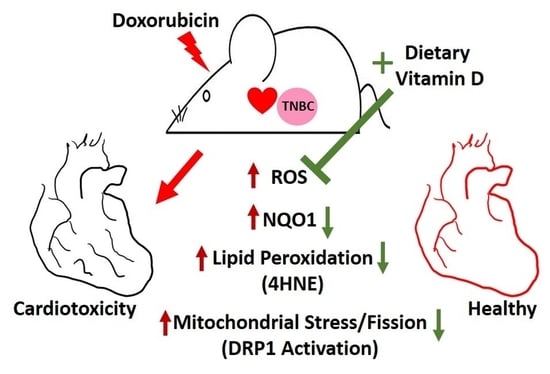
Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer
Abstract
:1. Introduction
2. Results
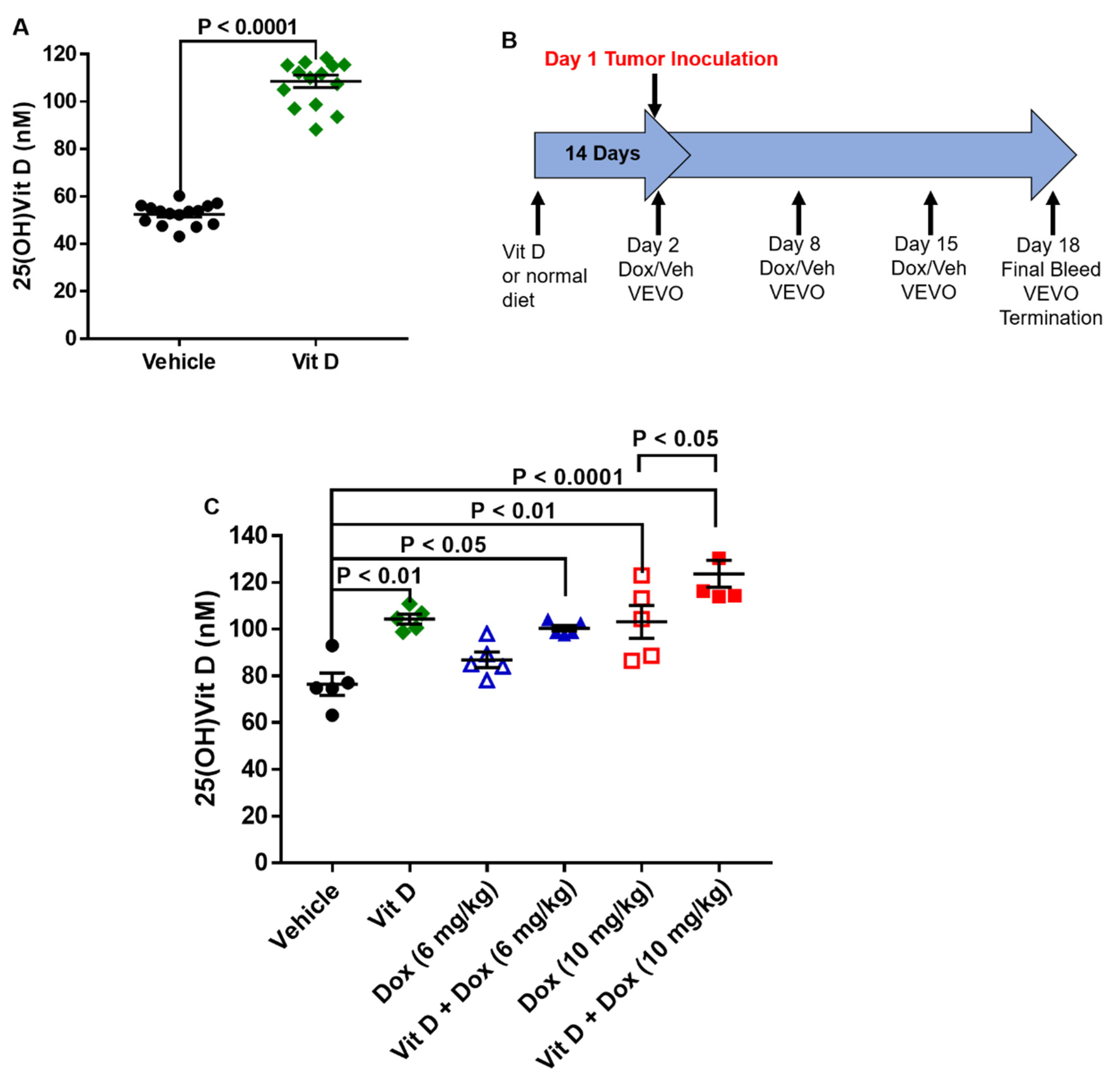
2.1. Dietary Vitamin D Supplementation
2.2. Doxorubicin-Induced Cardiotoxicity in a Triple Negative Breast Cancer Model
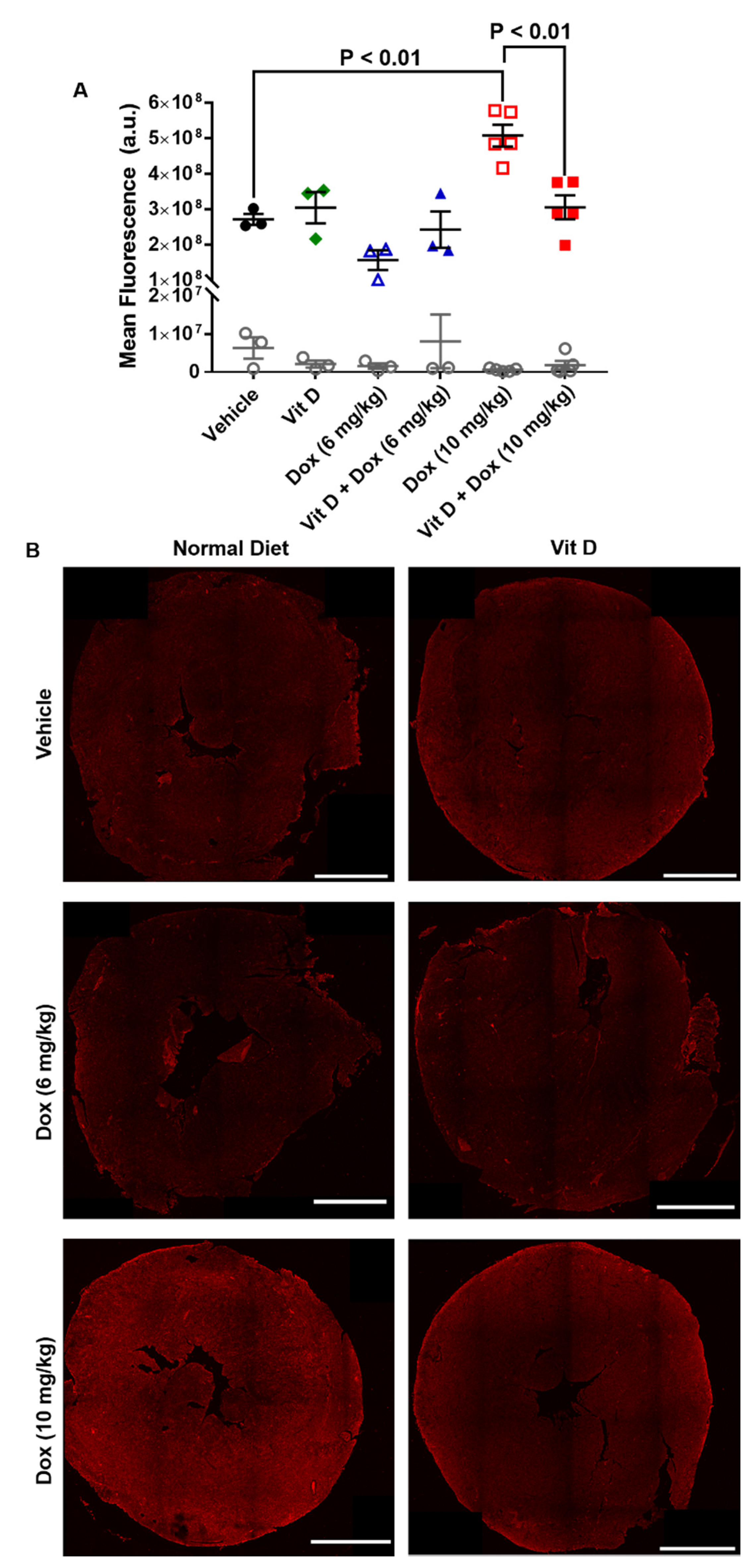
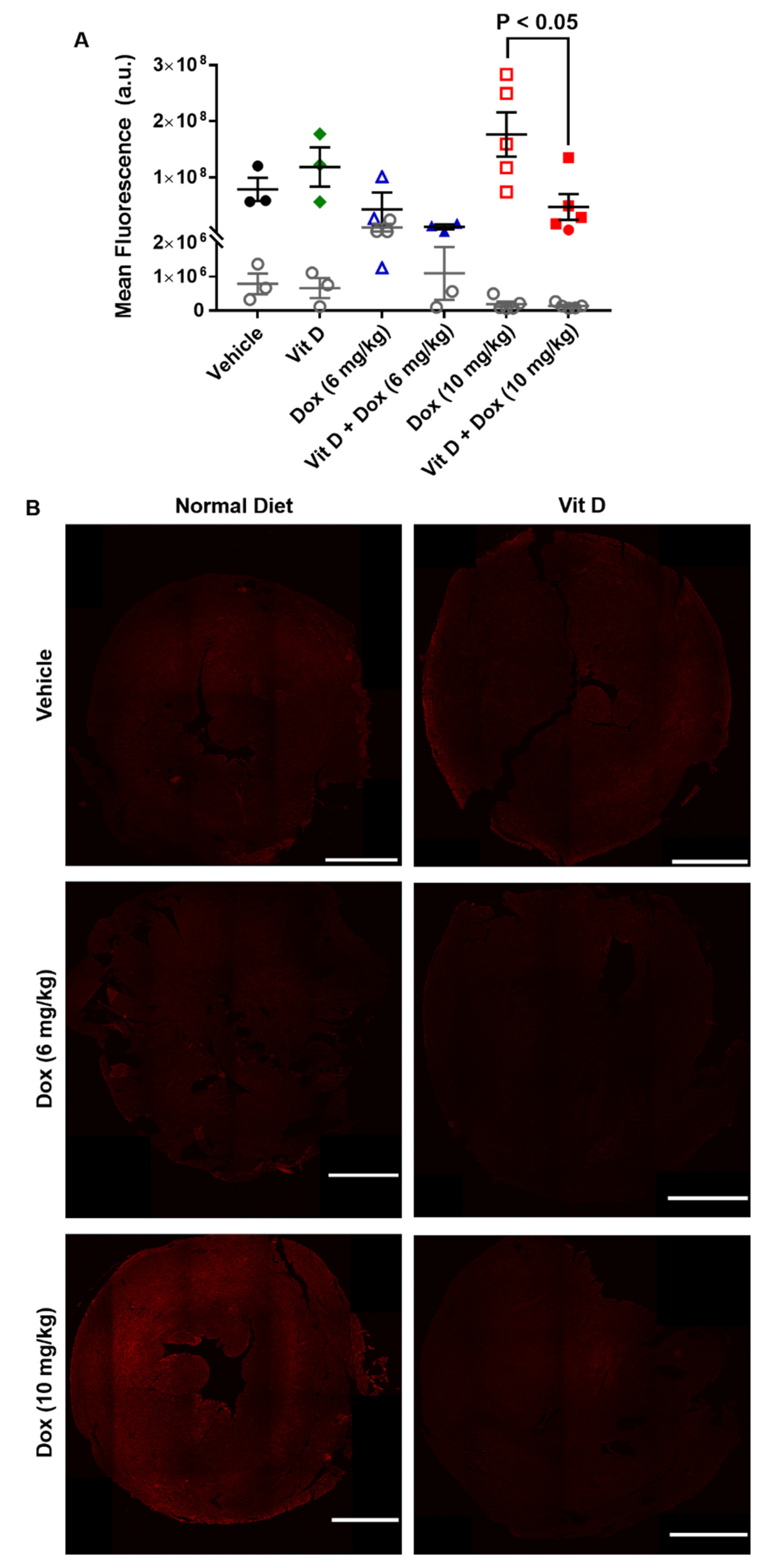
2.3. Dox-Induced Generation of Reactive Oxygen Species In Vivo
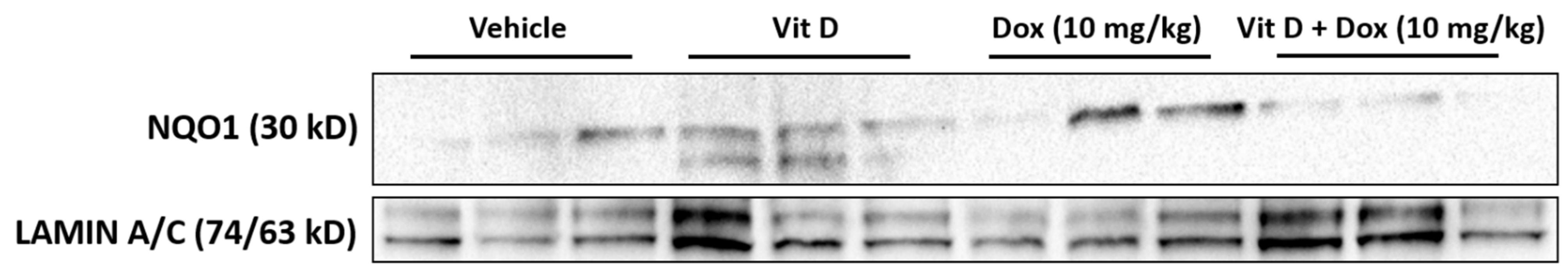
2.4. MYC Expression from Exposure to Doxorubicin
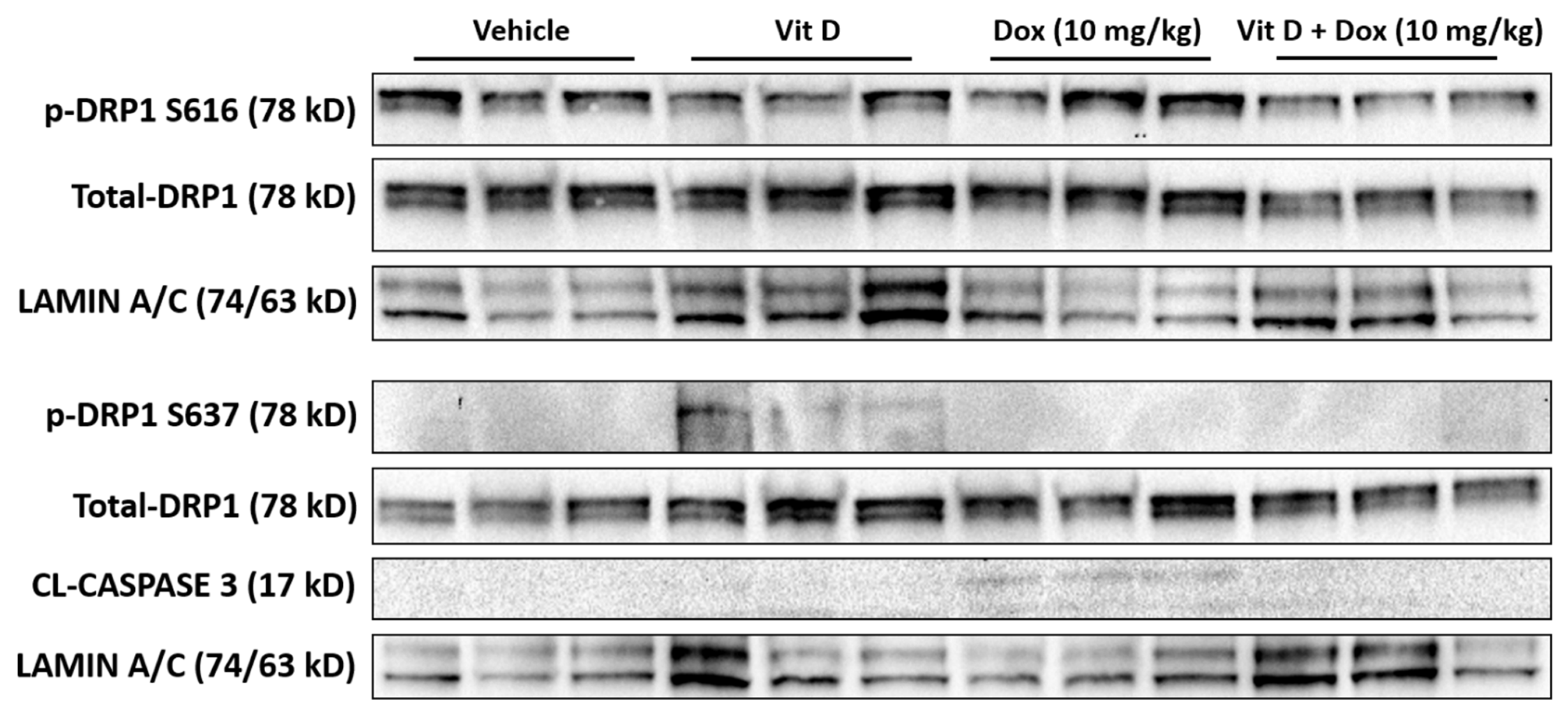
2.5. Doxorubicin-Induced Mitochondrial Dysfunction
2.6. Effects of Vitamin D Supplementation on Doxorubicin Treatment of TNBC Tumors
3. Discussion
4. Methods
4.1. Animal Care and Welfare
4.2. Dietary Vitamin D Supplementation
4.3. Blood Collection
4.4. Vitamin D Measurement
4.5. Tumor Establishment and Doxorubicin Treatment In Vivo
4.6. Transthoracic Echocardiography
4.7. Immunohistochemistry
4.8. Immunoblot
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin Cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Coppola, C.; Rienzo, A.; Piscopo, G.; Barbieri, A.; Arra, C.; Maurea, N. Management of QT Prolongation Induced by Anti-Cancer Drugs: Target Therapy and Old Agents. Different Algorithms for Different Drugs. Cancer Treat. Rev. 2018, 63, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, P.-Y.; Lu, Y.-S.; Ou, D.-L.; Cheng, A.-L. IκB Kinases Increase Myc Protein Stability and Enhance Progression of Breast Cancer Cells. Mol. Cancer 2011, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafa, O.; Wade, M.; Kern, S.; Beeche, M.; Pandita, T.K.; Hampton, G.M.; Wahl, G.M. C-Myc Can Induce DNA Damage, Increase Reactive Oxygen Species, and Mitigate P53 Function: A Mechanism for Oncogene-Induced Genetic Instability. Mol. Cell 2002, 9, 1031–1044. [Google Scholar] [CrossRef]
- Gupta, N.; Jung, K.; Wu, C.; Alshareef, A.; Alqahtani, H.; Damaraju, S.; Mackey, J.R.; Ghosh, S.; Sabri, S.; Abdulkarim, B.S.; et al. High Myc Expression and Transcription Activity Underlies Intra-Tumoral Heterogeneity in Triple-Negative Breast Cancer. Oncotarget 2017, 8, 28101–28115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuta, E.; Yan, L.; Takeshita, T.; McDonald, K.-A.; Dasgupta, S.; Opyrchal, M.; Takabe, K. High MYC MRNA Expression Is More Clinically Relevant than MYC DNA Amplification in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2019, 21, 217. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.; Franco, V.I.; Lipshultz, S.E. Anthracycline-Induced Cardiotoxicity: A Review of Pathophysiology, Diagnosis, and Treatment. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 315. [Google Scholar] [CrossRef] [PubMed]
- Pareja, F.; Geyer, F.C.; Marchiò, C.; Burke, K.A.; Weigelt, B.; Reis-Filho, J.S. Triple-Negative Breast Cancer: The Importance of Molecular and Histologic Subtyping, and Recognition of Low-Grade Variants. NPJ Breast Cancer 2016, 2, 16036. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the Systemic Treatment of Triple-Negative Breast Cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-Negative Breast Cancer: Is There a Treatment on the Horizon? Oncotarget 2016, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arola, O.J.; Saraste, A.; Pulkki, K.; Kallajoki, M.; Parvinen, M.; Voipio-Pulkki, L.-M. Acute Doxorubicin Cardiotoxicity Involves Cardiomyocyte Apoptosis. Cancer Res. 2000, 60, 1789–1792. [Google Scholar]
- Fernandez-Chas, M.; Curtis, M.J.; Niederer, S.A. Mechanism of Doxorubicin Cardiotoxicity Evaluated by Integrating Multiple Molecular Effects into a Biophysical Model. Br. J. Pharmacol. 2018, 175, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as Precision Weapons in War against Cancer Chemotherapy Induced Toxicity—Exploring the Armoury of Obscurity. Saudi Pharm. J. SPJ 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D Activates the Nrf2-Keap1 Antioxidant Pathway and Ameliorates Nephropathy in Diabetic Rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Rohan, J.N.P.; Weigel, N.L. 1α,25-Dihydroxyvitamin D3 Reduces c-Myc Expression, Inhibiting Proliferation and Causing G1 Accumulation in C4-2 Prostate Cancer Cells. Endocrinology 2009, 150, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.-M.; Shin, E.-A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef] [Green Version]
- Jones, G. Pharmacokinetics of Vitamin D Toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [Green Version]
- Shah, I.; Akhtar, M.K.; Hisaindee, S.; Rauf, M.A.; Sadig, M.; Ashraf, S.S. Clinical Diagnostic Tools for Vitamin D Assessment. J. Steroid Biochem. Mol. Biol. 2018, 180, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Breysse, D.H.; Boone, R.M.; Long, C.M.; Merrill, M.E.; Schaupp, C.M.; White, C.C.; Kavanagh, T.J.; Schmidt, E.E.; Merrill, G.F. Carbonyl Reductase 1 Plays a Significant Role in Converting Doxorubicin to Cardiotoxic Doxorubicinol in Mouse Liver, but the Majority of the Doxorubicinol-Forming Activity Remains Unidentified. Drug Metab. Dispos. 2020, 48, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of Lipid Peroxidation Derived 4-Hydroxynonenal (4-HNE) in Cancer: Focusing on Mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell Death and Diseases Related to Oxidative Stress:4-Hydroxynonenal (HNE) in the Balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Sharma, R.; McElhanon, K.; Allen, C.D.; Megyesi, J.K.; Beneš, H.; Singh, S.P. Sulforaphane Protects the Heart from Doxorubicin-Induced Toxicity. Free Radic. Biol. Med. 2015, 86, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Tadokoro, T.; Ikeda, M.; Ide, T.; Deguchi, H.; Ikeda, S.; Okabe, K.; Ishikita, A.; Matsushima, S.; Koumura, T.; Yamada, K.; et al. Mitochondria-Dependent Ferroptosis Plays a Pivotal Role in Doxorubicin Cardiotoxicity. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and Its Potential Role as a Redox Sensitive Molecular Switch. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Morrissy, S.; Strom, J.; Purdom-Dickinson, S.; Chen, Q.M. NAD(P)H: Quinone Oxidoreductase 1 Is Induced by Progesterone in Cardiomyocytes. Cardiovasc. Toxicol. 2012, 12, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Benassi, B.; Fanciulli, M.; Fiorentino, F.; Porrello, A.; Chiorino, G.; Loda, M.; Zupi, G.; Biroccio, A. C-Myc Phosphorylation Is Required for Cellular Response to Oxidative Stress. Mol. Cell 2006, 21, 509–519. [Google Scholar] [CrossRef]
- Gabay, M.; Li, Y.; Felsher, D.W. MYC Activation Is a Hallmark of Cancer Initiation and Maintenance. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Larriba, M.J.; Ordóñez-Morán, P.; Chicote, I.; Martín-Fernández, G.; Puig, I.; Muñoz, A.; Pálmer, H.G. Vitamin D Receptor Deficiency Enhances Wnt/β-Catenin Signaling and Tumor Burden in Colon Cancer. PLoS ONE 2011, 6, e23524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi-Tabar, R.; Nguyen-Yamamoto, L.; Tavera-Mendoza, L.E.; Quail, T.; Dimitrov, V.; An, B.-S.; Glass, L.; Goltzman, D.; White, J.H. Vitamin D Receptor as a Master Regulator of the C-MYC/MXD1 Network. Proc. Natl. Acad. Sci. USA 2012, 109, 18827–18832. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, M.P.; Weiner, A.; Kaminaris, A.; Li, C.; Cai, F.; Zhao, F.; Kobayashi, S.; Kobayashi, T.; Huang, Y.; Sesaki, H.; et al. Doxorubicin-Induced Cardiomyocyte Death Is Mediated by Unchecked Mitochondrial Fission and Mitophagy. FASEB J. 2019, 33, 11096–11108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jiang, H.; Chen, S.; Du, F.; Wang, X. The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways. Cell 2012, 148, 228–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, A.-R.; Hyun, H.-W.; Min, S.-J.; Kim, J.-E. The Differential DRP1 Phosphorylation and Mitochondrial Dynamics in the Regional Specific Astroglial Death Induced by Status Epilepticus. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeekpudsa, P.; Kukongviriyapan, V.; Senggunprai, L.; Sripa, B.; Prawan, A. Suppression of NAD(P)H-Quinone Oxidoreductase 1 Enhanced the Susceptibility of Cholangiocarcinoma Cells to Chemotherapeutic Agents. J. Exp. Clin. Cancer Res. 2014, 33, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quagliariello, V.; Coppola, C.; Mita, D.G.; Piscopo, G.; Iaffaioli, R.V.; Botti, G.; Maurea, N. Low Doses of Bisphenol A Have Pro-Inflammatory and pro-Oxidant Effects, Stimulate Lipid Peroxidation and Increase the Cardiotoxicity of Doxorubicin in Cardiomyoblasts. Environ. Toxicol. Pharmacol. 2019, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rainville, C.; Khan, Y.; Tisman, G. Triple Negative Breast Cancer Patients Presenting with Low Serum Vitamin D Levels: A Case Series. Cases J. 2009, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; He, L.; Wang, Z.; Dong, W.; Sun, W.; Qin, Y.; Zhang, P.; Zhang, H. Calcitriol Enhances Doxorubicin-Induced Apoptosis in Papillary Thyroid Carcinoma Cells via Regulating VDR/PTPN2/p-STAT3 Pathway. J. Cell. Mol. Med. 2020, 24, 5629–5639. [Google Scholar] [CrossRef] [Green Version]
- Maayah, Z.H.; Zhang, T.; Forrest, M.L.; Alrushaid, S.; Doschak, M.R.; Davies, N.M.; El-Kadi, A.O.S. DOX-Vit D, a Novel Doxorubicin Delivery Approach, Inhibits Human Osteosarcoma Cell Proliferation by Inducing Apoptosis While Inhibiting Akt and MTOR Signaling Pathways. Pharmaceutics 2018, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaly, S.; Kaakoush, N.O.; Lloyd, F.; McGonigle, T.; Mok, D.; Baird, A.; Klopcic, B.; Gordon, L.; Gorman, S.; Forest, C.; et al. High Dose Vitamin D Supplementation Alters Faecal Microbiome and Predisposes Mice to More Severe Colitis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Halade, G.V.; Kain, V.; Ingle, K.A. Heart Functional and Structural Compendium of Cardiosplenic and Cardiorenal Networks in Acute and Chronic Heart Failure Pathology. Am. J. Physiol. Heart Circ. Physiol. 2017, 314, H255–H267. [Google Scholar] [CrossRef]
- Respress, J.L.; Wehrens, X.H.T. Transthoracic Echocardiography in Mice. J. Vis. Exp. JoVE 2010. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Mann, E.; da Silva, L.M.; Scalici, J.; Gassman, N.R. DNA Damage Measurements within Tissue Samples with Repair Assisted Damage Detection (RADD). Curr. Res. Biotechnol. 2019, 1, 78–86. [Google Scholar] [CrossRef]
- Sonavane, M.; Sykora, P.; Andrews, J.F.; Sobol, R.W.; Gassman, N.R. Camptothecin Efficacy to Poison Top1 Is Altered by Bisphenol A in Mouse Embryonic Fibroblasts. Chem. Res. Toxicol. 2018, 31, 510–519. [Google Scholar] [CrossRef]
- Lee, K.J.; Piett, C.G.; Andrews, J.F.; Mann, E.; Nagel, Z.D.; Gassman, N.R. Defective Base Excision Repair in the Response to DNA Damaging Agents in Triple Negative Breast Cancer. PLoS ONE 2019, 14, e0223725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
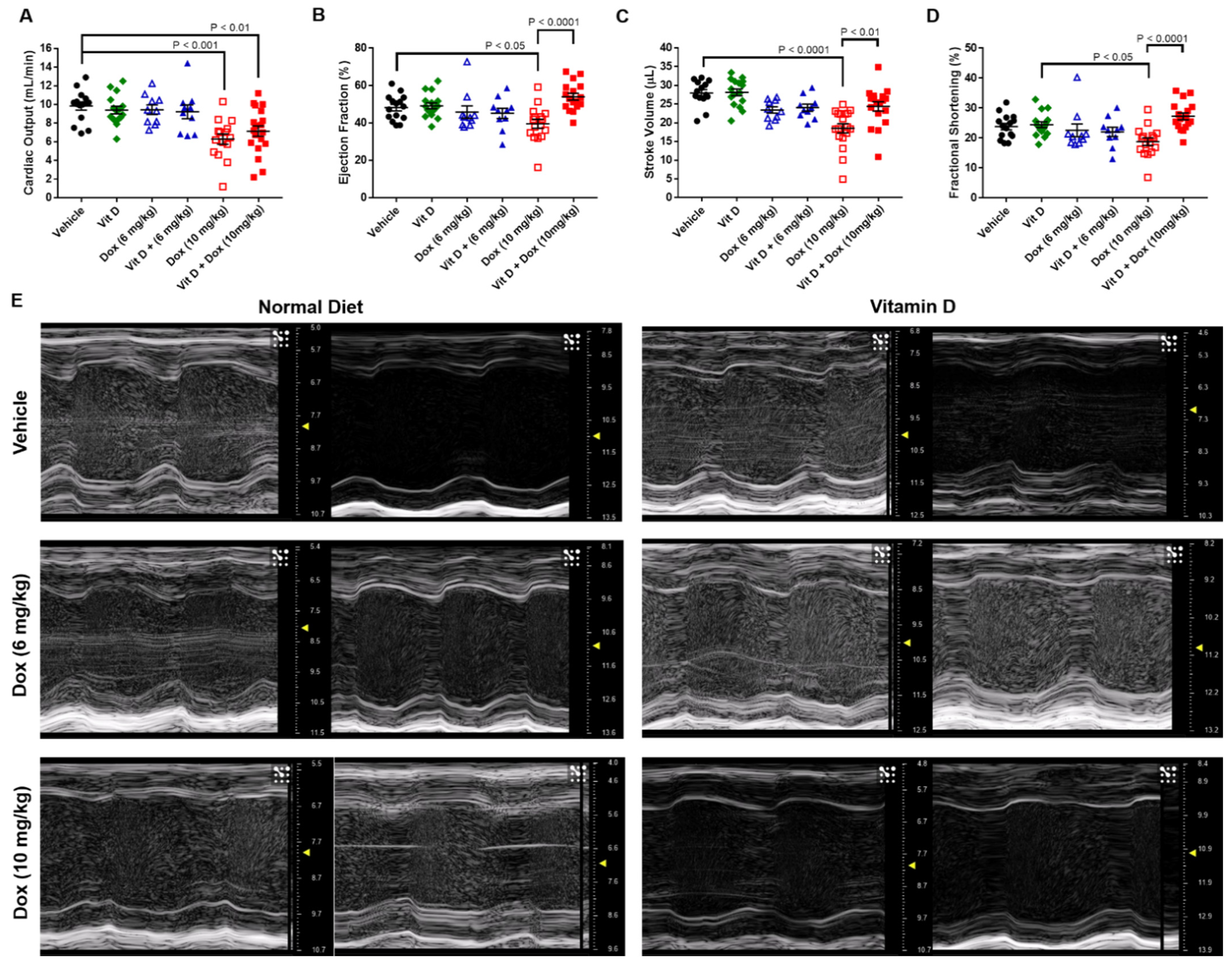

| CO (mL/min) | EF (%) | SV (µL) | FS (%) | |
|---|---|---|---|---|
| Vehicle | 9.8 ± 0.4 | 48.2 ± 1.8 | 27.9 ± 0.9 | 23.9 ± 1.1 |
| Vitamin D | 9.4 ± 0.4 | 49.2 ± 1.6 | 28.1 ± 0.9 | 24.5 ± 1.0 |
| Dox (6 mg/kg) | 9.4 ± 0.5 | 45.9 ± 3.3 | 23.4 ± 0.8 | 22.6 ± 2.1 |
| Vit D + Dox (6 mg/kg) | 9.2 ± 0.7 | 45.2 ± 2.7 | 24.0 ± 1.0 | 22.1 ± 1.6 |
| Dox (10 mg/kg) | 6.3 ± 0.6 *** | 39.6 ± 2.4 * | 18.5 ± 1.2 **** | 18.8 ± 1.3 & |
| Vit D + Dox (10 mg/kg) | 7.1 ± 0.6 ** | 54.0 ± 1.8 #### | 28.1 ± 0.9 ## | 27.3 ± 1.2 #### |
| Treatment | Tumor Volume Day 18 (mm3) |
|---|---|
| Vehicle | 1626 ± 166 |
| Vitamin D | 1244 ± 163 |
| Dox (6 mg/kg) | 1161 ± 175 |
| Vit D + Dox (6 mg/kg) | 771 ± 118 ** |
| Dox (10 mg/kg) | 415 ± 90 *** & |
| Vit D + Dox (10 mg/kg) | 687 ± 125 ** |
| Vehicle | Vitamin D Supplemented | |
|---|---|---|
| Protein (%) | 19.0 | 19.0 |
| Fat (%) | 9.0 | 9.0 |
| Vitamin D3 (Total IU/kg) | 1500 | 11,500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.J.; Wright, G.; Bryant, H.; Wiggins, L.A.; Dal Zotto, V.L.; Schuler, M.; Malozzi, C.; Cohen, M.V.; Gassman, N.R. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7439. https://doi.org/10.3390/ijms22147439
Lee KJ, Wright G, Bryant H, Wiggins LA, Dal Zotto VL, Schuler M, Malozzi C, Cohen MV, Gassman NR. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. International Journal of Molecular Sciences. 2021; 22(14):7439. https://doi.org/10.3390/ijms22147439
Chicago/Turabian StyleLee, Kevin J, Griffin Wright, Hannah Bryant, Leigh Ann Wiggins, Valeria L. Dal Zotto, Michele Schuler, Christopher Malozzi, Michael V Cohen, and Natalie R Gassman. 2021. "Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer" International Journal of Molecular Sciences 22, no. 14: 7439. https://doi.org/10.3390/ijms22147439
APA StyleLee, K. J., Wright, G., Bryant, H., Wiggins, L. A., Dal Zotto, V. L., Schuler, M., Malozzi, C., Cohen, M. V., & Gassman, N. R. (2021). Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. International Journal of Molecular Sciences, 22(14), 7439. https://doi.org/10.3390/ijms22147439