Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets?
Abstract
1. Introduction
2. ROS and Antioxidant Defense
3. Origin of Oxidative Stress in the Peritoneal Cavity
3.1. Erythrocytes and Hemoglobin
3.2. Iron Metabolism in the Pelvic Cavity
3.3. Cellular Damage and Adhesion and Growth of Ectopic Endometrial Tissue
3.4. Heme Oxygenase-1 Detoxification System
4. ROS as Potential Therapeutic Targets for Endometriosis Progression
4.1. Proliferative Phenotype
4.2. Iron Overload
5. Treatment of Chronic Pain by Decreasing Macrophage Activity
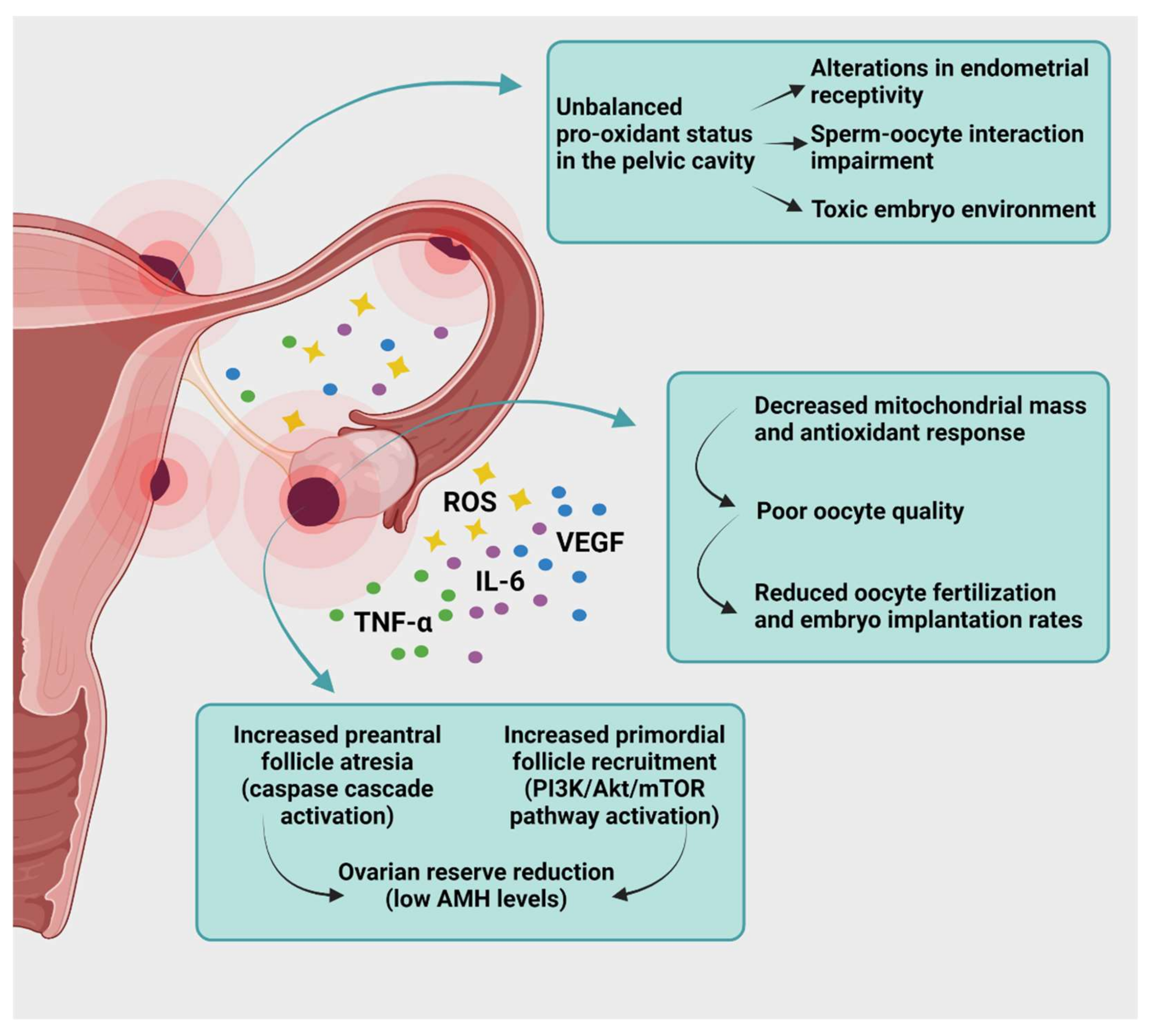
6. Targeting Oxidative Stress to Treat Endometriosis-Related Infertility
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Beliard, A.; Donnez, J.; Nisolle, M.; Foidart, J.M. Localization of laminin, fibronectin, E-cadherin and integrins in endometrium and endometriosis. Fertil. Steril. 1997, 67, 266–272. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef]
- Kokorine, I.; Nisolle, M.; Donnez, J.; Eeckhout, Y.; Courtoy, P.J.; Marbaix, E. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. Fertil. Steril. 1997, 68, 246–251. [Google Scholar] [CrossRef]
- Sillem, M.; Prifti, S.; Neher, M.; Runnebaum, B. Extracellular matrix remodelling in the endometrium and its possible relevance to the pathogenesis of endometriosis. Hum. Reprod. Update 1998, 4, 730–735. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef]
- Han, S.J.; Jung, S.Y.; Wu, S.-P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Donnez, J.; Smoes, P.; Gillerot, S.; Casanas-Roux, F.; Nisolle, M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998, 13, 1686–1690. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The immunopathophysiology of endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 91, 113–125. [Google Scholar] [CrossRef]
- Murphy, A.A.; Santanam, N.; Parthasarathy, S. Endometriosis: A disease of oxidative stress? Semin. Reprod. Endocrinol. 1998, 16, 263–273. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2000, 77, 861–870. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- American Society for Reproductive Medicine. Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Gayet, V.; Lafay-Pillet, M.C.; Korb, D.; Bourdon, M.; Marcellin, L.; de Ziegler, D.; Chapron, C. Prognostic factors for assisted reproductive technology in women with endometriosis-related infertility. Am. J. Obstet. Gynecol. 2017, 216, 280.e1–280.e9. [Google Scholar] [CrossRef] [PubMed]
- Maignien, C.; Santulli, P.; Marcellin, L.; Korb, D.; Bordonne, C.; Dousset, B.; Bourdon, M.; Chapron, C. Infertility in women with bowel endometriosis: First-line assisted reproductive technology results in satisfactory cumulative live-birth rates. Fertil. Steril. 2021, 115, 692–701. [Google Scholar] [CrossRef] [PubMed]
- The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar]
- Bostek, C.C. Oxygen toxicity: An introduction. AANA J. 1989, 57, 231–237. [Google Scholar]
- Ruder, E.H.; Hartman, T.J.; Blumberd, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008, 14, 345–357. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Redox-optimized ROS balance: A unifying hypothesis. Biochim. Biophys. Acta 2010, 1797, 865–877. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Yoshimoto, C.; Shigetomi, H.; Kobayashi, H. Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxid. Med. Cell Longev. 2015, 2015, 848595. [Google Scholar] [CrossRef]
- Martinez, V.D.; Vucic, E.A.; Thu, K.L.; Pikor, L.A.; Hubaux, R.; Lam, W.L. Unique Pattern of Component Gene Disruption in the NRF2 Inhibitor KEAP1/CUL3/RBX1 E3-Ubiquitin Ligase Complex in Serous Ovarian Cancer. Biomed. Res. Int. 2014, 2014, 159459. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.; Liu, T.; Kim, J.H.; Blank, V.; Li, H.; Kong, A.N.T. Heterodimerization with Small Maf Proteins Enhances Nuclear Retention of Nrf2 via Masking the NESzip Motif. Biochim. Biophys. Acta 2008, 1783, 1847–1856. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, R.; Donnez, J.; Defrere, S.; Leclercq, I.; Squifflet, J.; Lousse, J.C.; Van Langendonckt, A. Nuclear factor-kappa B (NF-kB) is constitutively activated in peritoneal endometriosis. Mol. Hum. Reprod. 2007, 13, 503–509. [Google Scholar] [CrossRef]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Gonzalez Ramos, R.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Lousse, J.C.; Van Langendonckt, A.; Gonzalez-Ramos, R.; Defrere, S.; Renkin, E.; Donnez, J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil. Steril. 2008, 90, 217–220. [Google Scholar] [CrossRef]
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Carvalho, L.F.; Samadder, A.N.; Agarwal, A.; Fernandes, L.F.; Abrao, M.S. Oxidative stress biomarkers in patients with endometriosis: Systematic review. Arch. Gynecol. Obstet. 2012, 286, 1033–1040. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.V.; Augoulea, A.; Christodoulakos, G.E.; Economou, E.V.; Kaparos, G.; Kontoravdis, A.; Papadias, C.; Creatsas, G. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil. Steril. 2009, 91, 46–50. [Google Scholar] [CrossRef]
- Yamawaki, H.; Pan, S.; Lee, R.T.; Berk, B.C. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J. Clin. Investig. 2005, 115, 733–738. [Google Scholar] [CrossRef]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.E.; Batteux, F.; Borderie, D.; Chapron, C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef]
- Defrere, S.; Van Langendonckt, A.; Vaesen, S.; Jouret, M.; Gonzalez Ramos, R.; Gonzalez, D.; Donnez, J. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum. Reprod. 2006, 21, 2810–2816. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Dolmans, M.M.; Donnez, J. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil. Steril. 2002, 77, 561–570. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil. Steril. 2002, 78, 712–718. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Eggermont, J.; Donnez, J. Characterization of iron deposition in endometriotic lesions induced in the nude mouse model. Hum. Reprod. 2004, 19, 1265–1271. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Donnez, J.; Brosens, I. Microscopic endometriosis: Impact on our understanding of the disease and its surgery. Fertil. Steril. 2016, 105, 305–306. [Google Scholar] [CrossRef][Green Version]
- Lousse, J.C.; Defrere, S.; Van Langendonckt, A.; Gras, J.; Gonzalez-Ramos, R.; Colette, S.; Donnez, J. Iron storage is significantly increased in peritoneal macrophages of patients with endometriosis and correlates with iron overload in the peritoneal fluid. Fertil. Steril. 2009, 91, 1668–1675. [Google Scholar] [CrossRef]
- Levy, A.P.; Levy, J.E.; Kalet-Litman, S.; Miller-Lotan, R.; Levy, N.S.; Asaf, R.; Guetta, J.; Yang, C.; Purushothaman, K.R.; Fuster, V.; et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 134–140. [Google Scholar] [CrossRef]
- Defrere, S.; Lousse, J.C.; Gonzalez-Ramos, R.; Colette, S.; Donnez, J.; Van Langendonckt, A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant stratagem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [CrossRef]
- Potts, M.B.; Koh, S.E.; Whetstone, W.D.; Walker, B.A.; Yoneyama, T.; Claus, C.P.; Manvelyan, H.M.; Noble-Haeusslein, L.J. Traumatic injury to the immature brain: Inflammation, oxidative injury, and iron-mediated damage as potential therapeutic targets. NeuroRx 2006, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Yip, Y.C. De novo formation of adhesions in endometriosis: The role of iron and free radical reactions. Fertil. Steril. 1995, 64, 62–64. [Google Scholar] [CrossRef]
- Buttke, T.M.; Sandstrom, P.A. Oxidative stress as a mediator of apoptosis. Immunol. Today 1994, 15, 7–10. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 1–21. [Google Scholar] [CrossRef]
- Wagener, F.A.; Volk, H.D.; Willis, D.; Abraham, N.G.; Soares, M.P.; Adema, G.J.; Figdor, C.G. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [CrossRef]
- Nakahira, K.; Takahashi, T.; Shimizu, H.; Maeshima, K.; Uehara, K.; Fujii, H.; Nakatsuka, H.; Yokoyama, M.; Akagi, R.; Morita, K. Protective role of heme oxygenase-1 induction in carbon tetrachlorideinduced hepatotoxicity. Biochem. Pharmacol. 2003, 15, 1091–1105. [Google Scholar] [CrossRef]
- Swiersz, L.M. Role of endometriosis in cancer and tumor development. Ann. N. Y. Acad. Sci. 2002, 955, 281–292, discussions 293–5, 396–406. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- National Guideline Alliance. Endometriosis: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2017. [Google Scholar]
- Bouchard, P.; Chabbert-Buffet, N.; Fauser, B.C. Selective progesterone receptor modulators in reproductive medicine: Pharmacology, clinical efficacy and safety. Fertil. Steril. 2011, 96, 1175–1189. [Google Scholar] [CrossRef]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lahair, M.M.; Franklin, R.A. Reactive oxygen speciesinduced activation of the MAP kinase signaling pathways. Antioxid. Redox. Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Murk, W.; Atabekoglu, C.S.; Cakmak, H.; Heper, A.; Ensari, A.; Kayisli, U.A.; Arici, A. ERK activity in the human endometrium: Possible roles in the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2008, 93, 3532–3540. [Google Scholar] [CrossRef]
- Gentilini, D.; Busacca, M.; Di Francesco, S.; Vignali, M.; Vigano, P.; Di Blasio, A.M. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol. Hum. Reprod. 2007, 13, 317–322. [Google Scholar] [CrossRef]
- Ngô, C.; Nicco, C.; Leconte, M.; Chéreau, C.; Arkwright, S.; Vacher-Lavenu, M.C.; Weill, B.; Chapron, C.; Batteux, F. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J. Pathol. 2010, 222, 148–157. [Google Scholar] [CrossRef]
- Leconte, M.; Nicco, C.; Ngô, C.; Arkwright, S.; Chéreau, C.; Guibourdenche, J.; Weill, B.; Chapron, C.; Dousset, B.; Batteux, F. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am. J. Pathol. 2010, 177, 2963–2970. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Hu, Y.Y.; Wang, H.; Zhang, C.J. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int. J. Mol. Med. 2011, 27, 87–94. [Google Scholar] [CrossRef]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2020, 36, 951–964. [Google Scholar] [CrossRef]
- Leconte, M.; Nicco, C.; Ngô, C.; Chéreau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef]
- Pittaluga, E.; Costa, G.; Krasnowska, E.; Brunelli, R.; Lundeberg, T.; Porpora, M.G.; Santucci, D.; Parasassi, T. More than antioxidant: N-acetyl-L-cysteine in a murine model of endometriosis. Fertil. Steril. 2010, 94, 2905–2908. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Brunelli, R.; Costa, G.; Imperiale, L.; Krasnowska, E.K.; Lundeberg, T.; Nofroni, I.; Piccioni, M.G.; Pittaluga, E.; Ticino, A.; et al. A promise in the treatment of endometriosis: An observational cohort study on ovarian endometrioma reduction by N-acetylcysteine. Evid. Based Complement. Alternat. Med. 2013, 2013, 240702. [Google Scholar] [CrossRef] [PubMed]
- Rudzitis-Auth, J.; Menger, M.D.; Laschke, M.W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 2013, 28, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Ozer, H.; Boztosun, A.; Acmaz, G.; Atilgan, R.; Akkar, O.B.; Kosar, M.I. The efficacy of bevacizumab, sorafenib, and retinoic acid on rat endometriosis model. Reprod. Sci. 2013, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Kacan, T.; Akkar, O.B.; Karakus, S.; Kacan, S.B.; Ozer, H.; Cetin, A. Effects of Pazopanib, Sunitinib, and Sorafenib, Anti-VEGF Agents, on the Growth of Experimental Endometriosis in Rats. Reprod. Sci. 2015, 22, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Leconte, M.; Santulli, P.; Chouzenoux, S.; Marcellin, L.; Cerles, O.; Chapron, C.; Dousset, B.; Batteux, F. Inhibition of MAPK and VEGFR by Sorafenib Controls the Progression of Endometriosis. Reprod. Sci. 2015, 22, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F.C.; Dayer, J.M. Leflunomide: Mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 841–849. [Google Scholar] [CrossRef]
- Aytan, H.; Caglar, P.; Uygur, D.; Zergeroglu, S.; Batioglu, S. Effect of the immunomodulator leflunomide on the induction of endometriosis in an experimental rat model. Fertil. Steril. 2007, 87, 698–701. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Yagyu, T.; Tsuji, Y.; Haruta, S.; Kitanaka, T.; Yamada, Y.; Kawaguchi, R.; Kanayama, S.; Tanase, Y.; Kurita, N.; Kobayashi, H. Activation of mammalian target of rapamycin in postmenopausal ovarian endometriosis. Int. J. Gynecol. Cancer 2006, 16, 1545–1551. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. Curcumin and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2440. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.N.; Park, J.K.; Lee, J.R.; Kim, J.H.; Choi, H.J.; Kim, B.S.; Lee, H.W.; Lee, K.S.; Yoon, S. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother. Res. 2012, 26, 1037–1047. [Google Scholar] [CrossRef]
- Parasassi, T.; Brunelli, R.; Bracci-Laudiero, L.; Greco, G.; Gustafsson, A.C.; Krasnowska, E.K.; Lundeberg, J.; Lundeberg, T.; Pittaluga, E.; Romano, M.C.; et al. Differentiation of normal and cancer cells induced by sulfhydryl reduction: Biochemical and molecular mechanisms. Cell Death Differ. 2005, 12, 1285–1296. [Google Scholar] [CrossRef]
- Alvarado-Díaz, C.P.; Núñez, M.T.; Devoto, L.; González-Ramos, R. Iron overload-modulated nuclear factor kappa-B activation in human endometrial stromal cells as a mechanism postulated in endometriosis pathogenesis. Fertil. Steril. 2015, 103, 439–447. [Google Scholar] [CrossRef]
- Alvarado-Díaz, C.P.; Núñez, M.T.; Devoto, L.; González-Ramos, R. Endometrial expression and in vitro modulation of the iron transporter divalent metal transporter-1: Implications for endometriosis. Fertil. Steril. 2016, 106, 393–401. [Google Scholar] [CrossRef]
- Abdelaal, G.; Veuger, S. Reversing oncogenic transformation with iron chelation. Oncotarget 2021, 12, 106–124. [Google Scholar] [CrossRef]
- Ibrahim, O.; O’Sullivan, J. Iron chelators in cancer therapy. Biometals 2020, 33, 201–215. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Capobianco, A.; Monno, A.; Cottone, L.; Venneri, M.A.; Biziato, D.; Di Puppo, F.; Ferrari, S.; De Palma, M.; Manfredi, A.A.; Rovere-Querini, P. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am. J. Pathol. 2011, 179, 2651–2659. [Google Scholar] [CrossRef]
- Zuo, Y.; Perkins, N.M.; Tracey, D.J.; Geczy, C.L. Inflammation and hyperalgesia induced by nerve injury in the rat: A key role of mast cells. Pain 2003, 105, 467–479. [Google Scholar] [CrossRef]
- Tran, L.V.; Tokushige, N.; Berbic, M.; Markham, R.; Fraser, I.S. Macrophages and nerve fibres in peritoneal endometriosis. Hum. Reprod. 2009, 24, 835–841. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast cell-mediated mechanisms of nociception. Int. J. Mol. Sci. 2015, 130, 395–412. [Google Scholar] [CrossRef]
- Binda, M.M.; Donnez, J.; Dolmans, M.M. Targeting mast cells: A new way to treat endometriosis. Expert Opin. Ther. Targets 2017, 21, 67–75. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Vardeh, D.; Wang, D.; Costigan, M.; Lazarus, M.; Saper, C.B.; Woolf, C.J.; Fitzgerald, G.A.; Samad, T.A. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J. Clin. Investig. 2009, 119, 287–294. [Google Scholar] [CrossRef]
- Greaves, E.; Horne, A.W.; Jerina, H.; Mikolajczak, M.; Hilferty, L.; Mitchell, R.; Fleetwood-Walker, S.M.; Saunders, P.T. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci. Rep. 2017, 7, 44169. [Google Scholar] [CrossRef]
- Acien, P.; Quereda, F.; Campos, A.; Gomez-Torres, M.J.; Velasco, I.; Gutierrez, M. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: A randomized clinical trial. Fertil. Steril. 2002, 78, 705–711. [Google Scholar] [CrossRef]
- Acien, P.; Quereda, F.J.; Gomez-Torres, M.J.; Bermejo, R.; Gutierrez, M. GnRH analogues, transvaginal ultrasound-guided drainage and intracystic injection of recombinant interleukin-2 in the treatment of endometriosis. Gynecol. Obstet. Investig. 2003, 55, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Haber, E.; Danenberg, H.D.; Koroukhov, N.; Ron-El, R.; Golomb, G.; Schachter, M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum. Reprod. 2009, 24, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Ihara, T.; Uchiide, I.; Sugamata, M. Light and electron microscopic evaluation of antileukotriene therapy for experimental rat endometriosis. Fertil. Steril. 2004, 81, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Guney, M.; Oral, B.; Karahan, N.; Mungan, T. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil. Steril. 2008, 89, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, G.; Attar, R.; Ozkan, F.; Kumbak, B.; Ficicioglu, C.; Yesildaglar, N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: A preliminary study. Fertil. Steril. 2010, 93, 1787–1792. [Google Scholar] [CrossRef]
- Schwertner, A.; Conceição Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase I.I.; randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef]
- Iuvone, T.; Affaitai, G.; De Filippis, D.; Lopopolo, M.; Grassia, G.; Lapenna, D.; Negro, L.; Costantini, R.; Vaia, M.; Cipollone, F.; et al. Ultramicronized palmitoyilethanolamide reduces viscervisceral hyperalgesia in a rat model of endometriosis plus ureteral calculosis: Role of mast cells. Pain 2016, 157, 80–91. [Google Scholar] [CrossRef]
- Indraccolo, U.; Indraccolo, S.R.; Mignini, F. Micronized palmitoylethanolamide/trans-polydatin treatment of endometriosis-related pain: A meta-analysis. Ann. Ist. Super. Sanita. 2017, 53, 125–134. [Google Scholar]
- Hart, D.A. Curbing inflammation in multiple sclerosis and endometriosis: Should mast cells be targeted? Int. J. Inflamm. 2015, 2015, 452095. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Tan, D.X.; Costantino, G.; Mazzon, E.; Caputi, A.P.; Reiter, R.J. The protective role of endogenous melatonin in carrageenan-induced pleurisy in the rat. FASEB J. 1999, 13, 1930–1938. [Google Scholar] [CrossRef]
- Blurton-Jones, M.; Kuan, P.N.; Tuszynski, M.H. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J. Comp. Neurol. 2004, 468, 347–360. [Google Scholar] [CrossRef]
- Allen, A.L.; McCarson, K.E. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology 2005, 81, 193–199. [Google Scholar] [CrossRef]
- Garcia-Fernandez, J.; García-Velasco, J.A. Endometriosis and Reproduction: What We Have Learned. Yale J. Biol. Med. 2020, 93, 571–577. [Google Scholar]
- Raffi, F.; Metwally, M.; Amer, S. The impact of excision of ovarian endometrioma on ovarian reserve: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 3146–3154. [Google Scholar] [CrossRef]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 932–940. [Google Scholar] [CrossRef]
- Kitajima, M.; Defrère, S.; Dolmans, M.M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pankhurst, M.W. Hyperactivation of dormant primordial follicles in ovarian endometrioma patients. Reproduction 2020, 160, R145–R153. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Cacciottola, L.; Courtoy, G.E.; Nguyen, T.Y.T.; Hossay, C.; Donnez, J.; Dolmans, M.M. Adipose tissue-derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2021, 38, 151–161. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010, 93, 2431–2432. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JN.K.; p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Papaleo, E.; Corti, L.; Santambrogio, P.; Levi, S.; Viganò, P.; Candiani, M.; Panina-Bordignon, P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum. Reprod. 2014, 29, 577–583. [Google Scholar] [CrossRef]
- Cobo, A.; Giles, J.; Paolelli, S.; Pellicer, A.; Remohí, J.; García-Velasco, J.A. Oocyte vitrification for fertility preservation in women with endometriosis: An observational study. Fertil. Steril. 2020, 113, 836–844. [Google Scholar] [CrossRef]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Oxidative stress, mitochondria, and infertility: Is the relationship fully established? Fertil. Steril. 2021, in press. [Google Scholar] [CrossRef]
- Mansour, G.; Sharma, R.K.; Agarwal, A.; Falcone, T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: A possible cause of infertility. Fertil. Steril. 2010, 94, 1894–1899. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Guo, N.; Zhang, X.M.; Shi, W.; Tong, X.H.; Iqbal, F.; Liu, Y.S. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci. Rep. 2015, 5, 10779. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Aguilar, A.; Quiñonero, A.; Carbajo-García, M.C.; Alamá, P.; Tejera, A.; Taboas, E.; Muñoz, E.; Pellicer, A.; et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 2019, 34, 1302–1312. [Google Scholar] [CrossRef]
- Tomassetti, C.; Meuleman, C.; Pexsters, A.; Mihalyi, A.; Kyama, C.; Simsa, P.; D’Hooghe, T.M. Endometriosis, recurrent miscarriage and implantation failure: Is there an immunological link? Reprod. Biomed. Online 2006, 13, 58–64. [Google Scholar] [CrossRef]
- Campos Petean, C.; Ferriani, R.A.; dos Reis, R.M.; de Moura, M.D.; Jordão, A.A., Jr.; Navarro, P.A. Lipid peroxidation and vitamin E in serum and follicular fluid of infertile women with peritoneal endometriosis submitted to controlled ovarian hyperstimulation: A pilot study. Fertil. Steril. 2008, 90, 2080–2085. [Google Scholar] [CrossRef]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef]
- Kishi, I.; Ohishi, M.; Akiba, Y.; Asada, H.; Konishi, Y.; Nakano, M.; Kamei, K.; Yoshimura, Y.; Maruyama, T. Thioredoxin, an antioxidant redox protein, in ovarian follicles of women undergoing in vitro fertilization. Endocr. J. 2016, 63, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod. Med. Biol. 2018, 17, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Creus, M.; Fábregues, F.; Carmona, F.; del Pino, M.; Manau, D.; Balasch, J. Combined laparoscopic surgery and pentoxifylline therapy for treatment of endometriosis-associated infertility: A preliminary trial. Hum. Reprod. 2008, 23, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Genera-García, M.; De la Jara-Díaz, J.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernández-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynaecol. Obstet. 2008, 100, 252–256. [Google Scholar] [CrossRef]
- Lu, X.; Wu, Z.; Wang, M.; Cheng, W. Effects of vitamin C on the outcome of in vitro fertilization-embryo transfer in endometriosis: A randomized controlled study. J. Int. Med. Res. 2018, 46, 4624–4633. [Google Scholar] [CrossRef]

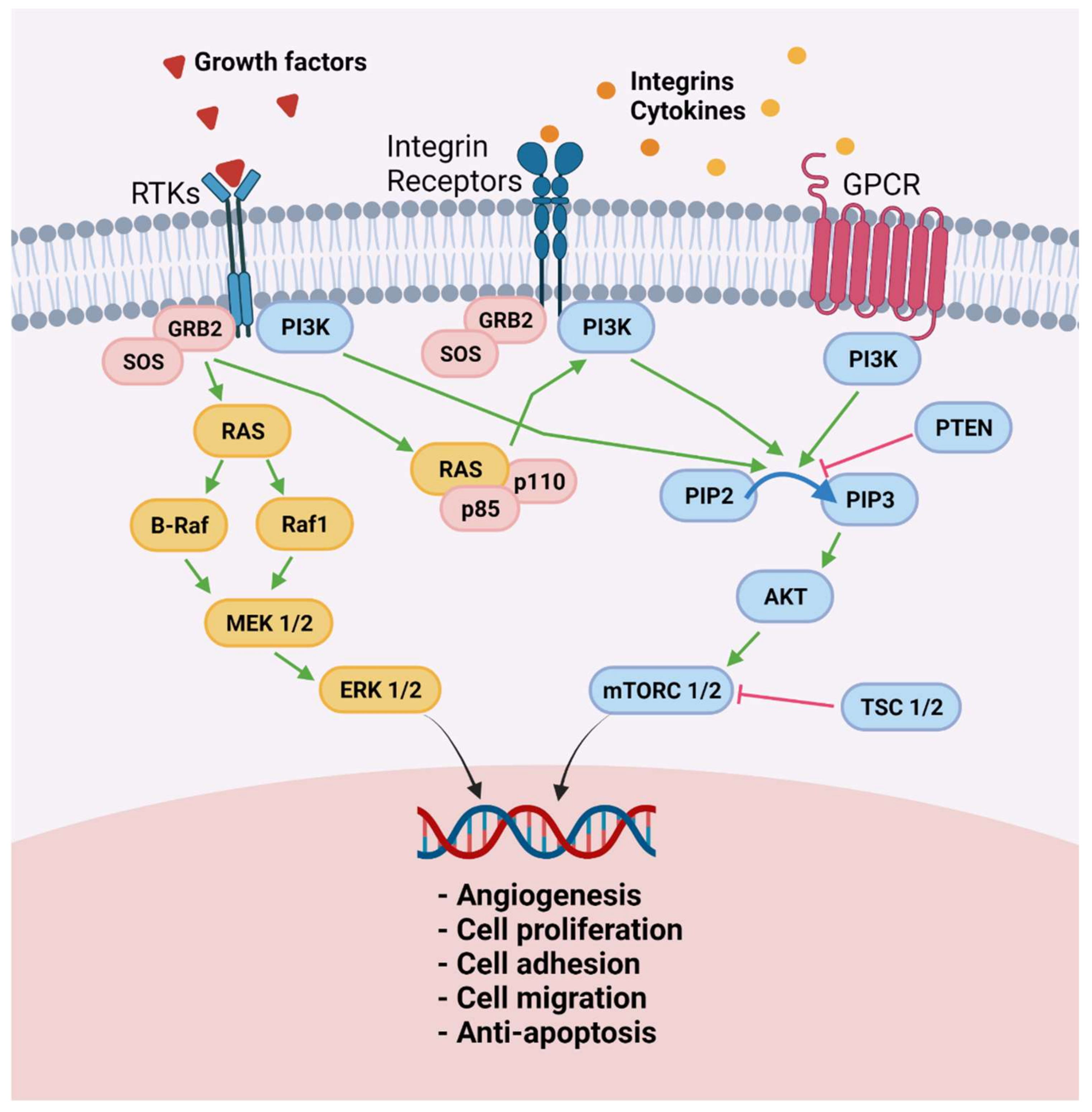
| Reference | Drug | Effect | Endometriosis Source | Experimental Model |
|---|---|---|---|---|
| Leconte et al., 2010 [65] | Cannabinoid agonists | Inhibition of PI3K/Akt/mTOR pathway | Human (DIE) | In vitro culture; xenotransplantation murine model |
| Drop in cell proliferation | ||||
| Reduced ROS generation | ||||
| Lower NO levels | ||||
| Decline in endometriotic lesion volume | ||||
| Zhang et al., 2011 [66] | Curcumin | Decline in endometriotic lesion volume | Rodent (rat) | Autotransplantation rat model |
| Lower VEGF levels | ||||
| Lower microvessel density | ||||
| Jana et al., 2012 [67] | Curcumin | Increase in apoptosis rates | Rodent (mouse) | Autotransplantation murine model |
| Higher MMP-3 levels | ||||
| Defrère et al., 2006 [40] | Desferrioxamine | Drop in cell proliferation | Human (menstrual endometrium) | Xenotransplantation murine model |
| lower iron levels in lesions, macrophages, and peritoneal fluid | ||||
| Li et al., 2020 [68] | Erastin | Increase in total and lipid ROS values | Human (endometriomas) | Xenotransplantation murine model |
| Upturn in iron levels | ||||
| Decline in endometriotic lesion volume | ||||
| Leconte et al., 2011 [69] | ERK inhibitors | Inhibition of Raf/MEK/ERK pathway | Human (DIE) | In vitro culture; xenotransplantation murine model |
| Temsirolimus | Drop in cell proliferation | |||
| Inhibition of PI3K/Akt/mTOR pathway | ||||
| NAC | Drop in cell proliferation | |||
| Reduced ROS generation | ||||
| Ngo et al., 2010 [64] | Leflunomide, MEK 1/2 inhibitors | Inhibition of Raf/MEK/ERK pathway | Human (endometriomas and DIE) | In vitro culture; xenotransplantation murine model |
| Drop in cell proliferation | ||||
| Decline in endometriotic lesion volume | ||||
| Park et al., 2017 [70] | Naringenin | Drop in cell proliferation | Human (pelvic endometriotic lesions) | In vitro culture |
| Inhibition of PI3K/Akt/mTOR pathway | ||||
| Increased levels of ER stress and ROS | ||||
| Kapoor et al., 2018 [71] | Naringenin | Decline in endometriotic lesion volume | Rodent (rat) | In vitro culture; autotransplantation rat model |
| Lower TNFα and NO levels | ||||
| Inhibition of Nrf2/KEAP1 pathway | ||||
| Increase in mitochondrial damage, ROS, and apoptosis | ||||
| Pittalunga et al., 2010 [72] | NAC | Decline in endometriotic lesion volume | Rodent (mouse) | Allotransplantation murine model |
| Decrease in COX-2 and MMP-9 expression | ||||
| Porpora et al., 2013 [73] | NAC | Decline in endometriotic lesion volume | Human (endometriomas >3 cm) | Endometriosis patients with chronic pain and infertility |
| Decrease in COX-2 expression | ||||
| Alleviation of endometriosis-related pain | ||||
| Rudzitis-Auth et al., 2013 [74] | Resveratrol | Drop in cell proliferation | Rodent (mouse) | Allotransplantation murine model |
| Decline in endometriotic lesion volume | ||||
| Lower microvessel density | ||||
| Taguchi et al., 2014 [75] | Resveratrol | No difference in cell proliferation | Human (endometriomas) | In vitro culture |
| Decrease in IL-8 expression | ||||
| Ozer et al., 2013 [76] | Sorafenib | No difference in implant volume | Rodent (rat) | Autotransplantation rat model |
| Lower microvessel density | ||||
| Yildiz et al., 2015 [77] | Sorafenib | No difference in proliferation rates | Rodent (rat) | Autotransplantation rat model |
| No difference in apoptosis rates | ||||
| Decrease in VEGF expression | ||||
| Leconte et al., 2015 [78] | Sorafenib | Inhibition of Raf/MEK/ERK pathway | Human (endometriomas and DIE) | In vitro culture; xenotransplantation murine model |
| Drop in cell proliferation |
| Reference | Drug | Effect | Endometriosis Source | Experimental Model |
|---|---|---|---|---|
| Haber et al., 2009 [102] | Liposomal bisphosphonate | Decline in endometriotic lesion volume | Rodent (rat) | Autotransplantation rat model |
| less macrophage infiltration | ||||
| Foster et al., 2019 [96] | Liposomal clodronate | Less macrophage infiltration | Human (pelvic endometriotic lesions) | In vitro culture; xenotransplantation murine model |
| Decrease in IGF-1 expression | ||||
| Linsitinib | Attenuation of hyperalgesia | |||
| Ihara et al., 2004 [103] | Leukotriene receptor antagonists | Lower stromal proliferation rates | Rodent (rat) | Autotransplantation rat model |
| Decline in mast cell infiltration and activation | ||||
| Guney et al., 2008 [104] | Melatonin | Decline in endometriotic lesion volume | Rodent (rat) | Autotransplantation rat model |
| Increased SOD and CAT activity | ||||
| Lower MDA levels | ||||
| Yildirim et al., 2009 [105] | Melatonin | Decline in endometriotic lesion volume | Rodent (rat) | Autotransplantation rat model |
| Increased SOD and CAT activity | ||||
| Schwertner et al., 2013 [106] | Melatonin | Alleviation of chronic pain | Human (pelvic endometriotic lesions) | Endometriosis patients with chronic pain |
| Improvement in dysmenorrhea and dyspareunia | ||||
| Improvement in dyschezia and dysuria | ||||
| Lower BDNF levels | ||||
| Iuvone et al., 2016 [107] | PEA | Decrease in mast cell numbers | Rodent (rat) | Autotransplantation rat model |
| Decline in endometriotic lesion volume | ||||
| Idraccolo et al., 2017 [108] | PEA + polydatin | Alleviation of chronic pain | Human (pelvic endometriotic lesions) | Endometriosis patients with chronic pain |
| Improvement in dysmenorrhea and dyspareunia | ||||
| No improvement in dyschezia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciottola, L.; Donnez, J.; Dolmans, M.-M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. https://doi.org/10.3390/ijms22137138
Cacciottola L, Donnez J, Dolmans M-M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? International Journal of Molecular Sciences. 2021; 22(13):7138. https://doi.org/10.3390/ijms22137138
Chicago/Turabian StyleCacciottola, Luciana, Jacques Donnez, and Marie-Madeleine Dolmans. 2021. "Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets?" International Journal of Molecular Sciences 22, no. 13: 7138. https://doi.org/10.3390/ijms22137138
APA StyleCacciottola, L., Donnez, J., & Dolmans, M.-M. (2021). Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? International Journal of Molecular Sciences, 22(13), 7138. https://doi.org/10.3390/ijms22137138







