The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma
Abstract
1. Introduction
2. Results
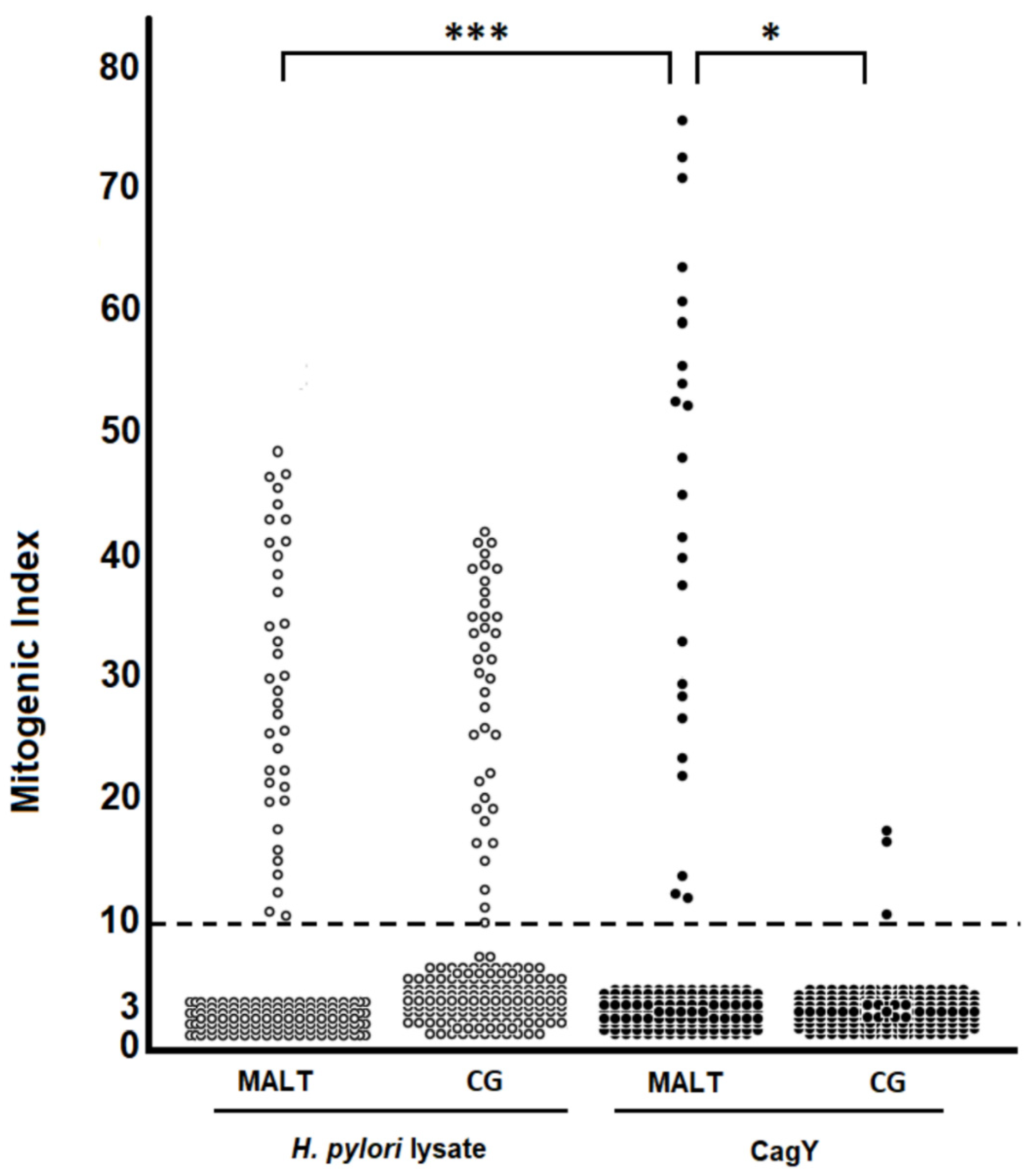
2.1. H. pylori CagY-Specific CD4+ T Cells Predominate in Gastric Low-Grade MALT Lymphoma
2.2. H. pylori CagY Predominantly Drives IFN-γ and IL-17 Secretion by Gastric CD4+ T Cells from H. pylori-Infected Patients with Gastric Low-Grade MALT Lymphoma
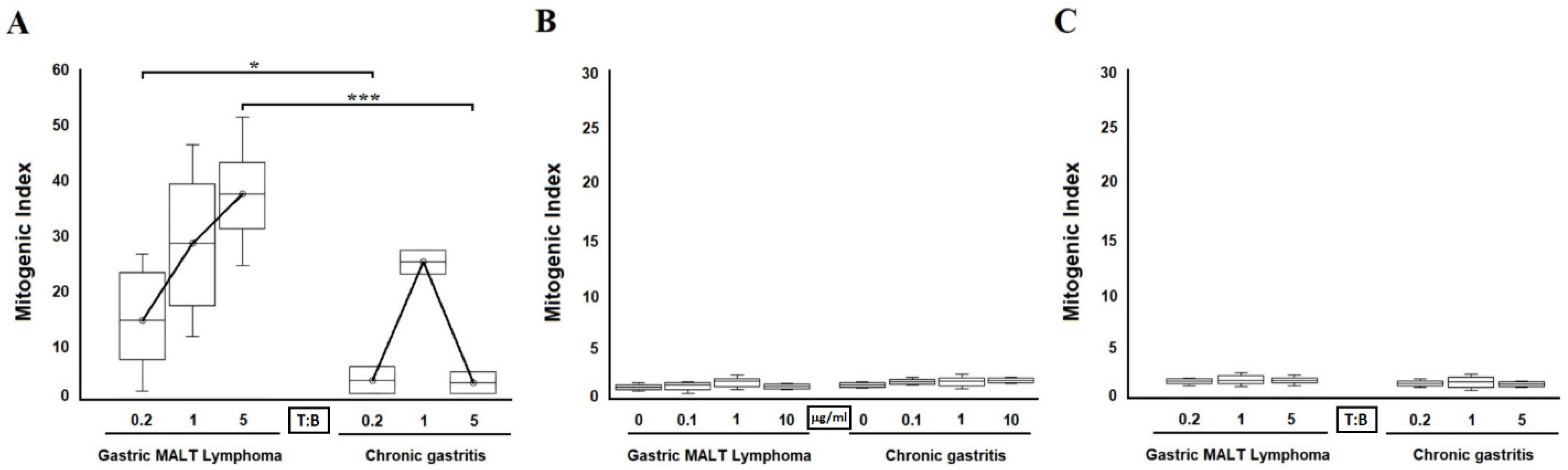
2.3. Antigen-Dependent B Cell Help by H. pylori CagY-Specific Th Clones
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Reagents
4.3. Generation of H. pylori-Specific T Cell Clones
4.4. Cytokine Profile of H. pylori CagY–Specific Gastric T Cell Clones
4.5. Helper Activity of T Cell Lones for B Cell Proliferation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, B.; Warren, J. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pyloriInfection and the Risk of Gastric Carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.; Diss, T.; Pan, L.; Isaacson, P.; Doglioni, C.; Moschini, A.; De Boni, M. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575–577. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric autoimmunity: The role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef]
- Pellicano, R.; Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Saracco, G.M.; Mégraud, F. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016, 58, 304–317. [Google Scholar] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Filip, P.V.; Cuciureanu, D.; Diaconu, L.S.; Vladareanu, A.M.; Pop, C.S. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J. Med. Life 2018, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Herlevic, V.; Morris, J.D. Gastric Lymphoma; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Greiner, A.; Knörr, C.; Qin, Y.; Sebald, W.; Schimpl, A.; Banchereau, J.; Müller-Hermelink, H.K. Low-grade B cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signaling and Th2-type cytokines for in vitro growth and differentiation. Am. J. Pathol. 1997, 150, 1583–1593. [Google Scholar]
- D’Elios, M.M.; Amedei, A.; Manghetti, M.; Costa, F.; Baldari, C.; Quazi, A.S.; Telford, J.L.; Romagnani, S.; del Prete, G. Impaired T-cell regulation of B-cell growth in Helicobacter pylori–related gastric low-grade MALT lymphoma. Gastroenterology 1999, 117, 1105–1112. [Google Scholar] [CrossRef]
- Hussell, T.; Isaacson, P.; Spencer, J.; Crabtree, J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993, 342, 571–574. [Google Scholar] [CrossRef]
- Hussell, T.; Isaacson, P.G.; Crabtree, J.E.; Spencer, J. Helicobacter pylori–specific tumor-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J. Pathol. 1996, 178, 122–127. [Google Scholar] [CrossRef]
- Capitani, N.; Codolo, G.; Vallese, F.; Minervini, G.; Grassi, A.; Cianchi, F.; Troilo, A.; Fischer, W.; Zanotti, G.; Baldari, C.T.; et al. The lipoprotein HP1454 of Helicobacter pylori regulates T-cell response by shaping T-cell receptor signalling. Cell Microbiol. 2019, 21, e13006. [Google Scholar] [CrossRef] [PubMed]
- Tomb, J.-F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Ling, L.-S.L.; Moir, D.T.; King, B.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; Dejonge, B.L.; et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M.; Püls, J.; Buhrdorf, R.; Fischer, W.; Haas, R. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol. Microbiol. 2003, 49, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J.; Perez, G.P.; Kleanthous, H.; Cover, T.; Peek, R.M.; Chyou, P.H.; Stemmermann, G.N.; Nomura, A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995, 55, 2111–2115. [Google Scholar]
- Kuipers, E.J.; Pérez-Pérez, G.I.; Meuwissen, S.G.M.; Blaser, M.J. Helicobacter pylori and Atrophic Gastritis: Importance of the cagA Status. J. Natl. Cancer Inst. 1995, 87, 1777–1780. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek, R.M. The Helicobacter pylori cag Pathogenicity Island. Methods Mol. Biol. 2012, 921, 41–50. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2019, 17, 50–63. [Google Scholar] [CrossRef]
- Soluri, M.F.; Puccio, S.; Caredda, G.; Edomi, P.; D’Elios, M.M.; Cianchi, F.; Troilo, A.; Santoro, C.; Sblattero, D.; Peano, C. Defining the Helicobacter pylori Disease-Specific Antigenic Repertoire. Front. Microbiol. 2020, 11, 1551. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Neddermann, M.; Lind, J.; Pachathundikandi, S.K.; Sharafutdinov, I.; Gutiérrez-Escobar, A.J.; Brönstrup, M.; Tegge, W.; Hong, M.; Rohde, M.; et al. Toll-like Receptor 5 Activation by the CagY Repeat Domains of Helicobacter pylori. Cell Rep. 2020, 32, 108–159. [Google Scholar] [CrossRef] [PubMed]
- Pachathundikandi, S.K.; Tegtmeyer, N.; Backert, S. Signal transduction of Helicobacter pylori during interaction with host cell protein receptors of epithelial and immune cells. Gut Microbes 2013, 4, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Barrozo, R.M.; Cooke, C.L.; Hansen, L.M.; Lam, A.M.; Gaddy, J.A.; Johnson, E.M.; Cariaga, T.A.; Suarez, G.; Peek, R.M., Jr.; Cover, T.; et al. Functional Plasticity in the Type IV Secretion System of Helicobacter pylori. PLoS Pathog. 2013, 9, e1003189. [Google Scholar] [CrossRef]
- Barrozo, R.M.; Hansen, L.M.; Lam, A.M.; Skoog, E.; Martin, M.E.; Cai, L.P.; Lin, Y.; Latoscha, A.; Suerbaum, S.; Canfield, D.R.; et al. CagY Is an Immune-Sensitive Regulator of the Helicobacter pylori Type IV Secretion System. Gastroenterology 2016, 151, 1164–1175.e3. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, P.G.; Wright, D.H. Malignant lymphoma of mucosaassociated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983, 52, 1410–1416. [Google Scholar] [CrossRef]
- Parsonnet, J.; Hansen, S.; Rodriguez, L.; Gelb, A.B.; Warnke, R.A.; Jellum, E.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Helicobacter pylori Infection and Gastric Lymphoma. N. Engl. J. Med. 1994, 330, 1267–1271. [Google Scholar] [CrossRef]
- Isaacson, P.G. Gastrointestinal lymphoma. Hum. Pathol. 1994, 25, 1020–1029. [Google Scholar] [CrossRef]
- Feng, G.; Chen, Y.; Li, K. Helicobacter pylori promote inflammation and host defense through the cagA-dependent activation of mTORC1. J. Cell. Physiol. 2020, 235, 10094–10108. [Google Scholar] [CrossRef]
- Munari, F.; Lonardi, S.; Cassatella, M.A.; Doglioni, C.; Cangi, M.G.; Amedei, A.; Facchetti, F.; Eishi, Y.; Rugge, M.; Fassan, M.; et al. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood 2011, 117, 6612–6616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munari, F.; Fassan, M.; Capitani, N.; Codolo, G.; Caballer, M.A.V.; Pizzi, M.; Rugge, M.; Della Bella, C.; Troilo, A.; D’Elios, S.; et al. Cytokine BAFF Released by Helicobacter pylori–Infected Macrophages Triggers the Th17 Response in Human Chronic Gastritis. J. Immunol. 2014, 193, 5584–5594. [Google Scholar] [CrossRef]
- Blosse, A.; Peru, S.; Levy, M.; Marteyn, B.; Floch, P.; Sifré, E.; Giese, A.; Prochazkova-Carlotti, M.; Martin, L.A.; Dubus, P.; et al. APRIL-producing eosinophils are involved in gastric MALT lymphomagenesis induced by Helicobacter sp infection. Sci. Rep. 2020, 10, 14858. [Google Scholar] [CrossRef]
- Chonwerawong, M.; Ferrand, J.; Chaudhry, H.M.; Higgins, C.; Tran, L.S.; Lim, S.S.; Walker, M.M.; Bhathal, P.S.; Dev, A.; Moore, G.T.; et al. Innate Immune Molecule NLRC5 Protects Mice from Helicobacter-induced Formation of Gastric Lymphoid Tissue. Gastroenterology 2020, 159, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Codolo, G.; Bossi, F.; Durigutto, P.; Della Bella, C.; Fischetti, F.; Amedei, A.; Tedesco, F.; D’Elios, S.; Cimmino, M.; Micheletti, A.; et al. Orchestration of Inflammation and Adaptive Immunity inBorrelia burgdorferi-Induced Arthritis by Neutrophil-Activating Protein A. Arthritis Rheum. 2013, 65, 1232–1242. [Google Scholar] [CrossRef]
- De Jong, D.; Van Der Hulst, R.W.; Pals, G.; Van Dijk, W.C.; Van Der Ende, A.; Tytgat, G.N.; Taal, B.G.; Boot, H. Gastric Non-Hodgkin Lymphomas of Mucosa-Associated Lymphoid Tissue Are not Associated with More Aggressive Helicobacter pyloriStrains as Identified by CagA. Am. J. Clin. Pathol. 1996, 106, 670–675. [Google Scholar] [CrossRef]
- Eck, M.; Schmausser, B.; Haas, R.; Greiner, A.; Czub, S.; Müller-Hermelink, H.K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 1997, 112, 1482–1486. [Google Scholar] [CrossRef]
- Enno, A.; O’Rourke, J.L.; Howlett, C.R.; Jack, A.; Dixon, M.F.; Lee, A. MALToma-like lesions in the murine gastric mucosa after longterminfection with Helicobacter felis. A mouse model of Helicobacter pylori–induced gastric lymphoma. Am. J. Pathol. 1995, 47, 217–222. [Google Scholar]
- Ferretti, E.; DI Carlo, E.; Ognio, E.; Guarnotta, C.; Bertoni, F.; Corcione, A.; Prigione, I.; Orcioni, G.F.; Ribatti, D.; Ravetti, J.L.; et al. Interleukin-17A promotes the growth of human germinal center derived non-Hodgkin B cell lymphoma. OncoImmunology 2015, 4, e1030560. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Manghetti, M.; De Carli, M.; Costa, F.; Baldari, C.; Burroni, D.; Telford, J.L.; Romagnani, S.; Del Prete, G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 1997, 158, 962–967. [Google Scholar] [PubMed]
- D’Elios, M.M.; Bergman, M.P.; Azzurri, A.; Amedei, A.; Benagiano, M.; De Pont, J.J.; Cianchi, F.; Vandenbroucke-Grauls, C.M.; Romagnani, S.; Appelmelk, B.J.; et al. H+, K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology 2001, 120, 377–386. [Google Scholar] [CrossRef]

| Patient ID | CagY-Specific T CD4+ Clones | H. pylori Lysate-Specific T CD4+ Clones | CagY-Specific T CD8+ Clones | H. pylori Lysate-Specific T CD8+ Clones |
|---|---|---|---|---|
| MALT 1 | 4/30 (13.3) | 5/30 (16.7) | 0/3 (0) | 0/3 (0) |
| MALT 2 | 5/28 (17.9) | 7/28 (25) | 0/4 (0) | 0/4 (0) |
| MALT 3 | 5/35 (14.3) | 8/35 (22.9) | 0/2 (0) | 0/2 (0) |
| MALT 4 | 4/22 (18.2) | 7/22 (31.8) | 0/4 (0) | 0/4 (0) |
| MALT 5 | 4/43 (9.3) | 10/43 (23.2) | 0/4 (0) | 0/4 (0) |
| Total | 22/158 (13.9) | 37/158 (23.4) | 0/17 (0) | 0/17 (0) |
| CG 1 | 1/37 (2.7) | 6/37 (16.2) | 0/3 (0) | 0/3 (0) |
| CG 2 | 1/28 (3.6) | 8/28 (28.6) | 0/4 (0) | 0/4 (0) |
| CG 3 | 0/35 (0) | 9/35 (25.7) | 0/5 (0) | 0/5 (0) |
| CG 4 | 1/44 (2.3) | 7/44 (15.9) | 0/5 (0) | 0/5 (0) |
| CG 5 | 0/35 (0) | 9/35 (25.7) | 0/5 (0) | 0/5 (0) |
| Total | 3/179 (1.7) | 39/179 (21.8) | 0/22 (0) | 0/22 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Bella, C.; Soluri, M.F.; Puccio, S.; Benagiano, M.; Grassi, A.; Bitetti, J.; Cianchi, F.; Sblattero, D.; Peano, C.; D’Elios, M.M. The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma. Int. J. Mol. Sci. 2021, 22, 9459. https://doi.org/10.3390/ijms22179459
Della Bella C, Soluri MF, Puccio S, Benagiano M, Grassi A, Bitetti J, Cianchi F, Sblattero D, Peano C, D’Elios MM. The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma. International Journal of Molecular Sciences. 2021; 22(17):9459. https://doi.org/10.3390/ijms22179459
Chicago/Turabian StyleDella Bella, Chiara, Maria Felicia Soluri, Simone Puccio, Marisa Benagiano, Alessia Grassi, Jacopo Bitetti, Fabio Cianchi, Daniele Sblattero, Clelia Peano, and Mario Milco D’Elios. 2021. "The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma" International Journal of Molecular Sciences 22, no. 17: 9459. https://doi.org/10.3390/ijms22179459
APA StyleDella Bella, C., Soluri, M. F., Puccio, S., Benagiano, M., Grassi, A., Bitetti, J., Cianchi, F., Sblattero, D., Peano, C., & D’Elios, M. M. (2021). The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma. International Journal of Molecular Sciences, 22(17), 9459. https://doi.org/10.3390/ijms22179459






