The Impact of Diet and Exercise on Drug Responses
Abstract
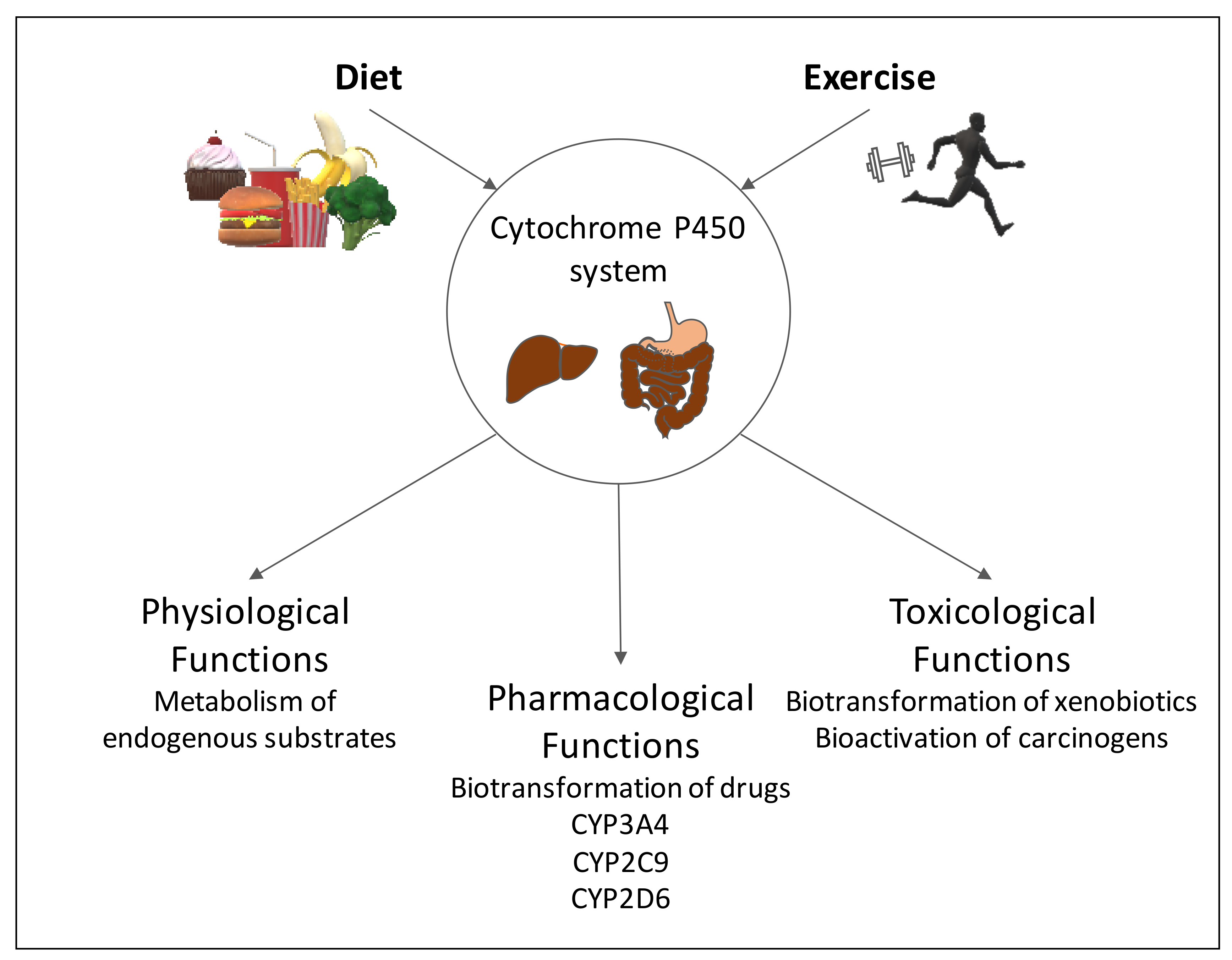
1. Introduction
2. Drug Pharmacokinetics and Metabolism
3. Diet
3.1. The Impact of Body Weight
3.2. The Impact of Nutrition
3.2.1. Effects of Macronutrients
3.2.2. Dietary Supplementation with Micronutrients and Bioactive Compounds
3.2.3. Phytochemicals
3.2.4. Minerals
3.2.5. Vitamins
3.3. The Role of the Microbiome
3.4. The Role of the Immune System
4. Exercise
4.1. Exercise and Drug Absorption
4.2. Effects on Drug Distribution
4.3. Exercise and Drug Metabolism
4.4. Effects of Physical Exercise on Drug Excretion
4.5. Clinical Studies on Exercise and Drug Effects
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADME | Adsorption, Distribution, Metabolism, Elimination |
| Cyp | Cytochrome |
| FDA | Food and Drug administration |
| FFA | Free fatty acid |
| GI | Gastrointestinal |
| GPCR | G-Protein coupled receptor |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
| Pgp | P-glycoprotein |
| PUFA | Polyunsaturated fatty acids |
References
- Parnham, M.J.; Geisslinger, G. Pharmacological plasticity-How do you hit a moving target? Pharmacol. Res. Perspect. 2019, 7, e00532. [Google Scholar] [CrossRef]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54.e19. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Cohen, J.L. Pharmacokinetic changes in aging. Am. J. Med. 1986, 80, 31–38. [Google Scholar] [CrossRef]
- Walter-Sack, I.; Klotz, U. Influence of diet and nutritional status on drug metabolism. Clin. Pharmacokinet. 1996, 31, 47–64. [Google Scholar] [CrossRef]
- Lenz, T.L.; Lenz, N.J.; Faulkner, M.A. Potential interactions between exercise and drug therapy. Sports Med. 2004, 34, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Saedisomeolia, A.; Shekarabi, M.; Khorshidi, M.; Emami, M.R.; Muller, D.J. The effect of obesity, macronutrients, fasting and nutritional status on drug-metabolizing cytochrome P450s: A systematic review of current evidence on human studies. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Van Vlack, T.; Schievink, B.P.; Doak, E.B.; Shane, J.S.; Dean, E. Lifestyle factors in hypertension drug research: Systematic analysis of articles in a leading cochrane report. Int. J. Hypertens. 2014, 2014, 835716. [Google Scholar] [CrossRef][Green Version]
- Miller, L.G. Cigarettes and drug therapy: Pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacol. 1990, 9, 125–135. [Google Scholar]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Cheymol, G. Clinical pharmacokinetics of drugs in obesity. An update. Clin. Pharmacokinet. 1993, 25, 103–114. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Jacobs, I. Exercise Is Medicine, But Does It Interfere With Medicine? Exerc. Sport Sci. Rev. 2017, 45, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.; Kaminski, D.L.; Rasmussen, A. Substrates of human hepatic cytochrome P450 3A4. Toxicology 1995, 104, 1–8. [Google Scholar] [CrossRef]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Knibbe, C.A.; Brill, M.J.; van Rongen, A.; Diepstraten, J.; van der Graaf, P.H.; Danhof, M. Drug disposition in obesity: Toward evidence-based dosing. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 149–167. [Google Scholar] [CrossRef]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Trobec, K.; Kerec Kos, M.; von Haehling, S.; Springer, J.; Anker, S.D.; Lainscak, M. Pharmacokinetics of drugs in cachectic patients: A systematic review. PLoS ONE 2013, 8, e79603. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.P.; Holm, R.; O’Driscoll, C.M.; Griffin, B.T. Food for thought: Formulating away the food effect—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 510–535. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Dalhoff, K. Food-drug interactions. Drugs 2002, 62, 1481–1502. [Google Scholar] [CrossRef]
- Liedholm, H.; Wahlin-Boll, E.; Melander, A. Mechanisms and variations in the food effect on propranolol bioavailability. Eur. J. Clin. Pharmacol. 1990, 38, 469–475. [Google Scholar] [CrossRef]
- Augustijns, P.; Wuyts, B.; Hens, B.; Annaert, P.; Butler, J.; Brouwers, J. A review of drug solubility in human intestinal fluids: Implications for the prediction of oral absorption. Eur. J. Pharmaceut. Sci. 2014, 57, 322–332. [Google Scholar] [CrossRef]
- Benet, L.Z.; Cummins, C.L.; Wu, C.Y. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int. J. Pharm. 2004, 277, 3–9. [Google Scholar] [CrossRef]
- Tamai, I. Oral drug delivery utilizing intestinal OATP transporters. Adv. Drug Deliv. Rev. 2012, 64, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Saleem, M.; Looke, D. Therapeutic drug monitoring to adjust dosing in morbid obesity—A new use for an old methodology. Br. J. Clin. Pharmacol. 2012, 73, 685–690. [Google Scholar] [CrossRef]
- Gagnon-Auger, M.; du Souich, P.; Baillargeon, J.P.; Martin, E.; Brassard, P.; Menard, J.; Ardilouze, J.L. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: A pharmacokinetic and pharmacodynamic study. Diabetes Care 2010, 33, 2502–2507. [Google Scholar] [CrossRef]
- Chan, C.C.; Ng, E.H.; Chan, M.M.; Tang, O.S.; Lau, E.Y.; Yeung, W.S.; Ho, P.C. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non-obese women. Hum. Reprod. 2003, 18, 2294–2297. [Google Scholar] [CrossRef]
- Brill, M.J.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Kotlyar, M.; Carson, S.W. Effects of obesity on the cytochrome P450 enzyme system. Int. J. Clin. Pharmacol. Ther. 1999, 37, 8–19. [Google Scholar] [PubMed]
- Sandvik, P.; Lydersen, S.; Hegstad, S.; Spigset, O. Association between low body weight and cytochrome P-450 enzyme activity in patients with anorexia nervosa. Pharmacol. Res. Perspect. 2020, 8, e00615. [Google Scholar] [CrossRef]
- Hole, K.; Heiberg, P.L.; Gjestad, C.; Mehus, L.L.; Ro, O.; Molden, E. Elevated 4beta-hydroxycholesterol/cholesterol ratio in anorexia nervosa patients. Pharmacol. Res. Perspect. 2018, 6, e00430. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Obesity and diabetic kidney disease. Med. Clin. N. Am. 2013, 97, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Kappas, A. Dietary regulation of cytochrome P450. Annu. Rev. Nutr. 1991, 11, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N. Effects of food on clinical pharmacokinetics. Clin. Pharmacokinet. 1999, 37, 213–255. [Google Scholar] [CrossRef]
- Welling, P.G. Effects of food on drug absorption. Pharmacol. Ther. 1989, 43, 425–441. [Google Scholar] [CrossRef]
- Stacher, G.; Granser, G.V.; Bergmann, H.; Kugi, A.; Stacher-Janotta, G.; Hobart, J. Slow gastric emptying induced by high fat content of meal accelerated by cisapride administered rectally. Dig. Dis. Sci. 1991, 36, 1259–1265. [Google Scholar] [CrossRef]
- Zimmermann, T.; Yeates, R.A.; Laufen, H.; Pfaff, G.; Wildfeuer, A. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur. J. Clin. Pharmacol. 1994, 46, 147–150. [Google Scholar] [CrossRef]
- Melander, A.; Stenberg, P.; Liedholm, H.; Schersten, B.; Wahlin-Boll, E. Food-induced reduction in bioavailability of atenolol. Eur. J. Clin. Pharmacol. 1979, 16, 327–330. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, X.; Chen, Z.; Fan, C.H.; Kwan, H.S.; Wong, C.H.; Shek, K.Y.; Zuo, Z.; Lam, T.N. A Review of Food-Drug Interactions on Oral Drug Absorption. Drugs 2017, 77, 1833–1855. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.K.; Trull, A.K.; Uttridge, J.A.; Metcalfe, S.; Heyes, C.S.; Facey, S.; Evans, D.B. Effect of dietary fat on the pharmacokinetics and pharmacodynamics of cyclosporine in kidney transplant recipients. Clin. Pharmacol. Ther. 1995, 57, 425–433. [Google Scholar] [CrossRef]
- Achterbergh, R.; Lammers, L.A.; van Nierop, S.; Klumpen, H.J.; Soeters, M.R.; Mathot, R.A.; Romijn, J.A. A short-term high fat diet increases exposure to midazolam and omeprazole in healthy subjects. Expert Opin. Drug Metab. Toxicol. 2016, 12, 715–720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spector, A.A.; Kim, H.Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Fetterman, J.W., Jr.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Oseid, D.E.; Song, L.; Lear, S.; Robinson, A.S. Nuclear translocation of the unliganded glucocorticoid receptor is influenced by membrane fluidity, but not A2AR agonism. Steroids 2020, 160, 108641. [Google Scholar] [CrossRef]
- Guixa-Gonzalez, R.; Javanainen, M.; Gomez-Soler, M.; Cordobilla, B.; Domingo, J.C.; Sanz, F.; Pastor, M.; Ciruela, F.; Martinez-Seara, H.; Selent, J. Membrane omega-3 fatty acids modulate the oligomerisation kinetics of adenosine A2A and dopamine D2 receptors. Sci. Rep. 2016, 6, 19839. [Google Scholar] [CrossRef]
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N., Jr.; Cicchetti, F.; Calon, F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef]
- Pardini, R.S. Nutritional intervention with omega-3 fatty acids enhances tumor response to anti-neoplastic agents. Chem. Biol. Interact. 2006, 162, 89–105. [Google Scholar] [CrossRef]
- Robertson, D.R.; Higginson, I.; Macklin, B.S.; Renwick, A.G.; Waller, D.G.; George, C.F. The influence of protein containing meals on the pharmacokinetics of levodopa in healthy volunteers. Br. J. Clin. Pharmacol. 1991, 31, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Fagan, T.C.; Walle, T.; Oexmann, M.J.; Walle, U.K.; Bai, S.A.; Gaffney, T.E. Increased clearance of propranolol and theophylline by high-protein compared with high-carbohydrate diet. Clin. Pharmacol. Ther. 1987, 41, 402–406. [Google Scholar] [CrossRef]
- Anderson, K.E.; McCleery, R.B.; Vesell, E.S.; Vickers, F.F.; Kappas, A. Diet and cimetidine induce comparable changes in theophylline metabolism in normal subjects. Hepatology 1991, 13, 941–946. [Google Scholar] [CrossRef]
- Feldman, C.H.; Hutchinson, V.E.; Sher, T.H.; Feldman, B.R.; Davis, W.J. Interaction between nutrition and theophylline metabolism in children. Ther. Drug Monit. 1982, 4, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.J.; Skypala, I.; Dawson, S.; McAllister, W.A.; Turner Warwick, M. The effect of diet upon serum concentrations of theophylline. Br. J. Clin. Pharmacol. 1983, 16, 267–270. [Google Scholar] [CrossRef]
- Bekersky, I.; Dressler, D.; Mekki, Q.A. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J. Clin. Pharmacol. 2001, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Balabaud, C.; Vinon, G.; Paccalin, J. Influence of dietary protein and carbohydrate on phenytoin metabolism in man. Br. J. Clin. Pharmacol. 1979, 8, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Lammers, L.A.; Achterbergh, R.; van Schaik, R.H.N.; Romijn, J.A.; Mathot, R.A.A. Effect of Short-Term Fasting on Systemic Cytochrome P450-Mediated Drug Metabolism in Healthy Subjects: A Randomized, Controlled, Crossover Study Using a Cocktail Approach. Clin. Pharmacokinet. 2017, 56, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Reidenberg, M.M.; Vesell, E.S. Unaltered metabolism of antipyrine and tolbutamide in fasting man. Clin. Pharmacol. Ther. 1975, 17, 650–656. [Google Scholar] [CrossRef]
- Lammers, L.A.; Achterbergh, R.; Romijn, J.A.; Mathot, R.A.A. Nutritional Status Differentially Alters Cytochrome P450 3A4 (CYP3A4) and Uridine 5′-Diphospho-Glucuronosyltransferase (UGT) Mediated Drug Metabolism: Effect of Short-Term Fasting and High Fat Diet on Midazolam Metabolism. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 751–767. [Google Scholar] [CrossRef]
- Opara, E.C.; Rockway, S.W. Antioxidants and micronutrients. Dis. Mon. 2006, 52, 151–163. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D. Nutritional genomics: Manipulating plant micronutrients to improve human health. Science 1999, 285, 375–379. [Google Scholar] [CrossRef]
- Boullata, J.I.; Hudson, L.M. Drug-nutrient interactions: A broad view with implications for practice. J. Acad. Nutr. Diet. 2012, 112, 506–517. [Google Scholar] [CrossRef]
- Gibaldi, M.; Grundhofer, B.; Levy, G. Effect of antacids on pH of urine. Clin. Pharmacol. Ther. 1974, 16, 520–525. [Google Scholar] [CrossRef]
- Neuvonen, P.J.; Kivisto, K.T. Enhancement of drug absorption by antacids. An unrecognised drug interaction. Clin. Pharmacokinet. 1994, 27, 120–128. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, P.F.; McElnay, J.C. Drug-antacid interactions: Assessment of clinical importance. Drug Intell. Clin. Pharm. 1987, 21, 607–617. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Reggi, R.; Yarla, N.S.; Palmery, M.; Peluso, I. Association of antioxidant nutraceuticals and acetaminophen (paracetamol): Friend or foe? J. Food Drug Anal. 2018, 26, S78–S87. [Google Scholar]
- Gougis, P.; Hilmi, M.; Geraud, A.; Mir, O.; Funck-Brentano, C. Potential Cytochrome P450-mediated pharmacokinetic interactions between herbs, food, and dietary supplements and cancer treatments. Crit. Rev. Oncol. Hematol. 2021, 10, 42. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines (Basel) 2020, 7, 26. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 3135. [Google Scholar] [CrossRef]
- Peluso, I.; Palmery, M.; Serafini, M. Association of flavonoid-rich foods and statins in the management of hypercholesterolemia: A dangerous or helpful combination? Curr. Drug Metab. 2015, 16, 833–846. [Google Scholar] [CrossRef]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef]
- Dresser, G.K.; Bailey, D.G.; Leake, B.F.; Schwarz, U.I.; Dawson, P.A.; Freeman, D.J.; Kim, R.B. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin. Pharmacol. Ther. 2002, 71, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Transporter-Mediated Drug-Drug Interactions and Their Significance. Adv. Exp. Med. Biol. 2019, 1141, 241–291. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.G.; Dresser, G.; Arnold, J.M. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? CMAJ 2013, 185, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Seden, K.; Dickinson, L.; Khoo, S.; Back, D. Grapefruit-drug interactions. Drugs 2010, 70, 2373–2407. [Google Scholar] [CrossRef]
- Lilja, J.J.; Backman, J.T.; Laitila, J.; Luurila, H.; Neuvonen, P.J. Itraconazole increases but grapefruit juice greatly decreases plasma concentrations of celiprolol. Clin. Pharmacol. Ther. 2003, 73, 192–198. [Google Scholar] [CrossRef]
- Petric, Z.; Zuntar, I.; Putnik, P.; Bursac Kovacevic, D. Food-Drug Interactions with Fruit Juices. Foods 2020, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, S.Y.; Fabriaga, E.; Zhang, P.H.; Zhou, Q. Food-drug interactions precipitated by fruit juices other than grapefruit juice: An update review. J. Food Drug Anal. 2018, 26, S61–S71. [Google Scholar] [CrossRef]
- Abernethy, D.R. Grapefruits and drugs: When is statistically significant clinically significant? J. Clin. Investig. 1997, 99, 2297–2298. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab. Rev. 2016, 48, 331–341. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, C.Q.; He, Y.J.; Chen, B.L.; Wang, G.; Zhou, G.; Zhang, W.; Tan, Z.R.; Cao, S.; Wang, L.P.; et al. Genistein alters caffeine exposure in healthy female volunteers. Eur. J. Clin. Pharmacol. 2011, 67, 347–353. [Google Scholar] [CrossRef]
- Nakajima, M.; Itoh, M.; Yamanaka, H.; Fukami, T.; Tokudome, S.; Yamamoto, Y.; Yamamoto, H.; Yokoi, T. Isoflavones inhibit nicotine C-oxidation catalyzed by human CYP2A6. J. Clin. Pharmacol. 2006, 46, 337–344. [Google Scholar] [CrossRef]
- Xiao, C.Q.; Chen, R.; Lin, J.; Wang, G.; Chen, Y.; Tan, Z.R.; Zhou, H.H. Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica 2012, 42, 173–178. [Google Scholar] [CrossRef]
- Cao, L.; Kwara, A.; Greenblatt, D.J. Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in-vitro inhibition studies. J. Pharm. Pharmacol. 2017, 69, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, A.; Mendonca, P.; Russell, T.D.; Soliman, K.F.A. The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9. Nutrients 2020, 12, 3651. [Google Scholar] [CrossRef] [PubMed]
- Gunne, S.; Heinicke, U.; Parnham, M.J.; Laux, V.; Zacharowski, K.; von Knethen, A. Nrf2-A Molecular Target for Sepsis Patients in Critical Care. Biomolecules 2020, 10, 1688. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Maucher, I.V.; Ruhl, M.; Kretschmer, S.B.; Hofmann, B.; Kuhn, B.; Fettel, J.; Vogel, A.; Flugel, K.T.; Manolikakes, G.; Hellmuth, N.; et al. Michael acceptor containing drugs are a novel class of 5-lipoxygenase inhibitor targeting the surface cysteines C416 and C418. Biochem. Pharmacol. 2017, 125, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Kensler, T.W. Nrf2 in liver toxicology. Arch. Pharm. Res. 2020, 43, 337–349. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants (Basel) 2020, 9, 973. [Google Scholar] [CrossRef]
- Pantuck, E.J.; Pantuck, C.B.; Anderson, K.E.; Wattenberg, L.W.; Conney, A.H.; Kappas, A. Effect of brussels sprouts and cabbage on drug conjugation. Clin. Pharmacol. Ther. 1984, 35, 161–169. [Google Scholar] [CrossRef]
- Pantuck, E.J.; Pantuck, C.B.; Garland, W.A.; Min, B.H.; Wattenberg, L.W.; Anderson, K.E.; Kappas, A.; Conney, A.H. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin. Pharmacol. Ther. 1979, 25, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kall, M.A.; Vang, O.; Clausen, J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: Evaluation of caffeine, oestrone and chlorzoxazone metabolism. Carcinogenesis 1996, 17, 793–799. [Google Scholar] [CrossRef]
- Kim, R.B.; O’Shea, D. Interindividual variability of chlorzoxazone 6-hydroxylation in men and women and its relationship to CYP2E1 genetic polymorphisms. Clin. Pharmacol. Ther. 1995, 57, 645–655. [Google Scholar] [CrossRef]
- Chen, L.; Mohr, S.N.; Yang, C.S. Decrease of plasma and urinary oxidative metabolites of acetaminophen after consumption of watercress by human volunteers. Clin. Pharmacol. Ther. 1996, 60, 651–660. [Google Scholar] [CrossRef]
- Neuvonen, P.J. Interactions with the absorption of tetracyclines. Drugs 1976, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J. Absorption of minocycline hydrochloride and tetracycline hydrochloride. Effect of food, milk, and iron. J. Am. Acad. Dermatol. 1985, 12, 308–312. [Google Scholar] [CrossRef]
- Karlson, B.; Leijd, B.; Hellstrom, K. On the influence of vitamin K-rich vegetables and wine on the effectiveness of warfarin treatment. Acta Med. Scand. 1986, 220, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Kempin, S.J. Warfarin resistance caused by broccoli. N. Engl. J. Med. 1983, 308, 1229–1230. [Google Scholar] [CrossRef]
- Baylis, E.M.; Crowley, J.M.; Preece, J.M.; Sylvester, P.E.; Marks, V. Influence of folic acid on blood-phenytoin levels. Lancet 1971, 1, 62–64. [Google Scholar] [CrossRef]
- Furlanut, M.; Benetello, P.; Avogaro, A.; Dainese, R. Effects of folic acid on phenytoin kinetics in healthy subjects. Clin. Pharmacol. Ther. 1978, 24, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Back, D.J.; Breckenridge, A.M.; MacIver, M.; Orme, M.L.; Purba, H.; Rowe, P.H. Interaction of ethinyloestradiol with ascorbic acid in man. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 1516. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.H. Megadose vitamin C and metabolic effects of the pill. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zelfand, E. Vitamin C, Pain and Opioid Use Disorder. Integr. Med. 2020, 19, 18–29. [Google Scholar]
- Murray, M. Altered CYP expression and function in response to dietary factors: Potential roles in disease pathogenesis. Curr. Drug Metab. 2006, 7, 67–81. [Google Scholar] [CrossRef]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef]
- Hegazy, A.M.; El-Sayed, E.M.; Ibrahim, K.S.; Abdel-Azeem, A.S. Dietary antioxidant for disease prevention corroborated by the Nrf2 pathway. J. Complement. Integr. Med. 2019, 16. [Google Scholar] [CrossRef]
- Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Cheng, S.L.; Klaassen, C.D.; Cui, J.Y. Regulation of Hepatic Drug-Metabolizing Enzymes in Germ-Free Mice by Conventionalization and Probiotics. Drug Metab. Dispos. 2016, 44, 262–274. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Cheng, S.L.; Bammler, T.K.; Prasad, B.; Vrana, M.; Klaassen, C.; Cui, J.Y. Developmental Regulation of Drug-Processing Genes in Livers of Germ-Free Mice. Toxicol. Sci. 2015, 147, 84–103. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.; Rizkallah, M.R.; Aziz, R.K. Gut Pharmacomicrobiomics: The tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012, 4, 16. [Google Scholar] [CrossRef]
- Sharma, A.; Buschmann, M.M.; Gilbert, J.A. Pharmacomicrobiomics: The Holy Grail to Variability in Drug Response? Clin. Pharmacol. Ther. 2019, 106, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ahrens, J.; Romani-Perez, M.; Watkins, C.; Sanz, Y.; Benitez-Paez, A.; Stanton, C.; Gunther, K. Dietary fat, the gut microbiota, and metabolic health—A systematic review conducted within the MyNewGut project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. (Lond.) 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuca, K.; Chopra, C.; Singh, R.; Kumar, H.; Sen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Anhe, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Stepanić, V.; Tafferner, N.; Panek, M.; Verbanac, D. Mild Plant and Dietary Immunomodulators. In Nijkamp and Parnham’s Principles of Immunopharmacology; Parnham, M.J., Nijkamp, F.P., Rossi, A.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 561–587. [Google Scholar]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Mendivil, C.O. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Front. Nutr. 2020, 7, 617652. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Celebi Sozener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 990. [Google Scholar] [CrossRef]
- Fournier, N.; Sayet, G.; Vedie, B.; Nowak, M.; Allaoui, F.; Solgadi, A.; Caudron, E.; Chaminade, P.; Benoist, J.F.; Paul, J.L. Eicosapentaenoic acid membrane incorporation impairs cholesterol efflux from cholesterol-loaded human macrophages by reducing the cholesteryl ester mobilization from lipid droplets. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The Role of Nutrients in Protecting Mitochondrial Function and Neurotransmitter Signaling: Implications for the Treatment of Depression, PTSD, and Suicidal Behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.P. HIV: A raft-targeting approach for prevention and therapy using plant-derived compounds (review). Curr. Drug Targets 2009, 10, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pepin, X.J.H.; Huckle, J.E.; Alluri, R.V.; Basu, S.; Dodd, S.; Parrott, N.; Emami Riedmaier, A. Understanding Mechanisms of Food Effect and Developing Reliable PBPK Models Using a Middle-out Approach. AAPS J. 2021, 23, 12. [Google Scholar] [CrossRef]
- Tistaert, C.; Heimbach, T.; Xia, B.; Parrott, N.; Samant, T.S.; Kesisoglou, F. Food Effect Projections via Physiologically Based Pharmacokinetic Modeling: Predictive Case Studies. J. Pharm. Sci. 2019, 108, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, P.; Pan, Y.; Wagner, C. Predictive Performance of Physiologically Based Pharmacokinetic Models for the Effect of Food on Oral Drug Absorption: Current Status. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Ioannidis, J.P. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 2013, 347, f5577. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef]
- Ter Steege, R.W.; Kolkman, J.J. Review article: The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment. Pharmacol. Ther. 2012, 35, 516–528. [Google Scholar] [CrossRef]
- Rowell, L.B. Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 1974, 54, 75–159. [Google Scholar] [CrossRef]
- Brock-Utne, J.G.; Gaffin, S.L.; Wells, M.T.; Gathiram, P.; Sohar, E.; James, M.F.; Morrell, D.F.; Norman, R.J. Endotoxaemia in exhausted runners after a long-distance race. S. Afr. Med. J. 1988, 73, 533–536. [Google Scholar]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Yki-Jarvinen, H.; DeFronzo, R.A. Physical training and insulin sensitivity. Diabetes Metab. Rev. 1986, 1, 445–481. [Google Scholar] [CrossRef]
- Klemsdal, T.O.; Gjesdal, K.; Bredesen, J.E. Heating and cooling of the nitroglycerin patch application area modify the plasma level of nitroglycerin. Eur. J. Clin. Pharmacol. 1992, 43, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.A. Proceedings: Some factors influencing the absorption of diazepam. Proc. R. Soc. Med. 1975, 68, 772. [Google Scholar]
- Schmidt, H.; Roholt, K. Penicillin serum concentrations in relation to exercise. Acta Pathol. Microbiol. Scand. 1966, 68, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Van Baak, M.A. Influence of exercise on the pharmacokinetics of drugs. Clin. Pharmacokinet. 1990, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, J. The changes in hematocrit, hemoglobin, plasma volume and proteins during and after different types of exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1977, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Van Baak, M.A.; Mooij, J.M.; Schiffers, P.M. Exercise and the pharmacokinetics of propranolol, verapamil and atenolol. Eur. J. Clin. Pharmacol. 1992, 43, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, G.A.; Webb, J.G.; Walle, T.; Bai, S.A.; Daniell, H.B.; Gourley, L.; Boyd Loadholt, C.; Gaffney, T.E. Exercise-induced increments in plasma levels of propranolol and noradrenaline. Br. J. Clin. Pharmacol. 1983, 16, 599–608. [Google Scholar] [CrossRef]
- Tesseromatis, C.; Trichilis, A.; Tsivos, E.; Messari, J.; Triantaphyllidis, H.; Varonos, D.D. Does stress influence ampicillin concentration in serum and tissues? Eur. J. Drug Metab. Pharmacokinet. 2001, 26, 167–171. [Google Scholar] [CrossRef]
- Saatmann, N.; Zaharia, O.P.; Loenneke, J.P.; Roden, M.; Pesta, D.H. Effects of Blood Flow Restriction Exercise and Possible Applications in Type 2 Diabetes. Trends Endocrinol. Metab. 2021, 32, 106–117. [Google Scholar] [CrossRef]
- Nies, A.S.; Shand, D.G.; Wilkinson, G.R. Altered hepatic blood flow and drug disposition. Clin. Pharmacokinet. 1976, 1, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Shek, P.N.; Shephard, R.J. Physical exercise as a human model of limited inflammatory response. Can. J. Physiol. Pharmacol. 1998, 76, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- King-Himmelreich, T.S.; Schramm, S.; Wolters, M.C.; Schmetzer, J.; Moser, C.V.; Knothe, C.; Resch, E.; Peil, J.; Geisslinger, G.; Niederberger, E. The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem. Biophys. Res. Commun. 2016, 474, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Yiamouyiannis, C.A.; Sanders, R.A.; Watkins, J.B., 3rd; Martin, B.J. Chronic physical activity: Hepatic hypertrophy and increased total biotransformation enzyme activity. Biochem. Pharmacol. 1992, 44, 121–127. [Google Scholar] [CrossRef]
- Hinderling, P.H.; Hartmann, D. The pH dependency of the binding of drugs to plasma proteins in man. Ther. Drug Monit. 2005, 27, 71–85. [Google Scholar] [CrossRef]
- Stoschitzky, K.; Lindner, W.; Klein, W. Stereoselective release of (S)-atenolol from adrenergic nerve endings at exercise. Lancet 1992, 340, 696–697. [Google Scholar] [CrossRef]
- Ylitalo, P.; Hinkka, H. Effect of exercise on plasma levels and urinary excretion of sulphadimidine and procainamide. Int. J. Clin. Pharmacol. Ther. Toxicol 1985, 23, 548–553. [Google Scholar]
- Moriguchi, T.; Shimomitsu, T.; Odagiri, Y.; Fukuda, J.; Hamano, K.; Kawai, T.; Tomoda, A. Marked increase in urinary bicarbonate and pH caused by heavy muscular exercise with dynamic knee extension. Tohoku J. Exp. Med. 2002, 198, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.; Lombardo, E.; Havinga, R.; Tietge, U.J.; Kuipers, F.; Groen, A.K. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011, 218, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Shendre, A.; Beasley, T.M.; Brown, T.M.; Hill, C.E.; Arnett, D.K.; Limdi, N.A. Influence of regular physical activity on warfarin dose and risk of hemorrhagic complications. Pharmacotherapy 2014, 34, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Rouleau-Mailloux, E.; Shahabi, P.; Dumas, S.; Feroz Zada, Y.; Provost, S.; Hu, J.; Nguyen, J.; Bouchama, N.; Mongrain, I.; Talajic, M.; et al. Impact of regular physical activity on weekly warfarin dose requirement. J. Thromb. Thrombolysis 2016, 41, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Susstrunk, H.; Morell, B.; Ziegler, W.H.; Froesch, E.R. Insulin absorption from the abdomen and the thigh in healthy subjects during rest and exercise: Blood glucose, plasma insulin, growth hormone, adrenaline and noradrenaline levels. Diabetologia 1982, 22, 171–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrannini, E.; Linde, B.; Faber, O. Effect of bicycle exercise on insulin absorption and subcutaneous blood flow in the normal subject. Clin. Physiol. 1982, 2, 59–70. [Google Scholar] [CrossRef]
- McAuley, S.A.; Horsburgh, J.C.; Ward, G.M.; La Gerche, A.; Gooley, J.L.; Jenkins, A.J.; MacIsaac, R.J.; O’Neal, D.N. Insulin pump basal adjustment for exercise in type 1 diabetes: A randomised crossover study. Diabetologia 2016, 59, 1636–1644. [Google Scholar] [CrossRef]
- Schlaeffer, F.; Engelberg, I.; Kaplanski, J.; Danon, A. Effect of exercise and environmental heat on theophylline kinetics. Respiration 1984, 45, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Lenz, T.L. The effects of high physical activity on pharmacokinetic drug interactions. Expert Opin. Drug Metab. Toxicol. 2011, 7, 257–266. [Google Scholar] [CrossRef]
- Miyauchi, S.; Oshima, S.; Asaka, M.; Kawano, H.; Torii, S.; Higuchi, M. Organ size increases with weight gain in power-trained athletes. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 617–623. [Google Scholar] [CrossRef]
| Changes in Physiology | Potential Effects | PK Effect | Examples | Ref. |
|---|---|---|---|---|
| Reduced gastric emptying | Decreased transport of drug to intestine | Increased Tmax | NSAIDs | [20,21] |
| Increased blood flow in GI tract | Saturation of liver enzymes, avoidance of first pass metabolism | AUC and Cmax increase | Propranolol | [22] |
| Increased pH in stomach | Altered solubility of drugs | AUC and Cmax for acids increase AUC and Cmax decrease for basic drugs | Cefuroxime, Dipyramidol | [20] |
| Food ingredients alter solubility of drugs (e.g., lipids) | Lipophilic drugs show increased solubility | AUC and Cmax increase | Fenofibrate | [23] |
| Inhibition of GI enzyme or transporter activity | Decrease in drug metabolism, decreased efflux Decreased drug uptake | AUC and Cmax increase AUC and Cmax decrease | Sirolimus, Midazolam Fexofenadine | [24,25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederberger, E.; Parnham, M.J. The Impact of Diet and Exercise on Drug Responses. Int. J. Mol. Sci. 2021, 22, 7692. https://doi.org/10.3390/ijms22147692
Niederberger E, Parnham MJ. The Impact of Diet and Exercise on Drug Responses. International Journal of Molecular Sciences. 2021; 22(14):7692. https://doi.org/10.3390/ijms22147692
Chicago/Turabian StyleNiederberger, Ellen, and Michael J. Parnham. 2021. "The Impact of Diet and Exercise on Drug Responses" International Journal of Molecular Sciences 22, no. 14: 7692. https://doi.org/10.3390/ijms22147692
APA StyleNiederberger, E., & Parnham, M. J. (2021). The Impact of Diet and Exercise on Drug Responses. International Journal of Molecular Sciences, 22(14), 7692. https://doi.org/10.3390/ijms22147692







