Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion
Abstract
:1. Introduction
2. Results
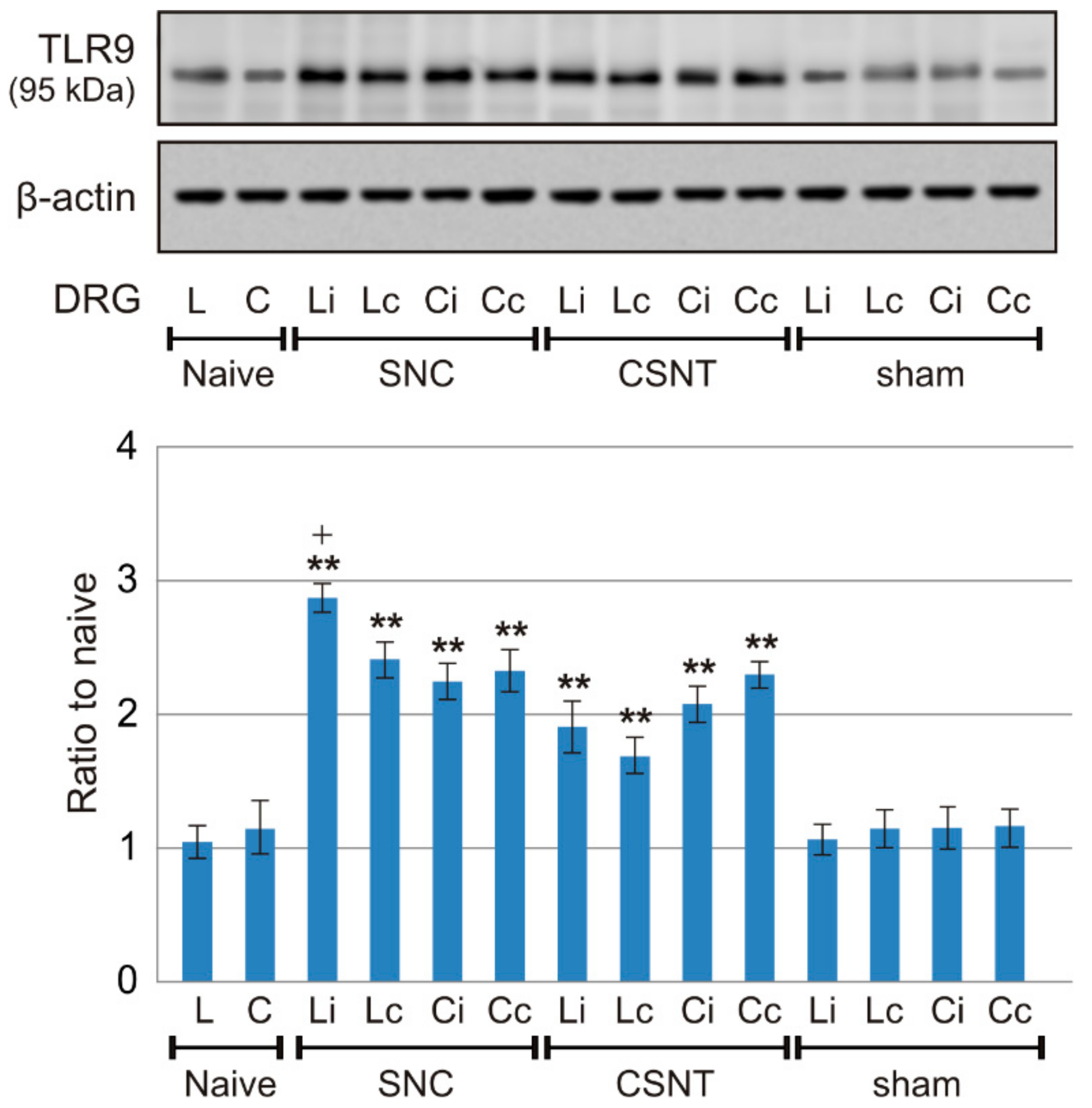
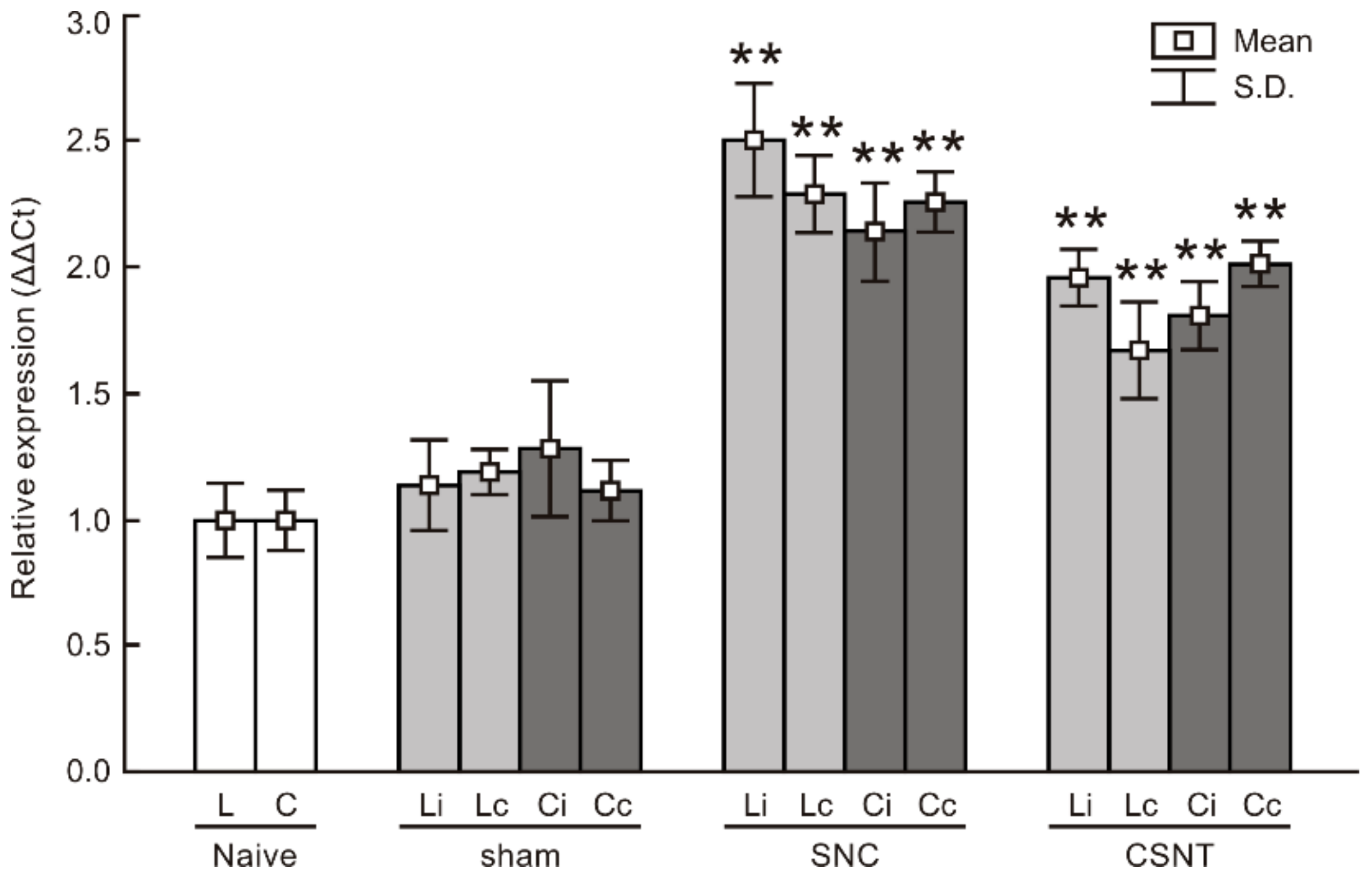
2.1. Increased Levels of TLR9 Protein and mRNA in DRG Following Sciatic Nerve Lesion
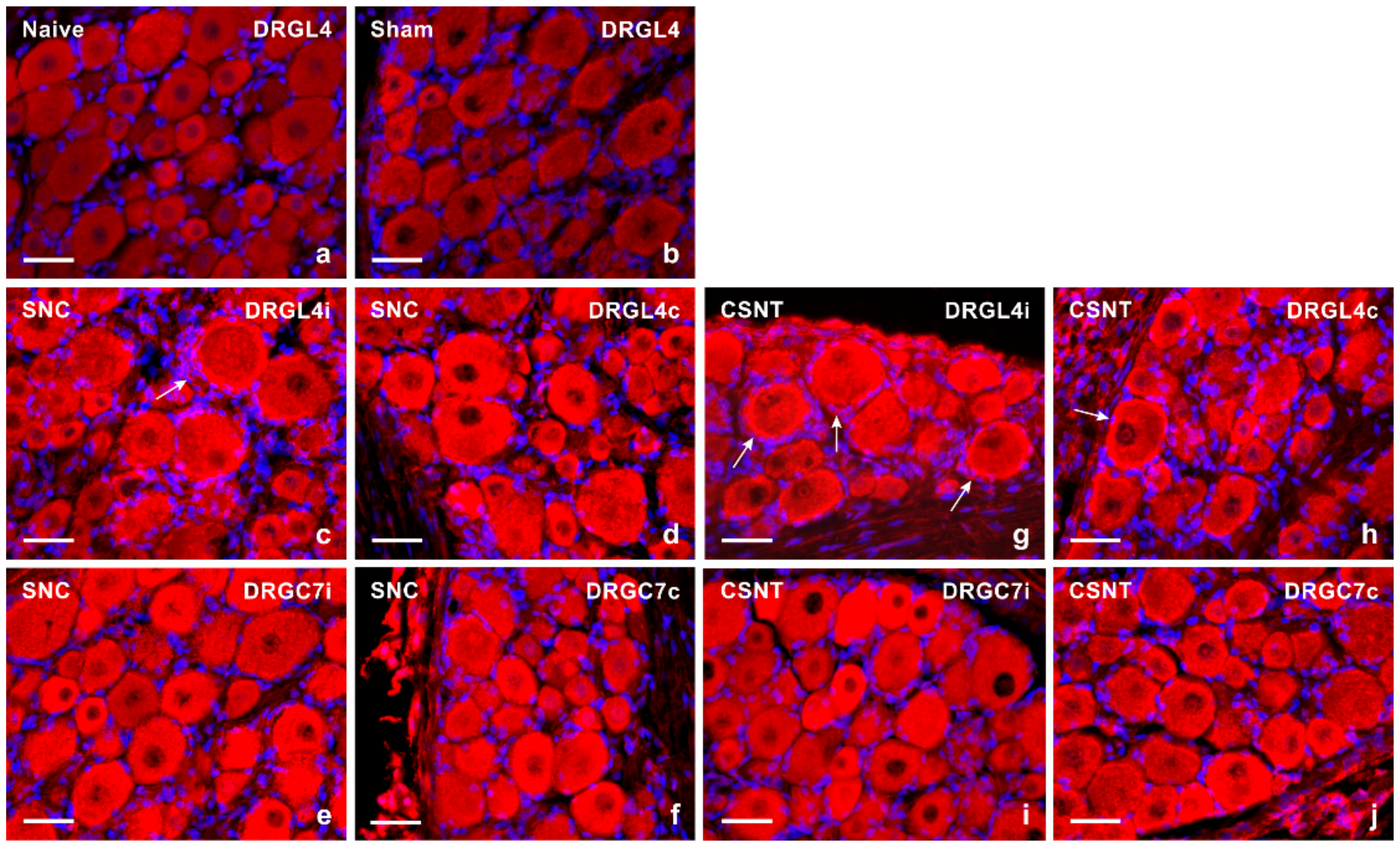
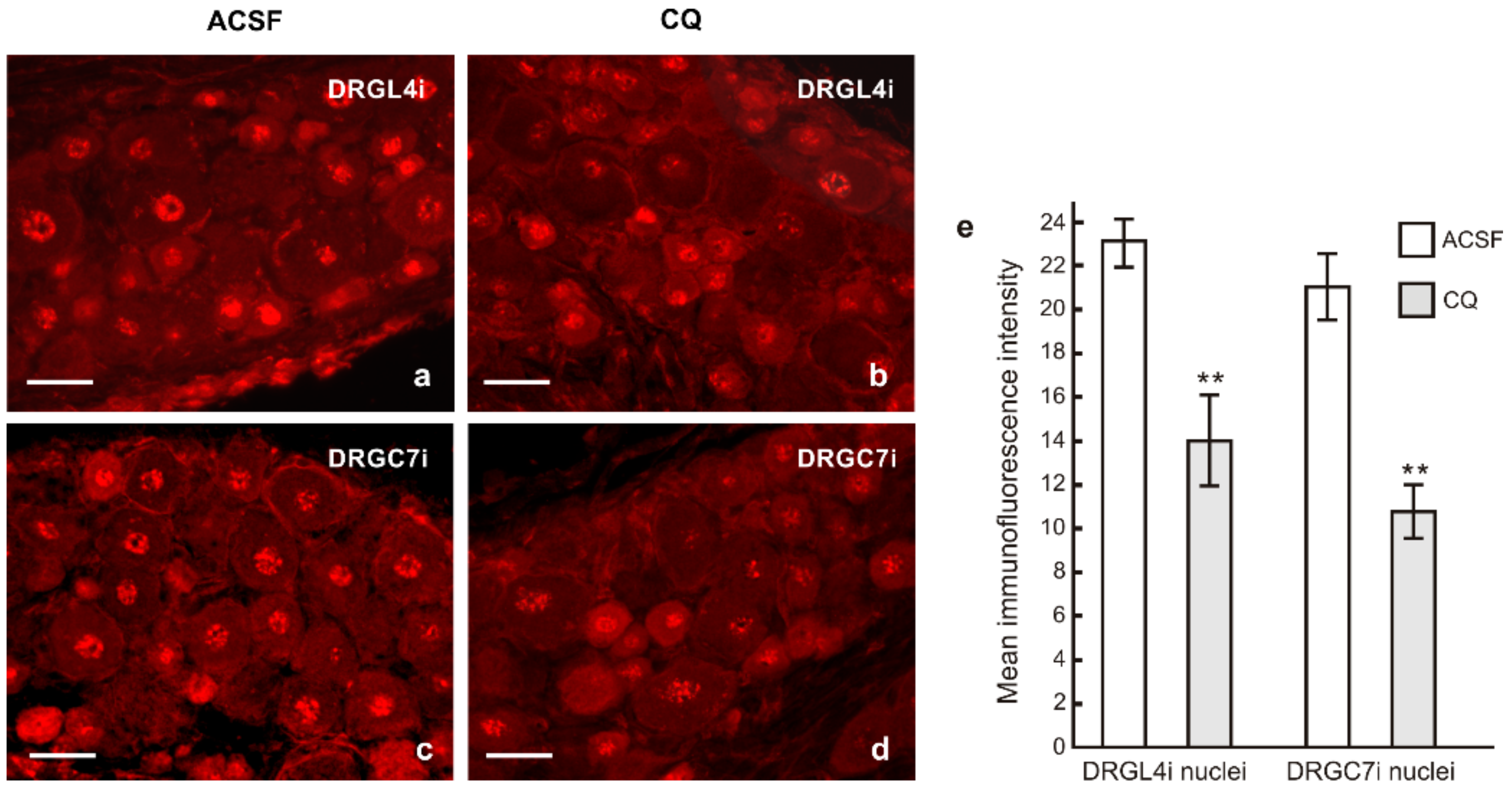
2.1.1. Immunofluorescence Staining
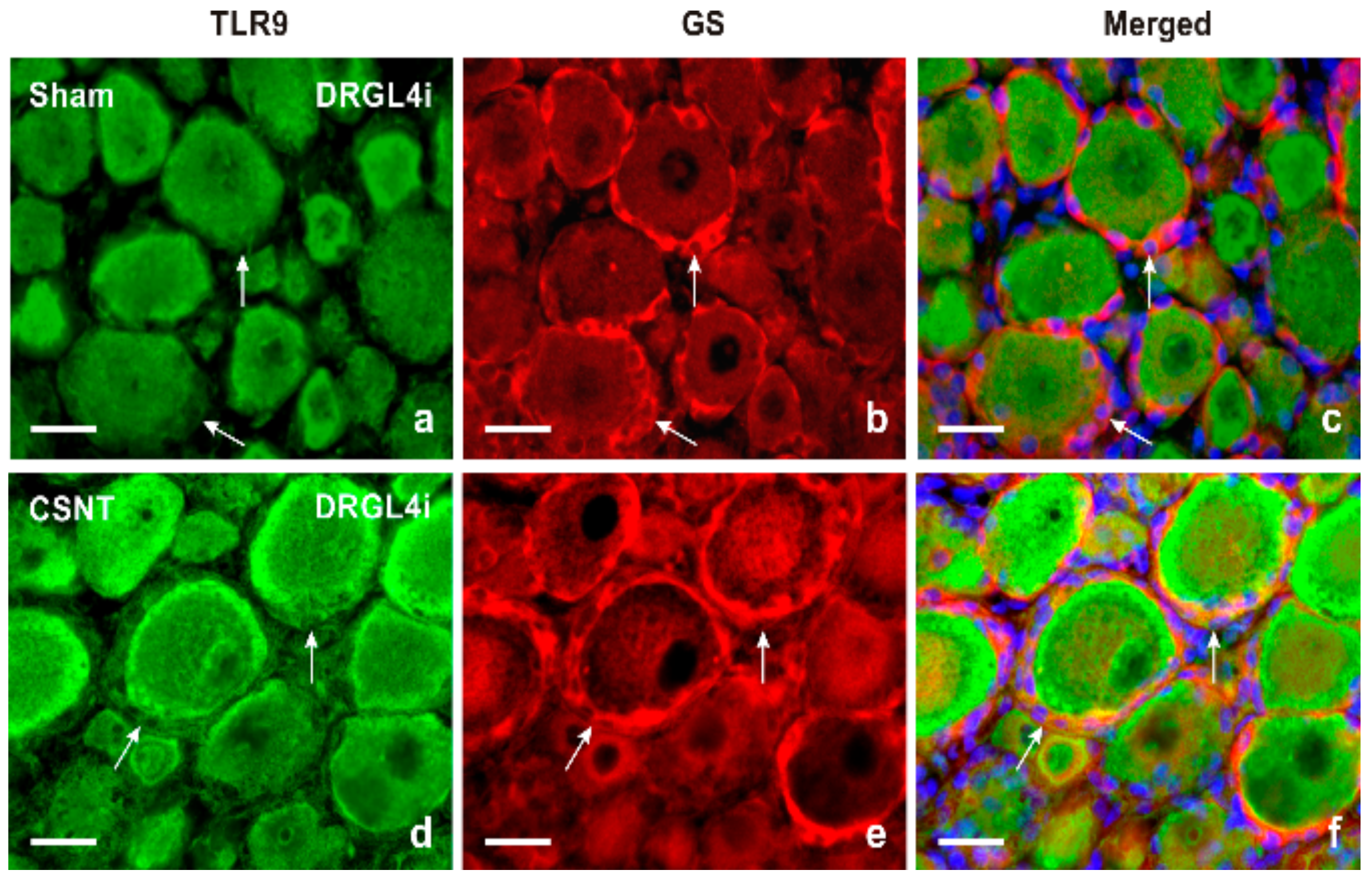
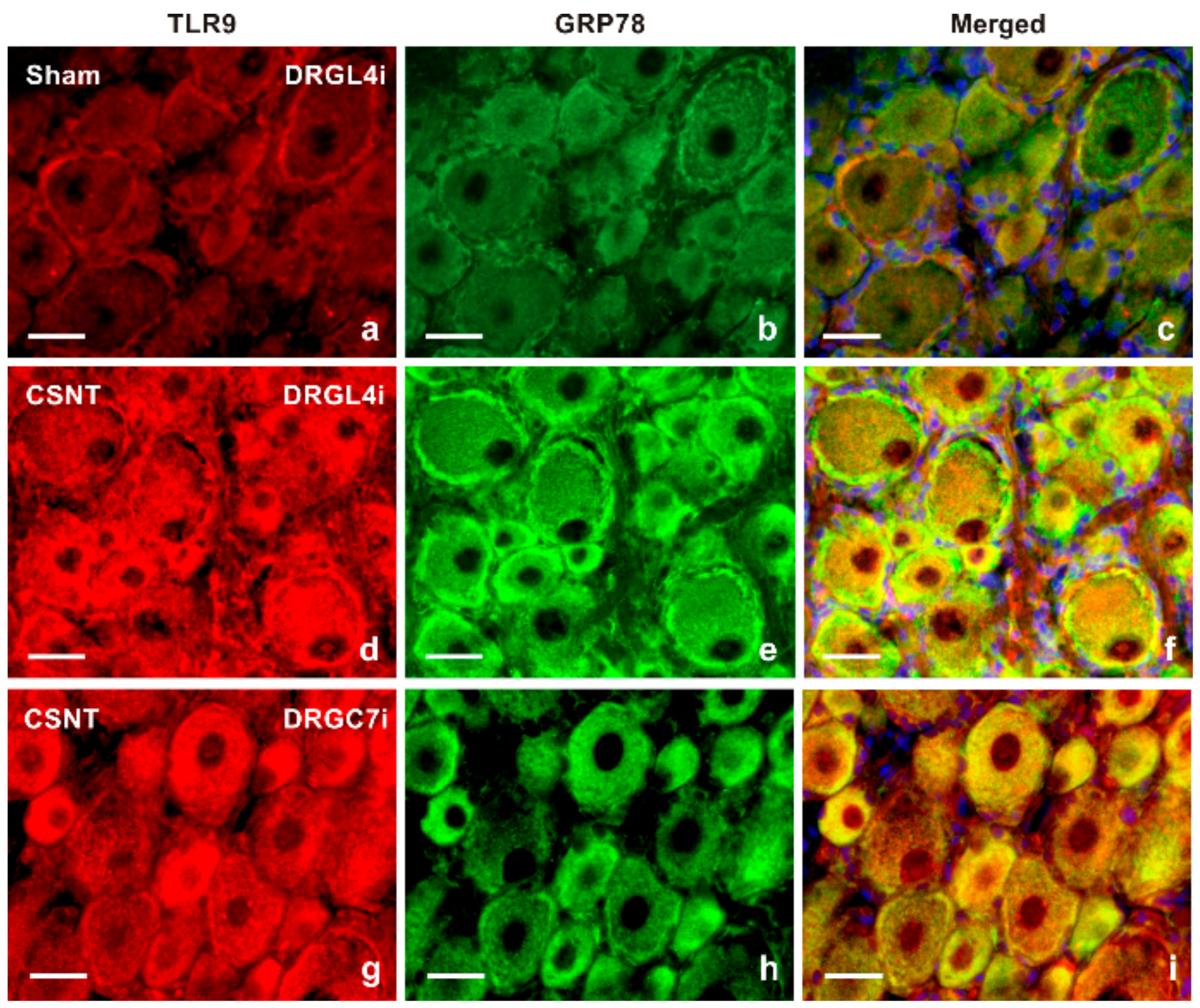
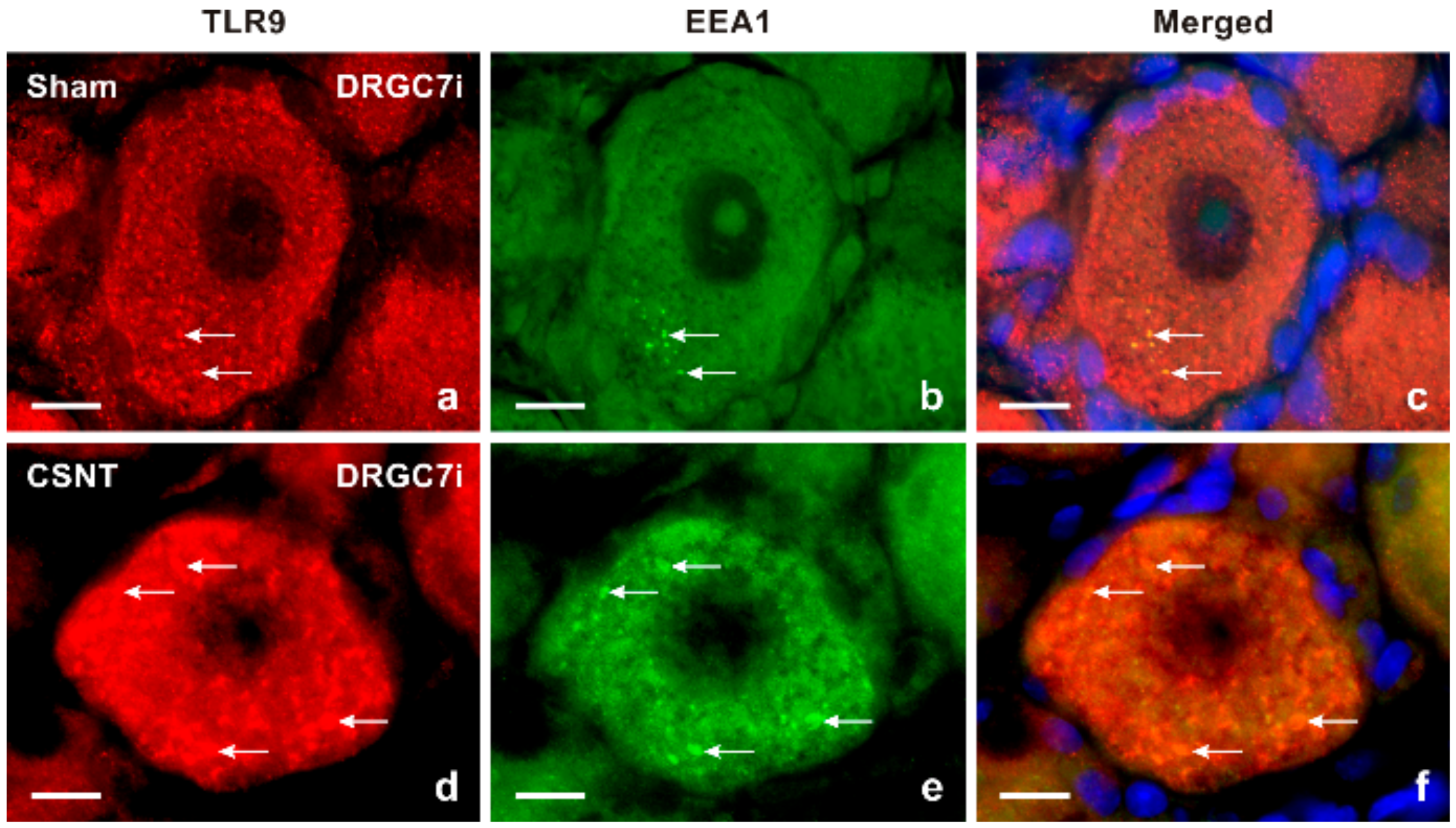
2.1.2. Double Immunostaining
2.1.3. Western Blot Analysis
2.1.4. Real-Time RT-PCR
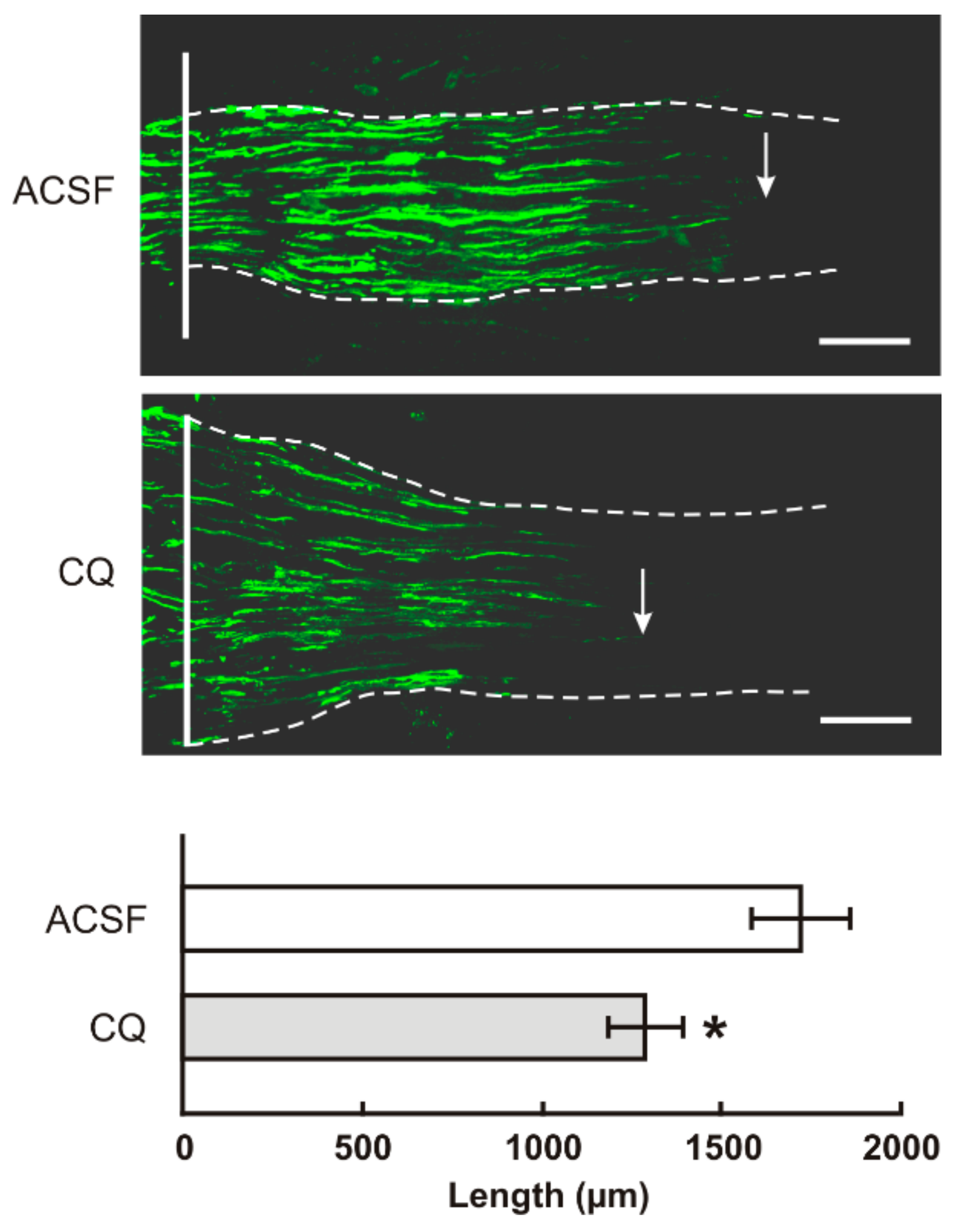
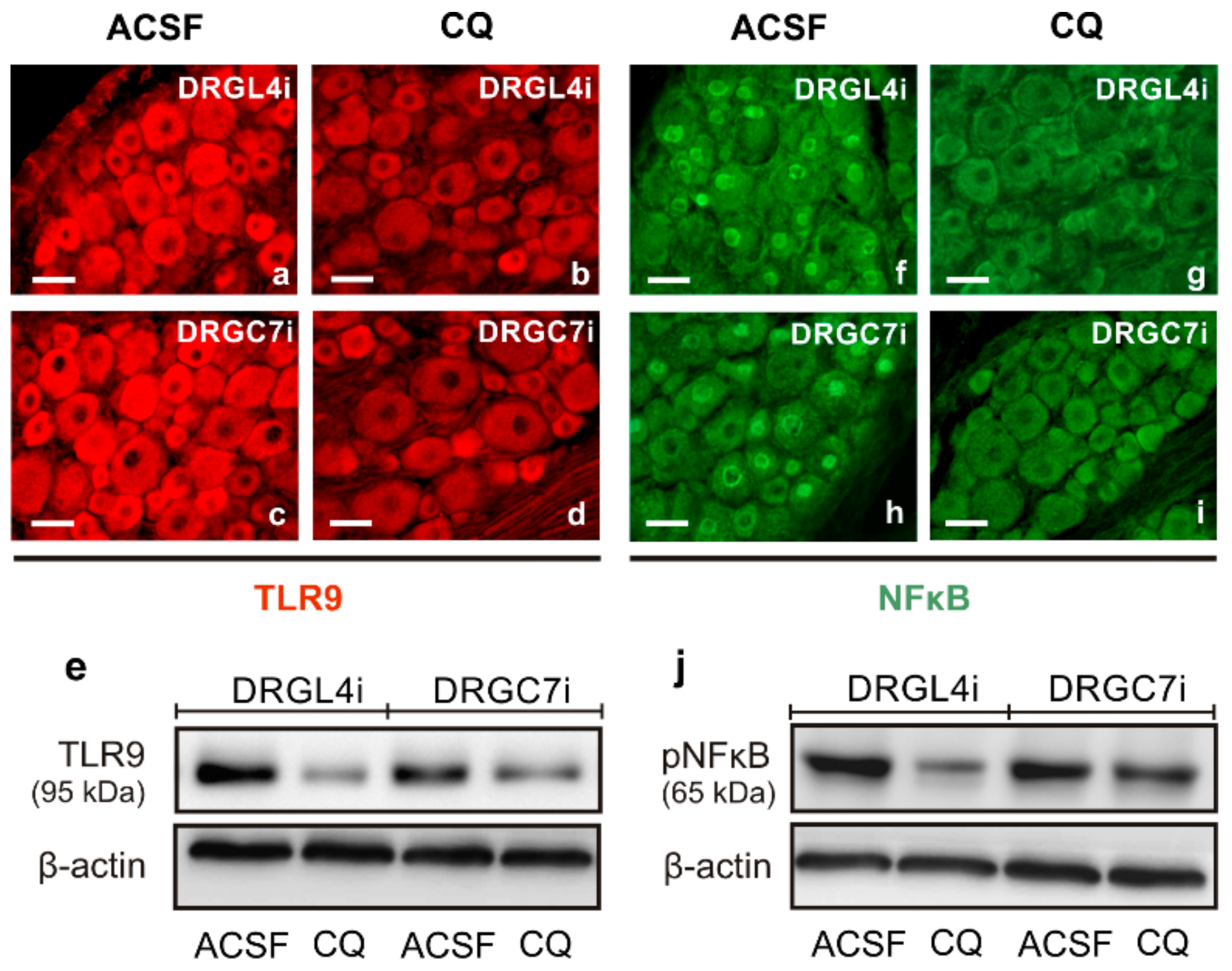
2.2. TLR9 and Neuronal Innate Immune Reactions Associated with Pro-Regenerative State of DRG Neurons
3. Discussion
3.1. Intracellular Localization of TLR9 in DRG Neurons
3.2. Neuronal Innate Immune Reaction and the Pro-Regenerative State of DRG Neurons
4. Materials and Methods
4.1. Animals and Surgical Treatment
4.2. Tissue Processing and Immunofluorescence Staining
4.3. Double Immunostaining
4.4. Western Blot Analysis
4.5. Real Time RT-PCR
4.6. Intrathecal Administration of Chloroquine, In Vivo Axon Regeneration Assay and Evaluation of Transforming Factors
4.6.1. Axon Regeneration Assay and Immunohistochemical Analysis
4.6.2. Western Blot Analysis
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubovy, P.; Klusakova, I.; Hradilova-Svizenska, I.; Joukal, M. Expression of regeneration-associated proteins in primary sensory neurons and regenerating axons after nerve injury—An overview. Anat. Rec. 2018, 301, 1618–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-H.; Jan, Y.-N. Mechanisms of neurite repair. Curr. Opin. Neurobiol. 2020, 63, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Woolf, C.J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 1999, 23, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, F.W.; Hager, G.; Schmitt, A.B.; Horvat, A.; Hager, G.; Streif, R.; Spitzer, C.; Gamal, S.; Breuer, S.; Brook, G.A.; et al. Peripheral but not central axotomy induces changes in janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur. J. Neurosci. 2000, 12, 1165–1176. [Google Scholar] [CrossRef]
- Qiu, J.; Cafferty, W.B.J.; McMahon, S.B.; Thompson, S.W.N. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Neurosci. 2005, 25, 1645–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubový, P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Court, F.A.; Coleman, M.P. Mitochondria as a central sensor for axonal degenerative stimuli. TINS 2012, 35, 364–372. [Google Scholar] [CrossRef]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, S.A.; Martinez, N.W.; Yoo, S.; Jara, J.S.; Zamorano, S.; Hetz, C.; Twiss, J.L.; Alvarez, J.; Court, F.A. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 2011, 31, 966–978. [Google Scholar] [CrossRef]
- Freeman, M.R. Signaling mechanisms regulating Wallerian degeneration. Curr. Opin. Neurobiol. 2014, 27, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catenaccio, A.; Hurtado, M.L.; Diaz, P.; Lamont, D.J.; Wishart, T.M.; Court, F.A. Molecular analysis of axonal-intrinsic and glial-associated co-regulation of axon degeneration. Cell Death Dis. 2017, 8, e3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef] [Green Version]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.A.; Beltrán, C.J.; Aguirre, I.M.; Silva, P.; Peralta, A.L.; Malinarich, F.; Hermoso, M.A. Toll-like receptors are key participants in innate immune responses. Biol. Res. 2007, 40. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, S.; Liu, T.; Wang, H.; Liu, K.; Wang, Q.; Zeng, W. Sarm1/Myd88-5 regulates neuronal intrinsic immune response to traumatic axonal injuries. Cell Rep. 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Dubový, P.; Hradilová-Svíženská, I.; Klusáková, I.; Kokošová, V.; Brázda, V.; Joukal, M. Bilateral activation of STAT3 by phosphorylation at the tyrosine-705 (Y705) and serine-727 (S727) positions and its nuclear translocation in primary sensory neurons following unilateral sciatic nerve injury. Histochem. Cell Biol. 2018, 150, 37–47. [Google Scholar] [CrossRef]
- Dubový, P.; Hradilová-Svíženská, I.; Klusáková, I.; Brázda, V.; Joukal, M. Interleukin-6 contributes to initiation of neuronal regeneration program in the remote dorsal root ganglia neurons after sciatic nerve injury. Histochem. Cell Biol. 2019, 152, 109–117. [Google Scholar] [CrossRef]
- Dubovy, P.; Klusakova, I.; Hradilova-Svizenska, I.; Brazda, V.; Kohoutkova, M.; Joukal, M. A conditioning sciatic nerve lesion triggers a pro-regenerative state in primary sensory neurons also of dorsal root ganglia non-associated with the damaged nerve. Front. Cell. Neurosci. 2019, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallières, N.; Rivest, S.; Lacroix, S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007, 27, 12565–12576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Weerasuriya, A.; Mizisin, A.P. The blood-nerve barrier: Structure and functional significance. In Blood-Brain and Other Neural Barriers: Reviews and Protocols; Nag, S., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2011; Volume 686, pp. 149–173. ISBN 9781607619376. [Google Scholar]
- Arvidson, B. Distribution of intravenously injected protein tracers in peripheral ganglia of adult mice. Exp. Neurol. 1979, 63, 388–410. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Macfarlane, R.M.; Cavanagh, J.B. Vascular leakage in the dorsal root ganglia of the rat, studied with horseradish peroxidase. J. Neurol. Sci. 1976, 29, 95–107. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Liu, Z.; Liu, J.; Ren, J.-X.; Sun, T.-S. Mitochondrial DNA induces inflammation and increases TLR9/NF-kB expression in lung tissue. Int. J. Mol. Med. 2014, 33, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Wei, X.; Wei, Y. Mitochondrial DNA in the regulation of innate immune responses. Protein Cell 2016, 7, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goethals, S.; Ydens, E.; Timmerman, V.; Janssens, S. Toll-like receptor expression in the peripheral nerve. Glia 2010, 58, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Buzas, K.; Fan, H.; Cohen, J.I.; Wang, K.; Mont, E.; Klinman, D.; Oppenheim, J.J.; Howard, O.M.Z. Painful pathways induced by Toll-like receptor stimulation of dorsal root ganglion neurons. J. Immunol. 2011, 186, 6417–6426. [Google Scholar] [CrossRef] [Green Version]
- Latz, E.; Schoenemeyer, A.; Visintin, A.; Fitzgerald, K.A.; Monks, B.G.; Knetter, C.F.; Lien, E.; Nilsen, N.J.; Espevik, T.; Golenbock, D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004, 5, 190–198. [Google Scholar] [CrossRef]
- Wang, M.; Ye, R.; Barron, E.; Baumeister, P.; Mao, C.; Luo, S.; Fu, Y.; Luo, B.; Dubeau, L.; Hinton, D.R.; et al. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010, 17, 488–498. [Google Scholar] [CrossRef]
- Dong, L.; Odeleye, A.O.; Jordan-Sciutto, K.L.; Winkelstein, B.A. Painful facet joint injury induces neuronal stress activation in the drg: Implications for cellular mechanisms of pain. Neurosci. Lett. 2008, 443, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Chockalingam, A.; Brooks, J.C.; Cameron, J.L.; Blum, L.K.; Leifer, C.A. TLR9 traffics through the golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol. Cell Biol. 2009, 87, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Dubový, P.; Klusáková, I.; Svíženská, I.; Brázda, V. Spatio-temporal changes of sdf1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem. Cell Biol. 2010, 133, 323–337. [Google Scholar] [CrossRef]
- Dubový, P.; Brázda, V.; Klusáková, I.; Hradilová-Svíženská, I. Bilateral elevation of interleukin-6 protein and mRNA in both lumbar and cervical dorsal root ganglia following unilateral chronic compression injury of the sciatic nerve. J. Neuroinflamm. 2013, 10, 824. [Google Scholar] [CrossRef] [Green Version]
- Dziennis, S.; Alkayed, N.J. Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev. Neurosci. 2008, 19, 341–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bareyre, F.M.; Garzorz, N.; Lang, C.; Misgeld, T.; Büning, H.; Kerschensteiner, M. In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6282–6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigmond, R.E. Gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci. 2012, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjuan, M.A.; Rao, N.; Lai, K.T.; Gu, Y.; Sun, S.; Fuchs, A.; Fung-Leung, W.P.; Colonna, M.; Karlsson, L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 2006, 172, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Rutz, M.; Metzger, J.; Gellert, T.; Luppa, P.; Lipford, G.B.; Wagner, H.; Bauer, S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 2004, 34, 2541–2550. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, D.; Shi, X.; Liu, S.; Li, A.; Wang, D.; Qin, G.; Ping, Y.; Qiao, Y.; Chen, X.; et al. Chloroquine inhibits stemness of esophageal squamous cell carcinoma cells through targeting CXCR4-STAT3 pathway. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Merrell, M.A.; Ilvesaro, J.M.; Lehtonen, N.; Sorsa, T.; Gehrs, B.; Rosenthal, E.; Chen, D.; Shackley, B.; Harris, K.W.; Selander, K.S. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol. Cancer Res. 2006, 4, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, A.; Cherryholmes, G.; Schroeder, A.; Phallen, J.; Alizadeh, D.; Xin, H.; Wang, T.; Lee, H.; Lahtz, C.; Swiderski, P.; et al. TLR9 is critical for glioma stem cell maintenance and targeting. Cancer Res. 2014, 74, 5218–5228. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Qiang, L.; Sample, A.; Shah, P.; He, Y.-Y. NF-ΚB signaling activation induced by chloroquine requires autophagosome, P62 protein, and c-jun N-terminal kinase (JNK) signaling and promotes tumor cell resistance. J. Biol. Chem. 2017, 292, 3379–3388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Y.; Li, Y.; Li, R.; Ma, Y.; Wang, H.; Wang, Y. Chloroquine inhibits MGC803 gastric cancer cell migration via the Toll-like Receptor 9/Nuclear Factor Kappa B signaling pathway. Mol. Med. Rep. 2015, 11, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Korimová, A.; Klusáková, I.; Hradilová-Svíženská, I.; Kohoutková, M.; Joukal, M.; Dubový, P. Mitochondrial damage-associated molecular patterns of injured axons induce outgrowth of Schwann cell processes. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Habbel, P.; Derkow, K.; Krüger, C.; Franzoni, E.; Wulczyn, F.G.; Bereswill, S.; Nitsch, R.; Schott, E.; Veh, R.; et al. Expression of Toll-like receptors in the developing brain. PLoS ONE 2012, 7, e37767. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.B.; Coppieters, M.W.; Ruitenberg, M.J.; McLachlan, E.M. Local and remote immune-mediated inflammation after mild peripheral nerve compression in rats. J. Neuropathol. Exp. Neurol. 2013, 72, 662–680. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, L.; Demartin, C. Buffered picric acid-formaldehyde—A new rapid fixative for electron microscopy. J. Cell. Biol. 1967, 35, A148. [Google Scholar]
- Swett, J.E.; Torigoe, Y.; Elie, V.R.; Bourassa, C.M.; Miller, P.G. Sensory neurons of the rat sciatic nerve. Exp. Neurol. 1991, 114, 82–103. [Google Scholar] [CrossRef]
- Hanani, M. Satellite glial cells in sensory ganglia: From form to function. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef]
- Poteryaev, D.; Datta, S.; Ackema, K.; Zerial, M.; Spang, A. Identification of the switch in early-to-late endosome transition. Cell 2010, 141, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Girard, E.; Chmiest, D.; Fournier, N.; Johannes, L.; Paul, J.-L.; Vedie, B.; Lamaze, C. Rab7 is functionally required for selective cargo sorting at the early endosome. Traffic 2014, 15, 309–326. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, G.; Nicolino, S.; Raimondo, S.; Tos, P.; Battiston, B.; Papalia, I.; Varejão, A.S.P.; Giacobini-Robecchi, M.G.; Perroteau, I.; Geuna, S. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J. Neurosci. Meth. 2009, 179, 51–57. [Google Scholar] [CrossRef]
- Hylden, J.L.K.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Abe, N.; Borson, S.H.; Gambello, M.J.; Wang, F.; Cavalli, V. Mammalian Target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J. Biol. Chem. 2010, 285, 28034–28043. [Google Scholar] [CrossRef] [Green Version]
| Naive | Cervical DRG | Lumbar DRG | ||
| 117.2 ± 20.7 | 127.9 + 18.2 | |||
| Ipsi | Contra | Ipsi | Contra | |
| Sham | 128.3 ± 16.8 | 125.3 ± 12.6 | 139.5 ± 16.2 | 134.5 ± 17.9 |
| SNC | 205.8 ± 14.6 ** | 197.2 ± 20.3 ** | 209.3 ± 19.3 ** | 199.7 ± 15.4 ** |
| CSNT | 206.5 ± 20.2 ** | 198.6 ± 21.3 ** | 207.9 ± 20.4 ** | 190.6 ± 17.4 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubový, P.; Hradilová-Svíženská, I.; Brázda, V.; Joukal, M. Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion. Int. J. Mol. Sci. 2021, 22, 7446. https://doi.org/10.3390/ijms22147446
Dubový P, Hradilová-Svíženská I, Brázda V, Joukal M. Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion. International Journal of Molecular Sciences. 2021; 22(14):7446. https://doi.org/10.3390/ijms22147446
Chicago/Turabian StyleDubový, Petr, Ivana Hradilová-Svíženská, Václav Brázda, and Marek Joukal. 2021. "Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion" International Journal of Molecular Sciences 22, no. 14: 7446. https://doi.org/10.3390/ijms22147446
APA StyleDubový, P., Hradilová-Svíženská, I., Brázda, V., & Joukal, M. (2021). Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion. International Journal of Molecular Sciences, 22(14), 7446. https://doi.org/10.3390/ijms22147446







