Hypoxia Enhances the Expression of RNASET2 in Human Monocyte-Derived Dendritic Cells: Role of PI3K/AKT Pathway
Abstract
:1. Introduction
2. Results
2.1. Hypoxia Enhances RNASET2 Expression Along with HIF-1α Dependent and Independent Signaling in Human Monocyte-Derived DCs
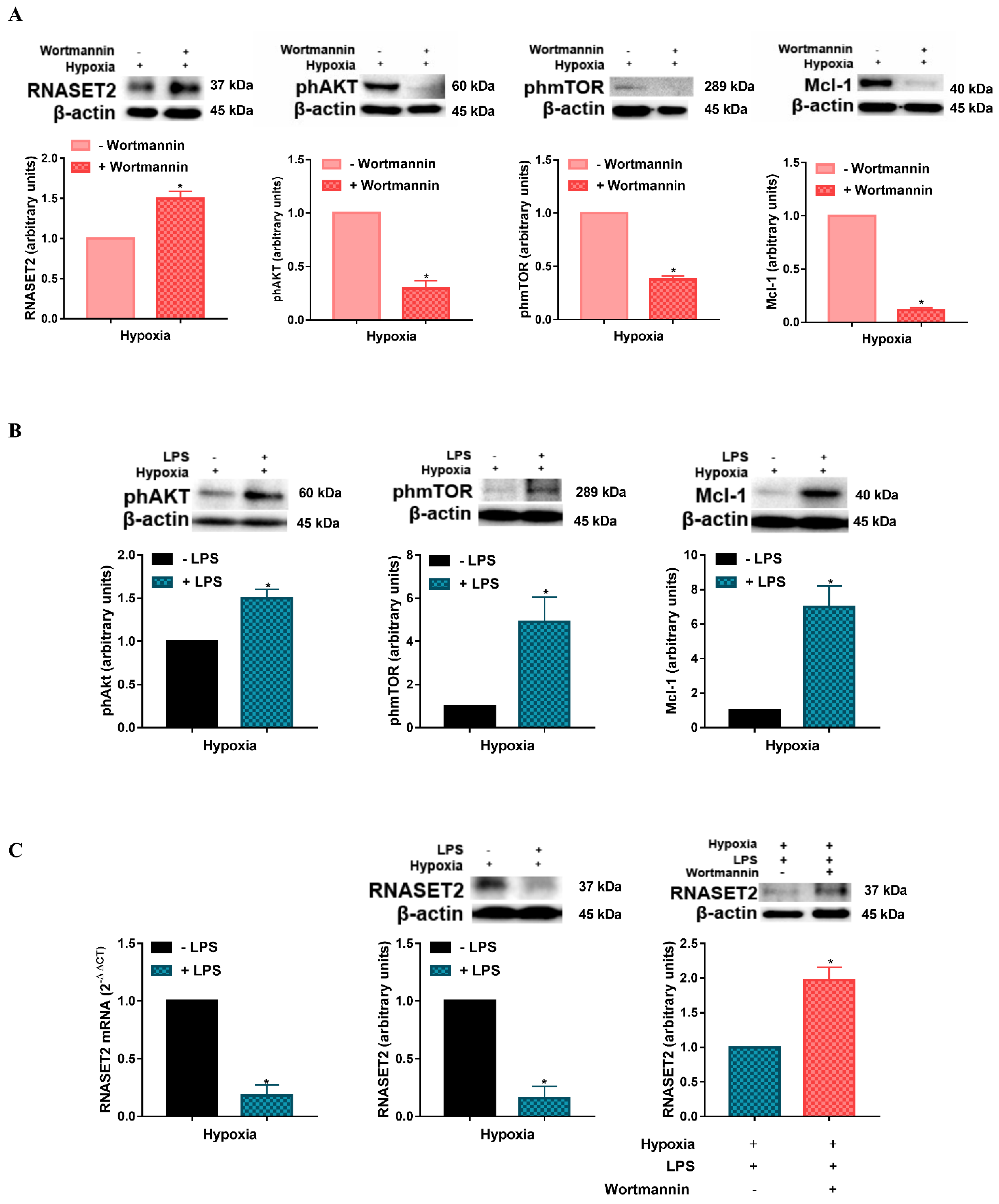
2.2. Modulation of Hypoxia-Induced RNASET2 Expression by PI3K/AKT Pathway and TLR4 Ligand LPS
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human Monocyte-Derived DC Preparation and Culture Conditions
4.3. Western Blot
4.4. RNA Isolation Extraction and RT-qPCR
4.5. ELISA
4.6. Cell Death/Viability Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| BNIP3 | BCL2 Interacting Protein 3 |
| CXCR | CXC-chemokine receptor |
| DC | Dendritic cell |
| GM-CSF | Granulocyte macrophage stimulating factor |
| HIF | Hypoxia-inducible factor |
| HREs | Hypoxic responsive elements |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| mTOR | Mammalian target of rapamycin |
| PI3K | Phosphatidylinositol 3-kinases |
| TLR | Toll-like receptor |
| VEGF-A | Vascular endothelial growth factor-A |
References
- Di Blasio, S.; van Wigcheren, G.F.; Becker, A.; van Duffelen, A.; Gorris, M.; Verrijp, K.; Stefanini, I.; Bakker, G.J.; Bloemendal, M.; Halilovic, A.; et al. The tumour microenvironment shapes dendritic cell plasticity in a human organotypic melanoma culture. Nat. Commun. 2020, 11, 2749. [Google Scholar] [CrossRef]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Wculek, S.K.; Khouili, S.C.; Priego, E.; Heras-Murillo, I.; Sancho, D. Metabolic Control of Dendritic Cell Functions: Digesting Information. Front. Immunol. 2019, 10, 775. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.D.; Lanzen, J.L.; Snyder, S.A.; Dewhirst, M.W. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2533–H2544. [Google Scholar] [CrossRef]
- Huang, J.H.; Cardenas-Navia, L.I.; Caldwell, C.C.; Plumb, T.J.; Radu, C.G.; Rocha, P.N.; Wilder, T.; Bromberg, J.S.; Cronstein, B.N.; Sitkovsky, M. Requirements for T lymphocyte migration in explanted lymph nodes. J. Immunol. 2007, 178, 7747–7755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paardekooper, L.M.; Vos, W.; van den Bogaart, G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget 2019, 10, 883–896. [Google Scholar] [CrossRef]
- Vaupel, P.; Schlenger, K.; Knoop, C.; Höckel, M. Oxygenation of human tumors: Evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991, 51, 3316–3322. [Google Scholar]
- Bosco, M.C.; Varesio, L. Dendritic cell reprogramming by the hypoxic environment. Immunobiology 2012, 217, 1241–1249. [Google Scholar] [CrossRef]
- Del Prete, A.; Sozio, F.; Barbazza, I.; Salvi, V.; Tiberio, L.; Laffranchi, M.; Gismondi, A.; Bosisio, D.; Schioppa, T.; Sozzani, S. Functional Role of Dendritic Cell Subsets in Cancer Progression and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 3930. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Morena, E.; Pucci, A.; Miglietta, D.; Riboldi, E.; Sozzani, S.; Carraro, F. Hypoxia affects dendritic cell survival: Role of the hypoxia-inducible factor-1alpha and lipopolysaccharide. J. Cell Physiol. 2012, 227, 587–595. [Google Scholar] [CrossRef]
- Sathaliyawala, T.; O’Gorman, W.E.; Greter, M.; Bogunovic, M.; Konjufca, V.; Hou, Z.E.; Nolan, G.P.; Miller, M.J.; Merad, M.; Reizis, B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity 2010, 33, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Hildeman, D.; Jorgensen, T.; Kappler, J.; Marrack, P. Apoptosis and the homeostatic control of immune responses. Curr. Opin. Immunol. 2007, 19, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrington, E.M.; Zhang, J.G.; Sutherland, R.M.; Vikstrom, I.B.; Brady, J.L.; Soo, P.; Vremec, D.; Allison, C.; Lee, E.F.; Fairlie, W.D.; et al. Prosurvival Bcl-2 family members reveal a distinct apoptotic identity between conventional and plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4044–4049. [Google Scholar] [CrossRef] [Green Version]
- Marsden, V.S.; Strasser, A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 2003, 21, 71–105. [Google Scholar] [CrossRef]
- Luhtala, N.; Parker, R. T2 Family ribonucleases: Ancient enzymes with diverse roles. Trends Biochem. Sci. 2010, 35, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Xu, Y.; Zhao, H.; Li, Y. RNase T2 in Inflammation and Cancer: Immunological and Biological Views. Front. Immunol. 2020, 11, 1554. [Google Scholar] [CrossRef]
- Lualdi, M.; Pedrini, E.; Rea, K.; Monti, L.; Scaldaferri, D.; Gariboldi, M.; Camporeale, A.; Ghia, P.; Monti, E.; Tomassetti, A.; et al. Pleiotropic modes of action in tumor cells of RNASET2, an evolutionary highly conserved extracellular RNase. Oncotarget 2015, 6, 7851–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquati, F.; Possati, L.; Ferrante, L.; Campomenosi, P.; Talevi, S.; Bardelli, S.; Margiotta, C.; Russo, A.; Bortoletto, E.; Rocchetti, R.; et al. Tumor and metastasis suppression by the human RNASET2 gene. Int. J. Oncol. 2005, 26, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Acquati, F.; Morelli, C.; Cinquetti, R.; Bianchi, M.G.; Porrini, D.; Varesco, L.; Gismondi, V.; Rocchetti, R.; Talevi, S.; Possati, L.; et al. Cloning and characterization of a senescence inducing and class II tumor suppressor gene in ovarian carcinoma at chromosome region 6q27. Oncogene 2001, 20, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Jiang, M.; Wu, J.; Ma, Y.; Li, T.; Chen, Q.; Zhang, X.; Xiang, L. Stress-induced RNASET2 overexpression mediates melanocyte apoptosis via the TRAF2 pathway in vitro. Cell Death Dis. 2014, 5, e1022. [Google Scholar] [CrossRef] [Green Version]
- Baranzini, N.; De Vito, A.; Orlandi, V.T.; Reguzzoni, M.; Monti, L.; de Eguileor, M.; Rosini, E.; Pollegioni, L.; Tettamanti, G.; Acquati, F.; et al. Antimicrobial Role of RNASET2 Protein During Innate Immune Response in the Medicinal Leech Hirudo verbana. Front. Immunol. 2020, 11, 370. [Google Scholar] [CrossRef]
- Ostendorf, T.; Zillinger, T.; Andryka, K.; Schlee-Guimaraes, T.M.; Schmitz, S.; Marx, S.; Bayrak, K.; Linke, R.; Salgert, S.; Wegner, J.; et al. Immune Sensing of Synthetic, Bacterial, and Protozoan RNA by Toll-like Receptor 8 Requires Coordinated Processing by RNase T2 and RNase 2. Immunity 2020, 52, 591–605.e596. [Google Scholar] [CrossRef]
- Greulich, W.; Wagner, M.; Gaidt, M.M.; Stafford, C.; Cheng, Y.; Linder, A.; Carell, T.; Hornung, V. TLR8 Is a Sensor of RNase T2 Degradation Products. Cell 2019, 179, 1264–1275.e1213. [Google Scholar] [CrossRef]
- Tran Janco, J.M.; Lamichhane, P.; Karyampudi, L.; Knutson, K.L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 2015, 194, 2985–2991. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Corzo, C.A.; Luetteke, N.; Yu, B.; Nagaraj, S.; Bui, M.M.; Ortiz, M.; Nacken, W.; Sorg, C.; Vogl, T.; et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 2008, 205, 2235–2249. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [Green Version]
- Carrington, E.M.; Zhan, Y.; Brady, J.L.; Zhang, J.G.; Sutherland, R.M.; Anstee, N.S.; Schenk, R.L.; Vikstrom, I.B.; Delconte, R.B.; Segal, D.; et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017, 24, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Hengstschlager, M.; Weichhart, T. mTOR-Mediated Regulation of Dendritic Cell Differentiation and Function. Trends Immunol. 2016, 37, 778–789. [Google Scholar] [CrossRef]
- Weichhart, T.; Saemann, M.D. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67 (Suppl. 3), iii70–iii74. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Scandurro, A.B. Tumors sound the alarmin(s). Cancer Res. 2008, 68, 6482–6485. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, W.; Bian, M.; Wang, X.; Sun, J.; Sun, H.; Jia, F.; Liang, C.; Li, X.; Zhou, X.; et al. Molecular characterization and immune modulation properties of Clonorchis sinensis-derived RNASET2. Parasit. Vectors 2013, 6, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granucci, F.; Vizzardelli, C.; Virzi, E.; Rescigno, M.; Ricciardi-Castagnoli, P. Transcriptional reprogramming of dendritic cells by differentiation stimuli. Eur. J. Immunol. 2001, 31, 2539–2546. [Google Scholar] [CrossRef]
- Mancino, A.; Schioppa, T.; Larghi, P.; Pasqualini, F.; Nebuloni, M.; Chen, I.H.; Sozzani, S.; Austyn, J.M.; Mantovani, A.; Sica, A. Divergent effects of hypoxia on dendritic cell functions. Blood 2008, 112, 3723–3734. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaci, S.; Coppola, F.; Giuntini, G.; Roncoroni, R.; Acquati, F.; Sozzani, S.; Carraro, F.; Naldini, A. Hypoxia Enhances the Expression of RNASET2 in Human Monocyte-Derived Dendritic Cells: Role of PI3K/AKT Pathway. Int. J. Mol. Sci. 2021, 22, 7564. https://doi.org/10.3390/ijms22147564
Monaci S, Coppola F, Giuntini G, Roncoroni R, Acquati F, Sozzani S, Carraro F, Naldini A. Hypoxia Enhances the Expression of RNASET2 in Human Monocyte-Derived Dendritic Cells: Role of PI3K/AKT Pathway. International Journal of Molecular Sciences. 2021; 22(14):7564. https://doi.org/10.3390/ijms22147564
Chicago/Turabian StyleMonaci, Sara, Federica Coppola, Gaia Giuntini, Rossella Roncoroni, Francesco Acquati, Silvano Sozzani, Fabio Carraro, and Antonella Naldini. 2021. "Hypoxia Enhances the Expression of RNASET2 in Human Monocyte-Derived Dendritic Cells: Role of PI3K/AKT Pathway" International Journal of Molecular Sciences 22, no. 14: 7564. https://doi.org/10.3390/ijms22147564
APA StyleMonaci, S., Coppola, F., Giuntini, G., Roncoroni, R., Acquati, F., Sozzani, S., Carraro, F., & Naldini, A. (2021). Hypoxia Enhances the Expression of RNASET2 in Human Monocyte-Derived Dendritic Cells: Role of PI3K/AKT Pathway. International Journal of Molecular Sciences, 22(14), 7564. https://doi.org/10.3390/ijms22147564






