.R. Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2016, 30, 1277–1285. [Google Scholar] [CrossRef]
- Wei, Q.J.; Ma, Q.L.; Ma, Z.Z.; Zhou, G.F.; Feng, F.F.; Le, S.; Le, C.; Gu, Q. Genome-wide identification and characterization of sweet orange (Citrus sinensis) aquaporin genes and their expression in two citrus cultivars differing in drought tolerance. Tree Genet. Genomes 2019, 15, 17. [Google Scholar] [CrossRef]
- Min, X.; Wu, H.; Zhang, Z.; Wei, X.; Jin, X.; Ndayambaza, B.; Wang, Y.; Liu, W. Genome-wide identification and characterization of the aquaporin gene family in Medicago truncatula. J. Plant Biochem. Biot. 2019, 28. [Google Scholar] [CrossRef]
- Zhao, P.C.; Xu, X.P.; Zhang, C.Y.; Chen, X.H.; Xu, S.; Lin, Y.L.; Lai, Z.X. Genome-wide identification of Aquaporin family and expression during early somatic embryogenesis in Dimocarpus longan Lour. Appl. Environ. Microb. 2020. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X. The regulation of light on flowering biological clock in Nymphaeaceae. Chin. Horticulture Abstr. 2018, 5, 21–25. [Google Scholar]
- Taro, H.; Yuka, T.; Shigeto, M.; Takehiro, M.; Shigeru, S. Differential expression of genes identified by suppression subtractive hybridization in petals of opening carnation flowers. J. Exp. Bot. 2010, 61, 2345–2354. [Google Scholar] [CrossRef]
- van Doorn, W.G.; van Meeteren, U. Flower opening and closure: A review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Barbara, A.M.P.; Rawan, Q.; Andrea, M.M.; Laura, T.; Andrea, S. TRANSPARENT TESTA GLABRA 1 participates in flowering time regulation in Arabidopsis thaliana. PeerJ 2020, 8, e8303. [Google Scholar] [CrossRef] [Green Version]
- Gan, E.S.; Xu, Y.F.; Wong, J.Y.; Jessamine, G.G.; Bo, S.; Wan, Y.W.; Huang, J.B.; Ito, T. Jumonji demethylases moderate precocious flowering at elevated temperature via regulation of FLC in Arabidopsis. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.C.; Zeng, Y.W. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.B.; Wu, J.Y.; Hua, Q.Z.; Tel-Zur, N.; Xie, F.F.; Zhang, Z.K.; Chen, J.Y.; Zhang, R.; Hu, G.B.; Zhao, J.T. Identification of reliable reference genes for quantitative real-time PCR normalization in pitaya. Plant Methods. 2019, 15, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.F.; Hua, Q.Z.; Chen, C.B.; Zhang, L.L.; Zhang, Z.K.; Chen, J.Y.; Zhang, R.; Zhao, J.T.; Hu, G.B. Transcriptomics-based identification and characterization of glucosyltransferases involved in betalain biosynthesis in Hylocereus megalanthus. Plant Physiol. Bioch. 2020, 152, 112–124. [Google Scholar] [CrossRef] [PubMed]
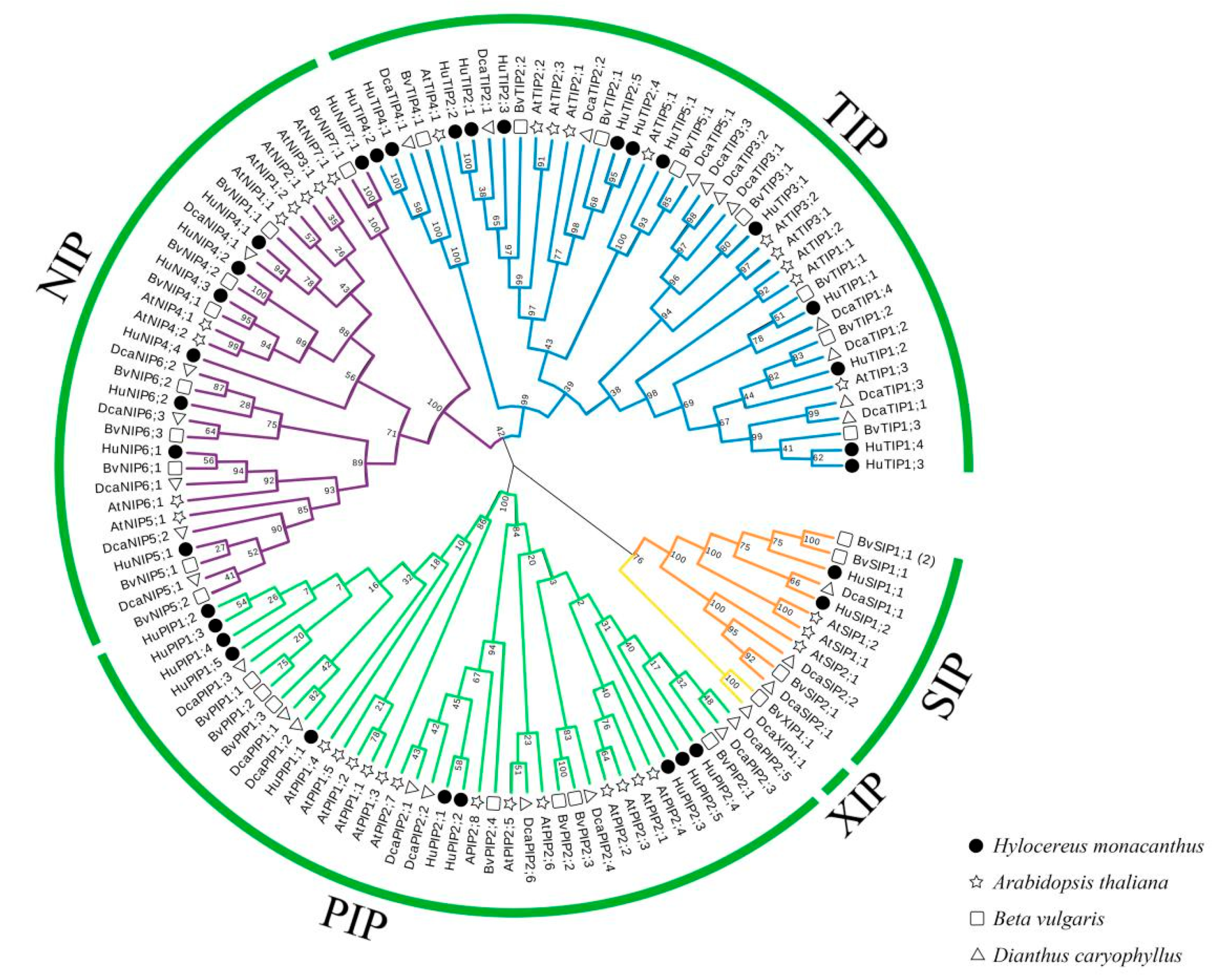

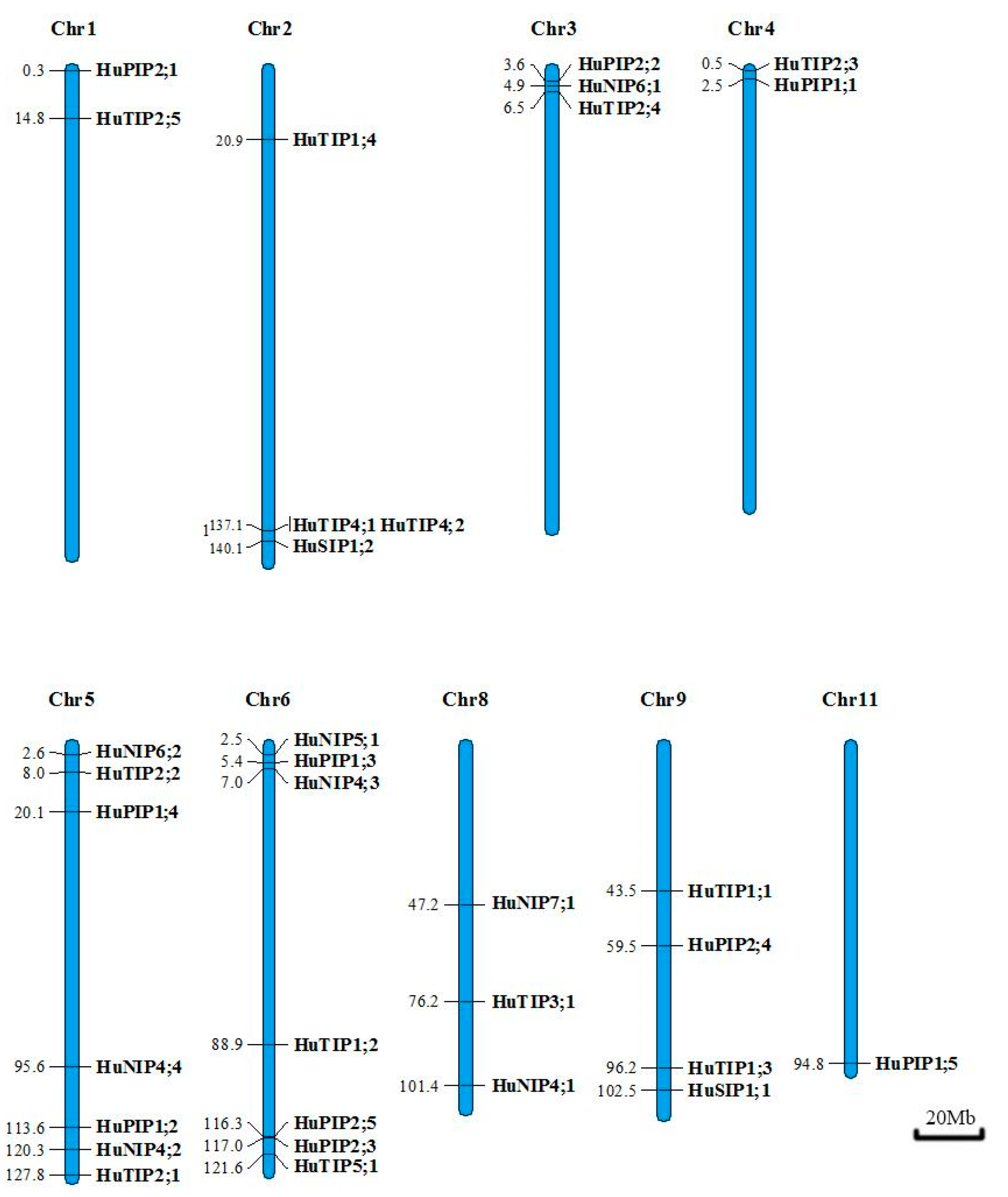
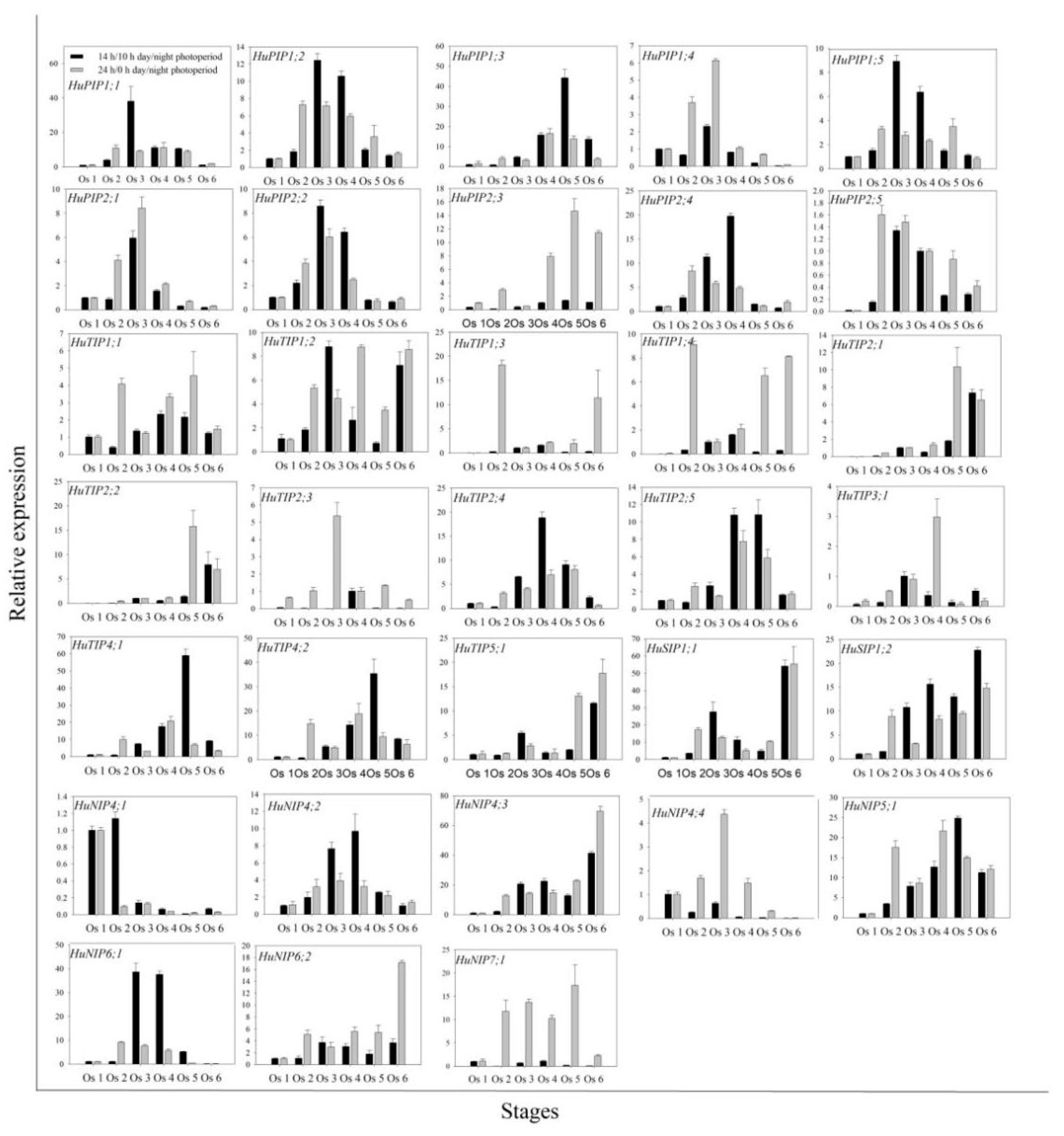
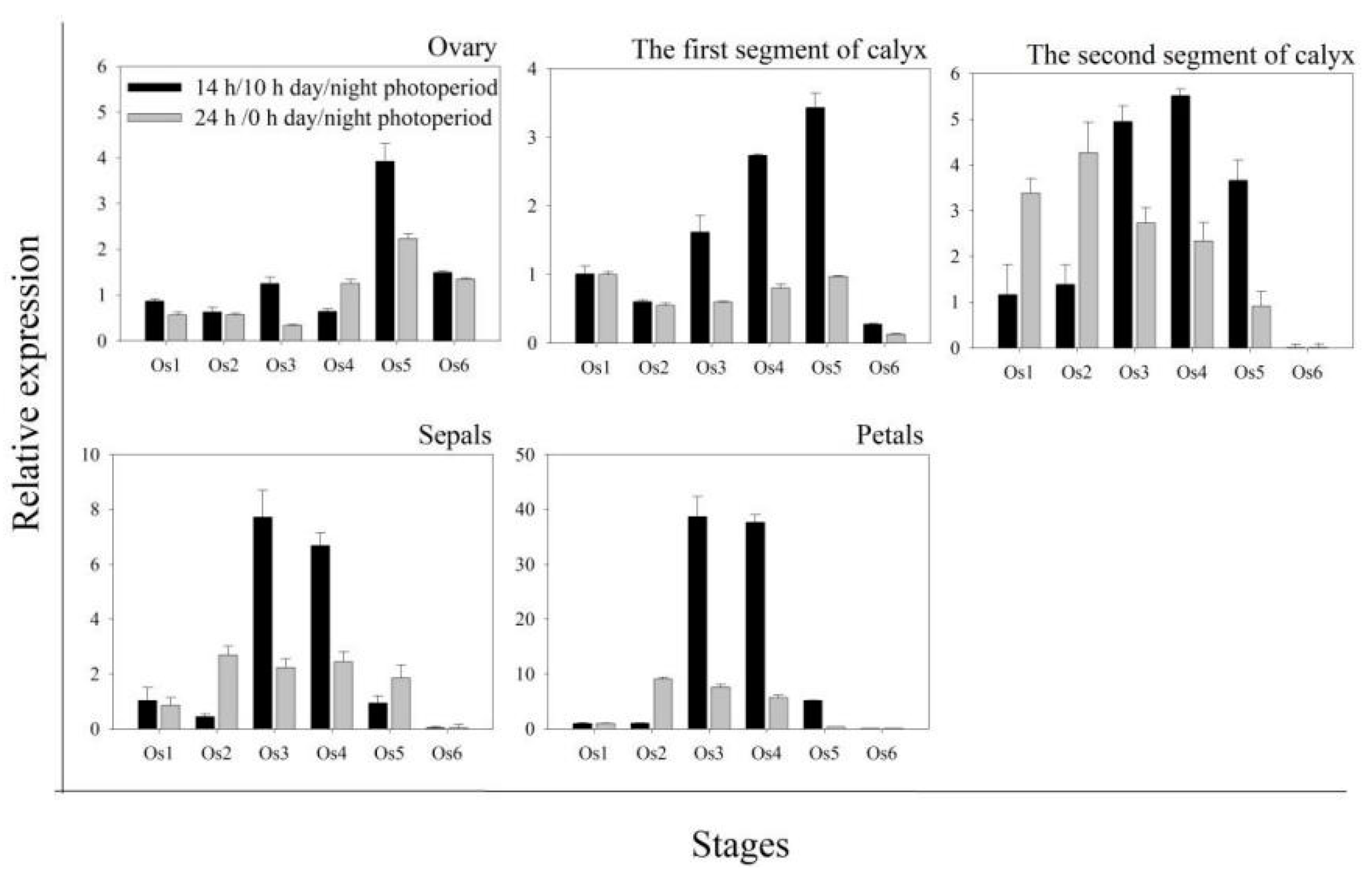


| Family | Gene Names | GenBank No. | Chromosomal Distribution | ORF (bp) | Length (AA) | pI | Molecular Weight (kDa) |
|---|---|---|---|---|---|---|---|
| PIP | HuPIP1;1 | HU04G00221.1 | 4 | 858 | 285 | 9.13 | 30.51 |
| HuPIP1;2 | HU05G01480.1 | 5 | 855 | 284 | 9.16 | 30.40 | |
| HuPIP1;3 | HU06G00483.1 | 6 | 858 | 285 | 9.11 | 30.48 | |
| HuPIP1;4 | HU05G00658.1 | 5 | 858 | 285 | 8.84 | 30.62 | |
| HuPIP1;5 | HU11G01779.1 | 11 | 858 | 285 | 9.19 | 30.75 | |
| HuPIP2;1 | HU01G00026.1 | 1 | 858 | 285 | 9.1 | 30.41 | |
| HuPIP2;2 | HU03G00407.1 | 3 | 852 | 283 | 8.98 | 30.20 | |
| HuPIP2;3 | HU06G02121.1 | 6 | 861 | 286 | 9.08 | 30.58 | |
| HuPIP2;4 | HU09G00753.1 | 9 | 861 | 286 | 8.59 | 30.53 | |
| HuPIP2;5 | HU06G02083.1 | 6 | 861 | 286 | 7.66 | 30.72 | |
| TIP | HuTIP1;1 | HU09G00676.1 | 9 | 768 | 255 | 5.84 | 26.22 |
| HuTIP1;2 | HU06G01671.1 | 6 | 756 | 251 | 6.09 | 26.03 | |
| HuTIP1;3 | HU09G01283.1 | 9 | 726 | 241 | 7.69 | 24.54 | |
| HuTIP1;4 | HU02G01483.1 | 2 | 753 | 250 | 5.35 | 25.74 | |
| HuTIP2;1 | HU05G02336.1 | 5 | 732 | 243 | 6 | 24.42 | |
| HuTIP2;2 | HU05G00450.1 | 5 | 732 | 243 | 6 | 24.42 | |
| HuTIP2;3 | HU04G00025.1 | 4 | 702 | 233 | 5.1 | 23.45 | |
| HuTIP2;4 | HU03G00687.1 | 3 | 750 | 249 | 5.76 | 25.29 | |
| HuTIP2;5 | HU01G00787.1 | 1 | 744 | 247 | 5.59 | 25.28 | |
| HuTIP3;1 | HU08G00860.1 | 8 | 774 | 257 | 7.1 | 27.27 | |
| HuTIP4;1 | HU02G02913.1 | 2 | 762 | 253 | 6.36 | 26.28 | |
| HuTIP4;2 | HU02G02914.1 | 2 | 762 | 253 | 6.57 | 26.30 | |
| HuTIP5;1 | HU06G02398.1 | 6 | 771 | 256 | 8.74 | 26.25 | |
| NIP | HuNIP4;1 | HU08G01901.1 | 8 | 882 | 293 | 9.51 | 31.12 |
| HuNIP4;2 | HU05G01754.1 | 5 | 906 | 301 | 7.7 | 32.23 | |
| HuNIP4;3 | HU06G00624.1 | 6 | 840 | 279 | 6.82 | 29.64 | |
| HuNIP4;4 | HU05G01185.1 | 5 | 867 | 288 | 9.48 | 30.34 | |
| HuNIP5;1 | HU06G00253.1 | 6 | 933 | 310 | 8.71 | 31.98 | |
| HuNIP6;1 | HU03G00523.1 | 3 | 915 | 304 | 6.07 | 31.43 | |
| HuNIP6;2 | HU05G00189.1 | 5 | 654 | 217 | 7.11 | 22.72 | |
| HuNIP7;1 | HU08G00592.1 | 8 | 864 | 287 | 8.3 | 29.78 | |
| SIP | HuSIP1;1 | HU09G01607.1 | 9 | 780 | 259 | 9.33 | 27.16 |
| HuSIP1;2 | HU02G03098.1 | 2 | 744 | 247 | 9.63 | 26.20 |
| Bolting Height (cm) | No. of Flower Buds | No. of Fruit Pods | No. of Rosette Leaves | No. of Bolting | |
|---|---|---|---|---|---|
| Wild-type | 0.90 d | 4.0 e | 0 c | 8.0 d | 0 e |
| 35S:HuNIP6;1 transgenic line 1 | 12.57 a | 19.0 ab | 3.0 a | 8.0 cd | 2.0 bcd |
| 35S:HuNIP6;1 transgenic line 2 | 8.74 b | 18.0 b | 1.0 c | 10.0 a | 2.0 cd |
| 35S:HuNIP6;1 transgenic line 3 | 9.27 b | 21.0 a | 2.0 b | 9.0 ab | 3.0 a |
| 35S:HuNIP6;1 transgenic line 4 | 6.30 c | 12.0 d | 0 c | 9.0 bcd | 2.0 d |
| 35S:HuNIP6;1 transgenic line 5 | 5.21 c | 14.0 cd | 0 c | 9.0 bc | 3.0 b |
| 35S:HuNIP6;1 transgenic line 6 | 8.27 b | 15.0 c | 0 c | 9.0 cd | 3.0 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Gao, Y.; Chen, C.; Xie, F.; Hua, Q.; Zhang, Z.; Zhang, R.; Zhao, J.; Hu, G.; Qin, Y. Genome-Wide Identification of Aquaporin Gene Family in Pitaya Reveals an HuNIP6;1 Involved in Flowering Process. Int. J. Mol. Sci. 2021, 22, 7689. https://doi.org/10.3390/ijms22147689
Ye X, Gao Y, Chen C, Xie F, Hua Q, Zhang Z, Zhang R, Zhao J, Hu G, Qin Y. Genome-Wide Identification of Aquaporin Gene Family in Pitaya Reveals an HuNIP6;1 Involved in Flowering Process. International Journal of Molecular Sciences. 2021; 22(14):7689. https://doi.org/10.3390/ijms22147689
Chicago/Turabian StyleYe, Xiaoying, Yongshun Gao, Canbin Chen, Fangfang Xie, Qingzhu Hua, Zhike Zhang, Rong Zhang, Jietang Zhao, Guibing Hu, and Yonghua Qin. 2021. "Genome-Wide Identification of Aquaporin Gene Family in Pitaya Reveals an HuNIP6;1 Involved in Flowering Process" International Journal of Molecular Sciences 22, no. 14: 7689. https://doi.org/10.3390/ijms22147689
APA StyleYe, X., Gao, Y., Chen, C., Xie, F., Hua, Q., Zhang, Z., Zhang, R., Zhao, J., Hu, G., & Qin, Y. (2021). Genome-Wide Identification of Aquaporin Gene Family in Pitaya Reveals an HuNIP6;1 Involved in Flowering Process. International Journal of Molecular Sciences, 22(14), 7689. https://doi.org/10.3390/ijms22147689








