Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogen Nuclear Magnetic Resonance Spectroscopy
2.2. Fourier Transform Infrared Spectroscopy
2.3. Size Exclusion Chromatography
2.4. Thermogravimetric Analysis
2.5. Differential Scanning Calorimetry
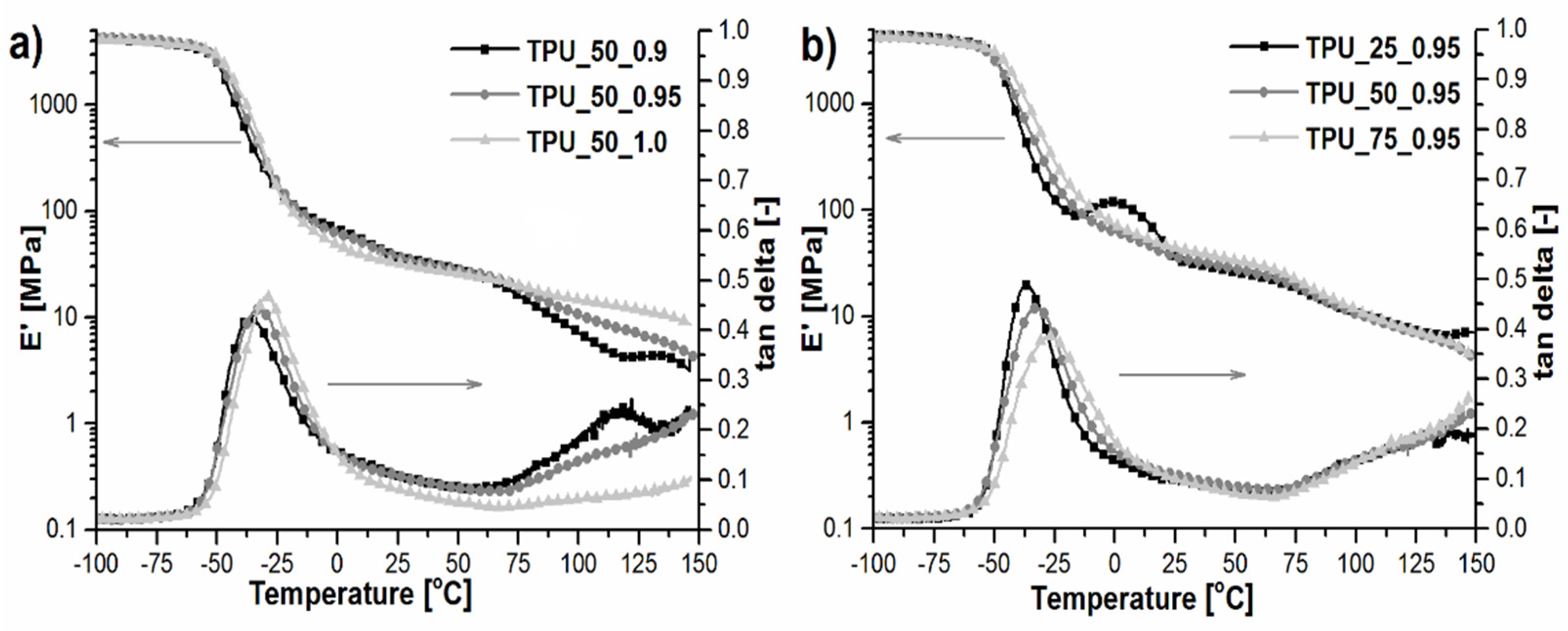
2.6. Dynamic Mechanical and Thermal Analysis
2.7. The Mechanical Properties
2.8. Melt Flow Index
3. Materials and Methods
3.1. Materials
- The bio-based poly(trimethylene glycol) (PO3G 1000) (Mn = 1000 g/mol, hydroxyl number 125–102 mgKOH/g, acid value <0.05 mgKOH/g, viscosity (at 40 °C) 200–300 mPa·s) was provided by Allesa (Frankfurt, Germany).
- The bio-based poly(trimethylene glycol) (PO3G 2700) (Mn = 2700 g/mol, hydroxyl number 43.2–40.1 mgKOH/g, acid value <0.05 mgKOH/g, viscosity (at 40 °C) 1450–1850 mPa·s) was provided by Allesa (Frankfurt, Germany).
- 4,4′-diphenylmethane diisocyanate (MDI) (NCO content: 33.5%, purity 99.5%) was purchased from Borsod Chem (Budapest, Hungary).
- The bio-based glycols: 1,4-butanediol (bio-BDO) (purity 99.8%)
- The catalyst 1,4-diacabicyclo [2.2.2]octane (DABCO) was purchased from Sigma-Aldrich (Warsaw, Poland).
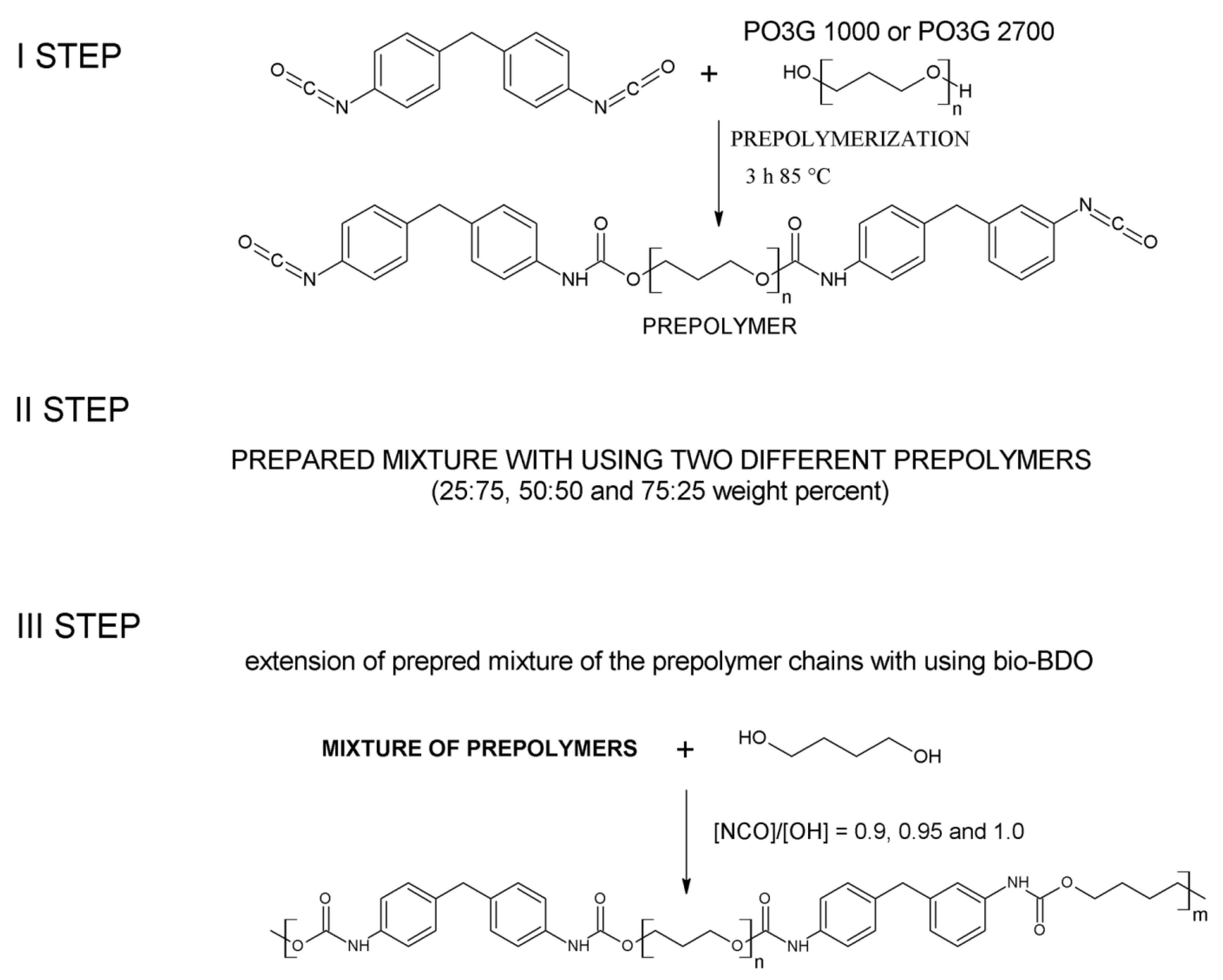
3.2. Preparation of Thermoplastic Polyurethane Elastomers
3.3. Characterization of Thermoplastic Polyurethane Elastomers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, J.; Kasprzyk, P. Thermoplastic Polyurethanes Derived From Petrochemical or Renewable Resources: A Comprehensive Review. Polym. Eng. Sci. 2017. [Google Scholar] [CrossRef] [Green Version]
- Kasprzyk, P.; Datta, J. Novel Bio-Based Thermoplastic Poly(Ether-Urethane)s. Correlations between the Structure, Processing and Properties. Polymer 2019. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Milić, J.; Zhang, F.; Ilavsky, J. Fast-Responding Bio-Based Shape Memory Thermoplastic Polyurethanes. Polymer 2017, 121, 26–37. [Google Scholar] [CrossRef]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of Bio-Based Components on the Chemical Structure, Thermal Stability and Mechanical Properties of Green Thermoplastic Polyurethane Elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, R.; Xiao, Q.; Guo, H.; Wang, Z.; Li, X.; Chen, J.; Zhu, J. Asynchronous Fracture of Hierarchical Microstructures in Hard Domain of Thermoplastic Polyurethane Elastomer: Effect of Chain Extender. Polymer 2018, 138, 242–254. [Google Scholar] [CrossRef]
- Eceiza, A.; Martin, M.D.; De La Caba, K.; Kortaberria, G.; Gabilondo, N.; Corcuera, M.A.; Mondragon, I. Thermoplastic Polyurethane Elastomers Based on Polycarbonate Diols With Different Soft Segment Molecular Weight and Chemical Structure: Mechanical and Thermal Properties. Polym. Eng. Sci. 2008. [Google Scholar] [CrossRef]
- Powers, D.S.; Vaia, R.A.; Koerner, H.; Serres, J.; Mirau, P.A. NMR Characterization of Low Hard Segment Thermoplastic Polyurethane/Carbon Nanofiber Composites. Macromolecules 2008, 41, 4290–4295. [Google Scholar] [CrossRef]
- Zhao, X.; Shou, T.; Liang, R.; Hu, S.; Yu, P.; Zhang, L. Bio-Based Thermoplastic Polyurethane Derived from Polylactic Acid with High-Damping Performance. Ind. Crop. Prod. 2020, 154, 112619. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable Natural Resources as Green Alternative Substrates to Obtain Bio-Based Non-Isocyanate Polyurethanes-Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Parcheta, P.; Datta, J. Environmental Impact and Industrial Development of Biorenewable Resources for Polyurethanes. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1986–2016. [Google Scholar] [CrossRef]
- Głowińska, E.; Kasprzyk, P.; Datta, J. The Green Approach to the Synthesis of Bio-Based Thermoplastic Polyurethane Elastomers with Partially Bio-Based Hard Blocks. Materials 2021, 14, 2334. [Google Scholar] [CrossRef] [PubMed]
- Głowińska, E.; Wolak, W.; Datta, J. Eco-Friendly Route for Thermoplastic Polyurethane Elastomers with Bio-Based Hard Segments Composed of Bio-Glycol and Mixtures of Aromatic–Aliphatic and Aliphatic–Aliphatic Diisocyanate. J. Polym. Environ. 2021. [Google Scholar] [CrossRef] [PubMed]
- Głowińska, E.; Parcheta, P.; Kasprzyk, P.; Janusz, D. Book chapter: Polyisocyanates from Sustainable Resources. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Publications: Washington, DC, USA, 2021. [Google Scholar]
- Kasprzyk, P.; Datta, J. Effect of Molar Ratio [NCO]/[OH] Groups during Prepolymer Chains Extending Step on the Morphology and Selected Mechanical Properties of Final Bio-Based Thermoplastic Poly ( Ether-Urethane ) Materials. Polym. Eng. Sci. 2018. [Google Scholar] [CrossRef]
- Zeng, B.; Li, Y.; Wang, L.; Zheng, Y.; Shen, J.; Guo, S. Body Temperature-Triggered Shape-Memory Effect via Toughening Sustainable Poly(Propylene Carbonate) with Thermoplastic Polyurethane: Toward Potential Application of Biomedical Stents. ACS Sustain. Chem. Eng. 2020, 8, 1538–1547. [Google Scholar] [CrossRef]
- Blache, H.; Méchin, F.; Rousseau, A.; Fleury, É.; Pascault, J.P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New Bio-Based Thermoplastic Polyurethane Elastomers from Isosorbide and Rapeseed Oil Derivatives. Ind. Crop. Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Ginzburg, V.V.; Schiller, K.S.; Wendt, B.L. Design, Polymerization, and Properties of High Performance Thermoplastic Polyurethane Elastomers from Seed-Oil Derived Soft Segments. Polymer 2013, 54, 1350–1360. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E. Effect of Hydroxylated Soybean Oil and Bio-Based Propanediol on the Structure and Thermal Properties of Synthesized Bio-Polyurethanes. Ind. Crop. Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Ghosal, K.; Bhattacharjee, U.; Sarkar, K. Facile Green Synthesis of Bioresorbable Polyester from Soybean Oil and Recycled Plastic Waste for Osteochondral Tissue Regeneration. Eur. Polym. J. 2019, 2, 109338. [Google Scholar] [CrossRef]
- Tawade, B.V.; Shingte, R.D.; Kuhire, S.; Sadavarte, N.; Garg, K.; Maher, D.; Ichake, A.; More, A.S.; Wadgaonkar, P.P. Bio-Based Di/ Polyisocyanates for Polyurethanes: An Overview. Polyurethanes Today 2017, 41–46. [Google Scholar] [CrossRef]
- Available online: https://www.weylchem.com (accessed on 15 January 2021).
- Pattamaprom, C.; Wu, C.H.; Chen, P.H.; Huang, Y.L.; Ranganathan, P.; Rwei, S.P.; Chuan, F.S. Solvent-Free One-Shot Synthesis of Thermoplastic Polyurethane Based on Bio-Poly(1,3-Propylene Succinate) Glycol with Temperature-Sensitive Shape Memory Behavior. ACS Omega 2020, 5, 4058–4066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk, P.; Sadowska, E.; Datta, J. Investigation of Thermoplastic Polyurethanes Synthesized via Two Different Prepolymers. J. Polym. Environ. 2019. [Google Scholar] [CrossRef] [Green Version]
- Kasprzyk, P.; Benes, H.; Keitel, R.; Datta, J. The Role of Hydrogen Bonding on Tuning Hard-Soft Segments in Bio- Based Thermoplastic Poly (Ether-Urethane) s. J. Clean. Prod. 2020, 274, 122678. [Google Scholar] [CrossRef]
- Tang, Q.; Gao, K. Structure Analysis of Polyether-Based Thermoplastic Polyurethane Elastomers by FTIR, 1H NMR and 13C NMR. Int. J. Polym. Anal. Charact. 2017, 22, 569–574. [Google Scholar] [CrossRef]
- Saralegi, A.; Rueda, L.; Fernández-D’Arlas, B.; Mondragon, I.; Eceiza, A.; Corcuera, M.A. Thermoplastic Polyurethanes from Renewable Resources: Effect of Soft Segment Chemical Structure and Molecular Weight on Morphology and Final Properties. Polym. Int. 2013, 62, 106–115. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-Change and Yellowing of Polyurethane as a Result of UV Irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J.; Rodríguez Romero, J.F.; Simón Herrero, D.; Carmona, M. Novel Cast Polyetherurethanes Based on Dispersed Polymeric Polyols. Polym. Test. 2018, 68. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J.; Parcheta, P. Effect of Sisal Fiber Filler on Thermal Properties of Bio-Based Polyurethane Composites. J. Therm. Anal. Calorim. 2017, 130, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Zajac, M.; Kahl, H.; Schade, B.; Rödel, T.; Dionisio, M.; Beiner, M. Relaxation Behavior of Polyurethane Networks with Different Composition and Crosslinking Density. Polymer 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Saiani, A.; Novak, A.; Rodier, L.; Eeckhaut, G.; Leenslag, J.W.; Higgins, J.S. Origin of Multiple Melting Endotherms in a High Hard Block Content Polyurethane. 1. Thermodynamic Investigation. Macromolecules 2007, 40, 7252–7262. [Google Scholar] [CrossRef]
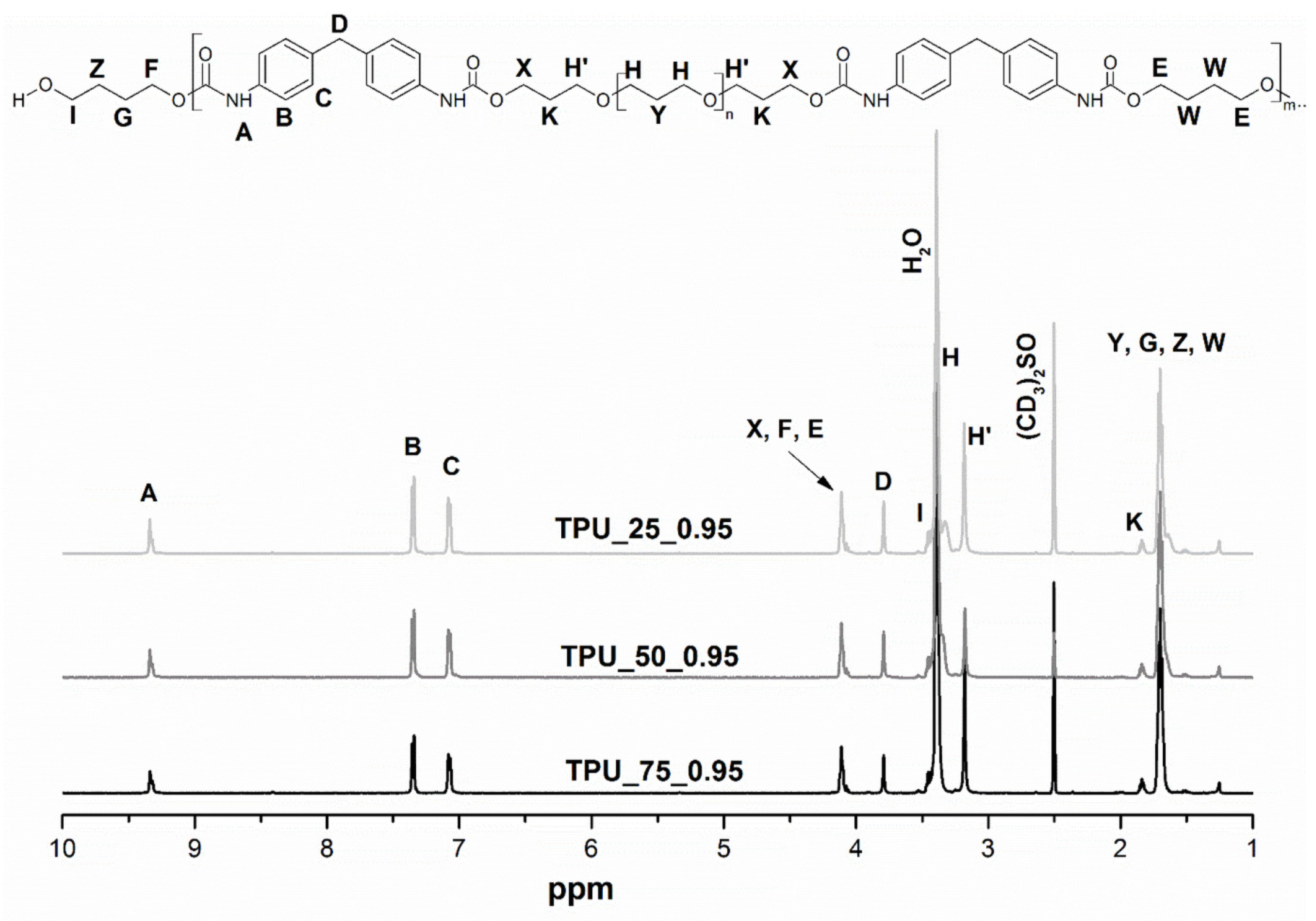

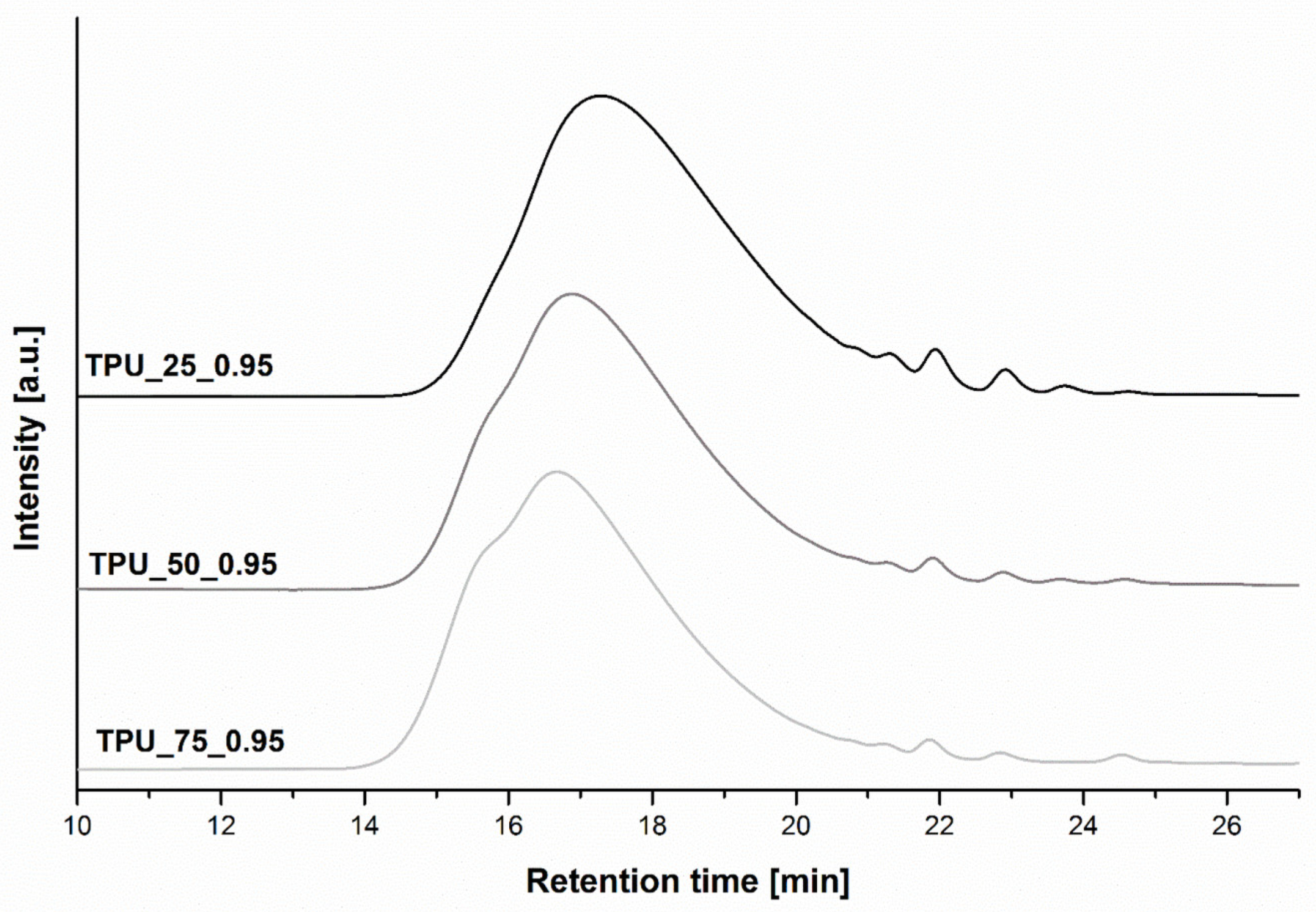


| Sample | [NCO]/[OH] | Mn | Mw | Đ | Retention Time of the Peak (min) |
|---|---|---|---|---|---|
| TPU_25_0.95 | 0.95 | 19,742 | 39,485 | 2.0 | 17.30 |
| TPU_50_0.9 | 0.9 | 22,075 | 41,944 | 1.9 | 17.02 |
| TPU_50_0.95 | 0.95 | 27,535 | 49,563 | 1.8 | 16.88 |
| TPU_50_1.0 | 1.0 | 45,342 | 90,684 | 2.0 | 15.58 |
| TPU_75_0.95 | 0.95 | 28,866 | 54,846 | 1.9 | 16.72 |
| Sample | T5% [°C] | T10% [°C] | T90% [°C] | Char Yield [%] | THS [°C] | DTGHS [%/min] | TSS [°C] | DTGSS [%/min] |
|---|---|---|---|---|---|---|---|---|
| TPU_25_0.95 | 332.1 | 342.1 | 441.2 | 1.77 | 362.8 | −6.5 | 426.2 | −15.4 |
| TPU_50_0.9 | 324.1 | 337.4 | 436.8 | 1.35 | 357.5 | −7.2 | 427.5 | −13.4 |
| TPU_50_0.95 | 326.0 | 338.9 | 436.9 | 1.89 | 354.9 | −7.0 | 427.4 | −13.7 |
| TPU_50_1.0 | 327.3 | 339.2 | 442.1 | 3.42 | 349.9 | −5.9 | 427.4 | −14.0 |
| TPU_75_0.95 | 317.2 | 336.2 | 429.3 | 4.21 | 351.8 | −10.4 | 429.8 | −8.2 |
| Sample | TgSS (°C) | Tc (°C) | ΔHc (J/g) | Total ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Total ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| TPU_25_0.95 | −63.7 | −8.0 123.2 | 3.52 9.12 | 12.64 | 11.30 170.4 182.5 203.2 218.7 | 3.47 9.05 | 12.52 |
| TPU_50_0.9 | −62.2 | −16.8 121.3 | 4.21 8.42 | 12.63 | 8.6 176.2 198.6 | 3.01 9.82 | 12.83 |
| TPU_50_0.95 | −61.3 | −14.1 116.5 | 2.10 8.29 | 10.39 | 10.4 180.7 202.9 | 2.22 8.53 | 10.75 |
| TPU_50_1.0 | −58.6 | 110.2 | 7.21 | 7.21 | 10.1 184.5 | 0.97 6.34 | 7.31 |
| TPU_75_0.95 | −54.9 | 101.8 | 8.90 | 8.90 | 168.7 | 8.57 | 8.57 |
| Sample | TgSS [°C] | tan δmax | ΔT [°C] | S−60–62.5 °C |
|---|---|---|---|---|
| TPU_25_0.95 | −35.8 | 0.49 | 23.3 | 15.6 |
| TPU_50_0.9 | −36.0 | 0.42 | 23.5 | 14.6 |
| TPU_50_0.95 | −32.0 | 0.44 | 24.6 | 16.3 |
| TPU_50_1.0 | −29.0 | 0.47 | 25.4 | 15.7 |
| TPU_75_0.95 | −27.0 | 0.39 | 23.5 | 15.2 |
| Sample | TSb [MPa] | εb [%] | H [°ShD] |
|---|---|---|---|
| TPU_25_0.95 | 13.3 ± 0.8 | 570 ± 12 | 23.1 ± 0.7 |
| TPU_50_0.9 | 8.2 ± 0.8 | 228 ± 13 | 23.1 ± 0.2 |
| TPU_50_0.95 | 17.5 ± 1.3 | 460 ± 21 | 27.5 ± 1.1 |
| TPU_50_1.0 | 24.7 ± 0.9 | 430 ± 19 | 29.8 ± 0.9 |
| TPU_75_0.95 | 24.5 ± 1.2 | 398 ± 10 | 32.3 ± 0.8 |
| Sample | MFR [g/10 min] | MVR [cm3/10 min] |
|---|---|---|
| TPU_25_0.95 | 14.4 ± 0.3 | 13.8 ± 0.2 |
| TPU_50_0.9 | 37.2 ± 0.4 | 36.8 ± 0.3 |
| TPU_50_0.95 | 22.1 ± 0.2 | 21.8 ± 0.3 |
| TPU_50_1.0 | 17.5 ± 0.3 | 17.2 ± 0.1 |
| TPU_75_0.95 | 33.7 ± 0.2 | 33.1 ± 0.1 |
| Sample | PO3G 1000 [wt.%] | PO3G 2700 [wt.%] | [NCO]/[OH] | Monomers Molar Ratio PO3G1000:PO3G2700:MDI:BDO | HS [wt.%] * | SS [wt.%] * | %BIO_BASED CONTENT ** |
|---|---|---|---|---|---|---|---|
| TPU_25_0.95 | 25 | 75 | 0.95 | 1:1.25:7.44:2.50 | 32.27 | 67.73 | 71.18 |
| TPU_50_0.9 | 50 | 50 | 0.9 | 1:0.41:4.00:1.31 | 34.60 | 65.40 | 69.04 |
| TPU_50_0.95 | 50 | 50 | 0.95 | 1:0.41:4.00:1.25 | 34.47 | 65.53 | 68.98 |
| TPU_50_1.0 | 50 | 50 | 1.0 | 1:0.41:4.00:1.19 | 34.36 | 65.64 | 68.92 |
| TPU_75_0.95 | 75 | 25 | 0.95 | 1:0.15:2.94:0.85 | 36.68 | 63.32 | 66.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzyk, P.; Głowińska, E.; Parcheta-Szwindowska, P.; Rohde, K.; Datta, J. Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments. Int. J. Mol. Sci. 2021, 22, 7438. https://doi.org/10.3390/ijms22147438
Kasprzyk P, Głowińska E, Parcheta-Szwindowska P, Rohde K, Datta J. Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments. International Journal of Molecular Sciences. 2021; 22(14):7438. https://doi.org/10.3390/ijms22147438
Chicago/Turabian StyleKasprzyk, Paulina, Ewa Głowińska, Paulina Parcheta-Szwindowska, Kamila Rohde, and Janusz Datta. 2021. "Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments" International Journal of Molecular Sciences 22, no. 14: 7438. https://doi.org/10.3390/ijms22147438
APA StyleKasprzyk, P., Głowińska, E., Parcheta-Szwindowska, P., Rohde, K., & Datta, J. (2021). Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments. International Journal of Molecular Sciences, 22(14), 7438. https://doi.org/10.3390/ijms22147438







