Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies
Abstract
:1. Endometriosis as a Chronic Inflammatory Disease
2. From Genetics to Inflammatory Pathways
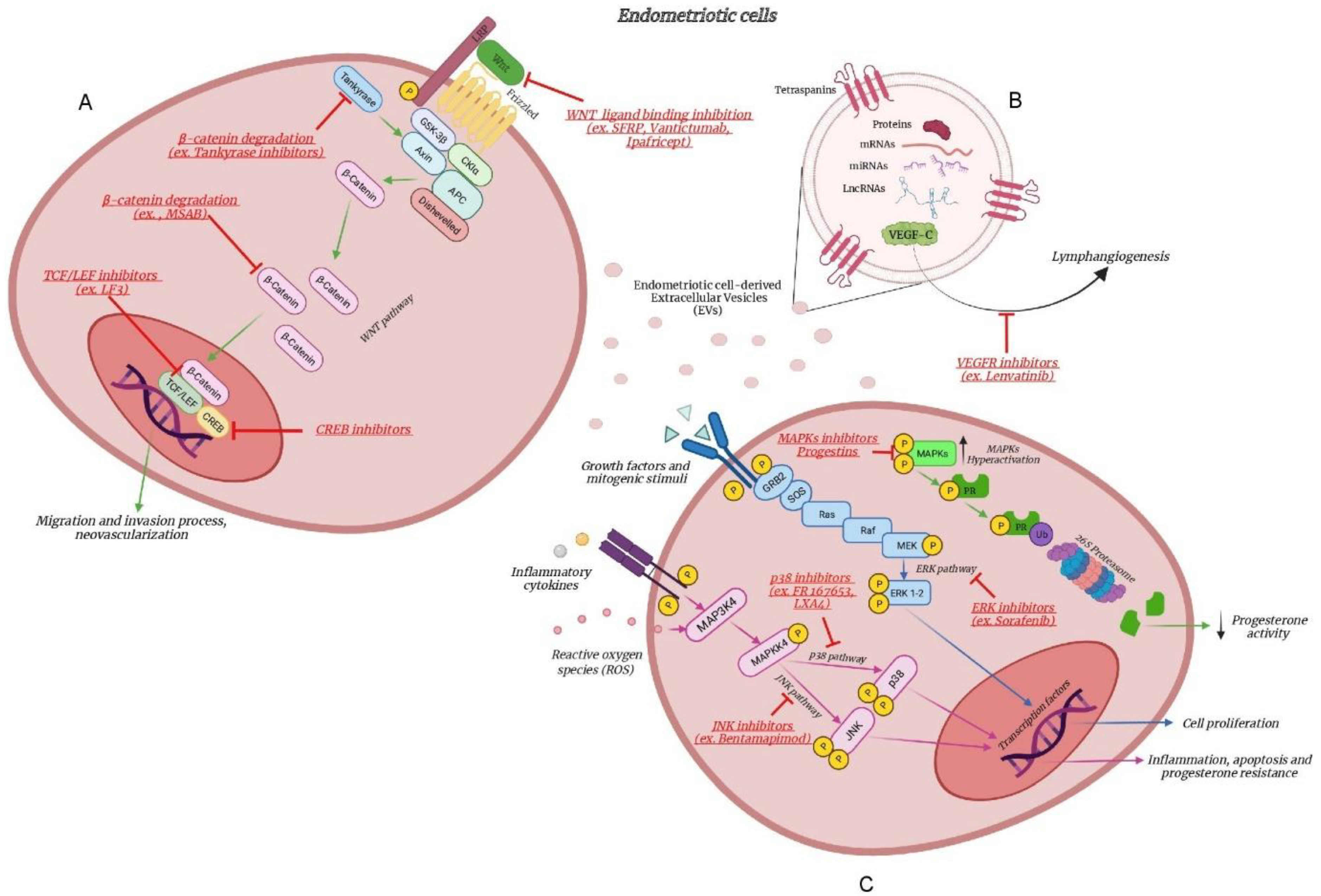
3. The MAPK Pathway
3.1. The MAPK Pathway between Genetics and Inflammation
- production of inflammatory substances;
- reactive oxygen species (ROS) production. The inflammatory environment typical of endometriosis is associated with alterations in ROS detoxification pathways and, consequently, determines an oxidative stress, both of which are involved in increased cell proliferation, particularly through activation of the MAPK ERK1/2 pathways [48];
- progesterone resistance. Endometriosis is known to be characterized by a condition of progesterone resistance [49]. In particular, hyperactivation of the MAPK pathways is thought to suppress progesterone receptor activity via proteasome-dependent degradation, impairing endometrial decidualization and increasing the establishment of ectopic endometrial implants [50].
3.2. The MAPK Pathway as Therapeutic Target
3.2.1. Inhibition of MAPKs
3.2.2. Inhibition of MAPKs and Progestins
4. The WNT Pathway
4.1. The WNT Pathway between Genetics and Inflammation
- migration and invasion process. Xiong et al. studied the effect of xeno-transplantation of human endometrium cells into NOD-SCID mice under estradiol treatment [65]. They observed that abnormal levels of estradiol were capable of inducing β-catenin expression in a dose- and time-dependent manner through the involvement of estrogen receptor (ER)-α. Moreover, along with previous evidence by Becker et al. in 2010, the authors showed that the estradiol-dependent increased level of β-catenin could up-regulate the production of MMPs, such as MMP-9, involved in extracellular matrix remodelling, allowing cellular migration and invasion [68].
- neovascularization. In 2008, Cheng et al. showed that the sFRP1 transcript, already known for its proangiogenic effects [69], was higher in the proliferative phase endometrium and significantly increased in endometriotic tissues compared with eutopic endometrium [69]. In human endometrial stromal cells, Zhang et al. proved that the regulation of vascular endothelial growth factor (VEGF) by estradiol involved the multifunctional protein β-catenin and the transcription factors TCF3/LEF1 [67]. In detail, at a genomic level, the binding of ERα to estrogen responsive elements in the β-catenin promoter induced its transcription, on one hand; on the other hand, aberrant estrogen levels inactivated GSK3β, preventing β-catenin from its degradation. Both ways converged into the enhanced level of β-catenin in the nucleus, modulating the expression of VEGF [67]. VEGF is thought to be a crucial factor in endometriosis establishment, as ectopic and eutopic endometrium of endometriosis patients have high VEGF mRNA levels [70].
- production of inflammatory cytokines. Jiang and coworkers suggested that the anti-inflammatory cytokine IL-37 suppressed the proliferation, adhesion, migration and invasion of human ectopic endometriotic stromal cells from ovarian endometrioma samples, through multiple signaling pathways, with the β-catenin pathway as one of the most critical. IL-37 overexpression significantly suppressed IL-1β, IL-6, IL-10 and and TNF-α, whereas knockdown of IL-37 significantly upregulated their protein and mRNA expression, compared to the control. From the in vivo experiment, the lesion size of endometriosis mice treated with recombinant IL-37 was significantly decreased, compared with the control mice [71].
4.2. The WNT Pathway as Therapeutic Target
4.2.1. β-Catenin Degradation
4.2.2. Inhibition of WNT Ligand Binding
4.2.3. Transcriptional Activity Inhibition
5. The Extracellular Vesicles Modulating Inflammation
5.1. The Extracellular Vesicle Cargo as Therapeutic Target
5.2. The Extracellular Vesicles Cargo as Therapeutic Tool
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Viganò, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Riccio, L.D.G.C.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- Capobianco, A.; Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front. Immunol. 2013, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeljeli, M.; Riccio, L.G.C.; Chouzenoux, S.; Moresi, F.; Toullec, L.; Doridot, L.; Nicco, C.; Bourdon, M.; Marcellin, L.; Santulli, P.; et al. Macrophage Immune Memory Controls Endometriosis in Mice and Humans. Cell Rep. 2020, 33, 108325. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Panir, K.; Dhami, P.; Rosser, M.; Mack, M.; Soong, D.; Pollard, J.W.; Jenkins, S.J.; Horne, A.W.; Greaves, E. Macrophages inhibit and enhance endometriosis depending on their origin. Proc. Natl. Acad. Sci. USA 2021, 118, e2013776118. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, D.; Perino, A.; Viganò, P.; Chiodo, I.; Cucinella, G.; Vignali, M.; Di-Blasio, A.M.; Busacca, M. Gene expression profiling of peripheral blood mononuclear cells in endometriosis identifies genes altered in non-gynaecologic chronic inflammatory diseases. Hum. Reprod. 2011, 26, 3109–3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hey-Cunningham, A.J.; Wong, C.; Hsu, J.; Fromm, P.D.; Clark, G.J.; Kupresanin, F.; Miller, E.J.; Markham, R.; McGuire, H.M. Comprehensive analysis utilizing flow cytometry and immunohistochemistry reveals inflammatory changes in local endometrial and systemic dendritic cell populations in endometriosis. Hum. Reprod. 2021, 36, 415–428. [Google Scholar] [CrossRef]
- Villanacci, R.; Bandini, V.; Ottolina, J.; Pagliardini, L.; Candiani, M.; Viganò, P. The pathogenesis of endometriosis: Clues from the immunological evidence. Minerva Obstet. Gynecol. 2021, 73, 275–282. [Google Scholar] [CrossRef]
- Vignali, M.; Pisoni, S.; Gentilini, D.; Spada, E.; Solima, E.; Viganò, P.; Candiani, M.; Busacca, M.; di Blasio, A.M. Hormonal therapy potentiates the effect of surgery on gene expression profile of peripheral blood mononuclear cells in patients affected by endometriosis. Minerva Endocrinol. 2021, 46, 90–98. [Google Scholar] [CrossRef]
- Brenhouse, H.C.; Schwarz, J.M. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci. Biobehav. Rev. 2016, 70, 288–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmioglu, N.; Banasik, K.; Paraskevi, C.; Danning, R.; Galarneau, G.; GirI, A.; MacGregor, S.; Mortlock, S.; Sapkota, Y.; Schork, J.A.; et al. Large-scale genome-wide association meta-analysis of endometriosis reveals 13 novel loci and genetically-associated comorbidity with other pain conditions. BioRxiv 2018, 406967. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, C.; Balduit, A.; Mangogna, A.; Zito, G.; Romano, F.; Ricci, G.; Kishore, U.; Bulla, R. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front. Immunol. 2021, 11, 599117. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Abbamonte, L.H.; Anserini, P.; Remorgida, V.; Ragni, N. Future perspectives in the medical treatment of endometriosis. Obstet. Gynecol. Surv. 2005, 60, 817–826. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Frattaruolo, M.P.; Borghi, A.; Dridi, D.; Somigliana, E. Medical treatment of endometriosis-related pain. Best. Pr. Res. Clin. Obstet. Gynaecol. 2018, 51, 68–91. [Google Scholar] [CrossRef]
- Surrey, E.S.; Soliman, A.M.; Johns, B.; Vora, J.B.; Taylor, H.S.; Agarwal, S.K. Real-World Characterization of Women with Diagnosed Endometriosis Initiating Therapy with Elagolix Using a US Claims Database. Clinicoecon. Outcomes. Res. 2020, 12, 473–479. [Google Scholar] [CrossRef]
- Donnez, J.; Chantraine, F.; Nisolle, M. The efficacy of medical and surgical treatment of endometriosis-associated infertility: Arguments in favour of a medico-surgical approach. Hum. Reprod. Update 2002, 8, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Donnez, J.; Dolmans, M.M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J. Clin. Med. 2021, 10, 1085. [Google Scholar] [CrossRef]
- Simpson, J.L.; Elias, S.; Malinak, L.R.; Buttram, V.C., Jr. Heritable aspects of endometriosis: I. Genetic studies. Am. J. Obstet. Gynecol. 1980, 137, 327–331. [Google Scholar] [CrossRef]
- Treloar, S.A.; O’Connor, D.T.; O’Connor, V.M.; Martin, N.G. Genetic influences on endometriosis in an Australian twin sample. Fertil. Steril. 1999, 71, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Vassilopoulou, L.; Matalliotakis, M.; Zervou, M.I.; Matalliotaki, C.; Krithinakis, K.; Matalliotakis, I.; Spandidos, D.A.; Goulielmos, G.N. Defining the genetic profile of endometriosis. Exp. Ther. Med. 2019, 17, 3267–3281. [Google Scholar]
- Nyholt, D.R.; Low, S.K.; Anderson, C.A.; Painter, J.N.; Uno, S.; Morris, A.P.; MacGregor, S.; Gordon, S.D.; Henders, A.K.; Martin, N.G.; et al. Genome-wide association meta-analysis identified new endometriosis risk loci. Nat. Genet. 2012, 44, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalami, I.; Abo, C.; Borghese, B.; Chapron, C.; Vaiman, D. Genomics of Endometriosis: From Genome Wide Association Studies to Exome Sequencing. Int. J. Mol. Sci. 2021, 22, 7297. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, Y.; Low, S.K.; Attia, J.; Gordon, S.D.; Henders, A.K.; Holliday, E.G.; MacGregor, S.; Martin, N.G.; McEvoy, M.; Morris, A.P.; et al. Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod. 2015, 30, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdsworth-Carson, S.J.; Fung, J.N.; Luong, H.T.; Sapkota, Y.; Bowdler, L.M.; Wallace, L.; The, W.T.; Powell, J.E.; Girling, J.E.; Healey, M.; et al. Endometrial vezatin and its association with endometriosis risk. Hum. Reprod. 2016, 31, 999–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azimzadeh, P.; Khorram-Khorshid, H.R.; Akhondi, M.M.; Shirazi, A. Association of interleukin-16 polymorphisms with disease progression and susceptibility in endometriosis. Int. J. Immunogenet. 2016, 43, 297–302. [Google Scholar] [CrossRef]
- Peluso, C.; Christofolini, D.M.; Goldman, C.S.; Mafra, F.A.; Cavalcanti, V.; Barbosa, C.P.; Bianco, B. TYK2 rs34536443 polymorphism is associated with a decreased susceptibility to endometriosis-related infertility. Hum. Immunol. 2013, 74, 93–97. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Li, G.; Wang, S.; Zhang, S.; Xie, B. Association of FCRL3 Genetic Polymorphisms With Endometriosis-Related Infertility Risk: An Independent Study in Han Chinese. Medicine 2015, 94, e1168. [Google Scholar] [CrossRef]
- Uimari, O.; Rahmioglu, N.; Nyholt, D.R.; Vincent, K.; Missmer, S.A.; Becker, C.; Morris, A.P.; Montgomery, G.W.; Zondervan, K.T. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 2017, 32, 780–793. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Nyholt, D.R.; Morris, A.P.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 2014, 20, 702–716. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Papaconstantinou, J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef] [Green Version]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in Synaptic Function and Dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Khalaj, K.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Immune-inflammation gene signatures in endometriosis patients. Fertil. Steril. 2016, 106, 1420–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, G.; Yaba, A. The role of mitogen-activated protein kinase signaling pathway in endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Boulton, T.G.; Nye, S.H.; Robbins, D.J.; Ip, N.Y.; Radziejewska, E.; Morgenbesser, S.D.; DePinho, R.A.; Panayotatos, N.; Cobb, M.H.; Yancopoulos, G.D. ERKs: A family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 1991, 65, 663–675. [Google Scholar] [CrossRef]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Li, H.H.; Li, M.; Wang, S.; Jiang, X.R.; Li, Y.; Ping, G.F.; Cao, Q.; Liu, X.; Fang, W.H.; et al. SL4, a chalcone-based compound, induces apoptosis in human cancer cells by activation of the ROS/MAPK signalling pathway. Cell Prolif. 2015, 48, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Leconte, M.; Santulli, P.; Chouzenoux, S.; Marcellin, L.; Cerles, O.; Chapron, C.; Dousset, B.; Batteux, F. Inhibition of MAPK and VEGFR by Sorafenib Controls the Progression of Endometriosis. Reprod. Sci. 2015, 22, 1171–1180. [Google Scholar] [CrossRef]
- Yotova, I.Y.; Quan, P.; Leditznig, N.; Beer, U.; Wenzl, R.; Tschugguel, W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum. Reprod. 2011, 26, 885–897. [Google Scholar] [CrossRef] [Green Version]
- De-La-Garza, E.M.; Binkley, P.A.; Ganapathy, M.; Krishnegowda, N.K.; Tekmal, R.R.; Schenken, R.S.; Kirma, N.B. Raf-1, a potential therapeutic target, mediates early steps in endometriosis lesion development by endometrial epithelial and stromal cells. Endocrinology 2012, 153, 3911–3921. [Google Scholar] [CrossRef] [Green Version]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta. Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, B.; Wu, L.; Xu, H.; Cheung, C.W.; Fung, W.Y.; Wong, S.W.; Wang, C.C. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: A comparison study in mice. Reprod. Biol. Endocrinol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Yoshino, O.; Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Ruimeng, X.; Na, L.; Yano, T.; Tsutsumi, O.; Taketani, Y. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J. Reprod. Immunol. 2006, 72, 85–93. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br. J. Pharmacol. 2014, 171, 4927–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Zhu, M.; Wu, R.; Lin, D.; Huang, Z.; Ren, L.; Huang, S.; Cheng, L.; Chen, Q. Lipoxin A4 Suppresses IL-1β-Induced Cyclooxygenase-2 Expression through Inhibition of p38 MAPK Activation in Endometriosis. Reprod. Sci. 2019, 26, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.S.; Altan, M.; Denis, D.; Tos, E.G.; Gotteland, J.P.; Osteen, K.G.; Bruner-Tran, K.L.; Nataraja, S.G. Bentamapimod (JNK Inhibitor AS602801) Induces Regression of Endometriotic Lesions in Animal Models. Reprod. Sci. 2016, 23, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, T.; Andoh, A.; Nishida, A.; Shioya, M.; Koizumi, Y.; Tsujikawa, T.; Fujiyama, Y. FR167653, a p38 mitogen-activated protein kinase inhibitor, aggravates experimental colitis in mice. World J. Gastroenterol. 2008, 14, 5851–5856. [Google Scholar] [CrossRef] [PubMed]
- Gezer, A.; Oral, E. Progestin therapy in endometriosis. Womens Health 2015, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Osiński, M.; Mostowska, A.; Wirstlein, P.; Wender-Ożegowska, E.; Jagodziński, P.P.; Szczepańska, M. The assessment of GWAS-identified polymorphisms associated with infertility risk in Polish women with endometriosis. Ginekol. Pol. 2018, 89, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Mafra, F.; Catto, M.; Bianco, B.; Barbosa, C.P.; Christofolini, D. Association of WNT4 polymorphisms with endometriosis in infertile patients. J. Assist. Reprod. Genet. 2015, 32, 1359–1364. [Google Scholar] [CrossRef] [Green Version]
- Rahmioglu, N.; Macgregor, S.; Drong, A.W.; Hedman, Å.K.; Harris, H.R.; Randall, J.C.; Prokopenko, I.; Nyholt, D.R.; Morris, A.P.; Montgomery, G.W.; et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet. 2015, 24, 1185–1199. [Google Scholar] [CrossRef] [Green Version]
- Freese, J.L.; Pino, D.; Pleasure, S.J. Wnt signaling in development and disease. Neurobiol. Dis. 2010, 38, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, W.J.; Bothwell, A.L.M. Dickkopf1: An immunomodulatory ligand and Wnt antagonist in pathological inflammation. Differentiation 2019, 108, 33–39. [Google Scholar] [CrossRef]
- Pabona, J.M.; Simmen, F.A.; Nikiforov, M.A.; Zhuang, D.; Shankar, K.; Velarde, M.C.; Zelenko, Z.; Giudice, L.C.; Simmen, R.C. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: Implications for the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, E376–E392. [Google Scholar] [CrossRef] [Green Version]
- Hundt, J. Involvement of the WNT Pathway in Endometriosis. Ph.D. Thesis, Technical University of Dortmund, Dortmund, Germany, 2016. Available online: https://eldorado.tu-dortmund.de/bitstream/2003/35727/1/Dissertation%20Hundt.pdf (accessed on 23 September 2016).
- Heinosalo, T.; Gabriel, M.; Kallio, L.; Adhikari, P.; Huhtinen, K.; Laajala, T.D.; Kaikkonen, E.; Mehmood, A.; Suvitie, P.; Kujari, H.; et al. Secreted frizzled-related protein 2 (SFRP2) expression promotes lesion proliferation via canonical WNT signaling and indicates lesion borders in extraovarian endometriosis. Hum. Reprod. 2018, 33, 817–831. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Yu, L.; Xie, W.; Man, Y.; Xiong, Y.; Liu, H.; Liu, Y. Estradiol promotes cells invasion by activating β-catenin signaling pathway in endometriosis. Reproduction 2015, 150, 507–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xiong, W.; Xiong, Y.; Liu, H.; Liu, Y. 17β-Estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. Mol. Hum. Reprod. 2016, 22, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufourcq, P.; Couffinhal, T.; Ezan, J.; Barandon, L.; Moreau, C.; Daret, D.; Duplàa, C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation 2002, 106, 3097–3103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.M.; Louis, G.; Exarhopoulos, A.; Mechsner, S.; Ebert, A.D.; Zurakowski, D.; Moses, M.A. Matrix metalloproteinases are elevated in the urine of patients with endometriosis. Fertil. Steril. 2010, 94, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Smith, S.K.; Charnock-Jones, D.S. Transcript profile and localization of Wnt signaling-related molecules in human endometrium. Fertil. Steril. 2008, 90, 201–204. [Google Scholar] [CrossRef]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, K.; Jiang, Z.; Xue, M. IL-37 affects the occurrence and development of endometriosis by regulating the biological behavior of endometrial stromal cells through multiple signaling pathways. Biol. Chem. 2018, 399, 1325–1337. [Google Scholar] [CrossRef]
- Biechele, T.L.; Kulikauskas, R.M.; Toroni, R.A.; Lucero, O.M.; Swift, R.D.; James, R.G.; Robin, N.C.; Dawson, D.W.; Moon, R.T.; Chien, A.J. Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci. Signal. 2012, 5, ra3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, W.-J.; Yoon, J.-B.; Park, J.-C.; Lee, S.-H.; Kaduwal, S.; Kim, H.; Choi, K.-Y. Ras Stabilization Through Aberrant Activation of Wnt/ -Catenin Signaling Promotes Intestinal Tumorigenesis. Sci. Signal. 2012, 5, ra30. [Google Scholar] [CrossRef] [PubMed]
- Guardavaccaro, D.; Clevers, H. Wnt/β-catenin and MAPK signaling: Allies and enemies in different battlefields. Sci. Signal. 2012, 5, pe15. [Google Scholar] [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, L.; Pollock, K.; Guettler, S. Regulation of Wnt/beta-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br. J. Pharm. 2017, 174, 4611–4636. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.Y.; Deng, X.; Byun, S.; Lee, C.; Lee, S.J.; Suh, H.; Zhang, J.; Kang, Q.; Zhang, T.; Westover, K.D.; et al. Direct Targeting of β-Catenin by a Small Molecule Stimulates Proteasomal Degradation and Suppresses Oncogenic Wnt/β-Catenin Signaling. Cell Rep. 2016, 16, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.K.; Li, L.; Cheng, S.H.; Ng, K.; Chan, N.P.; Ip, R.K.; Wong, R.S.; Shing, M.M.; Li, C.K.; Ng, M.H. Secreted-frizzled related protein 1 is a transcriptional repression target of the t(8;21) fusion protein in acute myeloid leukemia. Blood 2011, 118, 6638–6648. [Google Scholar] [CrossRef]
- Pavlovic, Z.; Adams, J.J.; Blazer, L.L.; Gakhal, A.K.; Jarvik, N.; Steinhart, Z.; Robitaille, M.; Mascall, K.; Pan, J.; Angers, S.; et al. A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties. mAbs 2018, 10, 1157–1167. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.N.; Gunderson, C.C.; Sabbatini, P.; McMeekin, D.S.; Mantia-Smaldone, G.; Burger, R.A.; Morgan, M.A.; Kapoun, A.M.; Brachmann, R.K.; Stagg, R.; et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2019, 154, 294–301. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhang, H.; Wang, Z.; Nan, G.; Li, Y.; Zhang, F.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: Implications in targeted cancer therapies. Lab. Investig. 2016, 96, 116–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Zhu, Q.; Neuenschwander, M.; Specker, E.; Wulf-Goldenberg, A.; Weis, W.I.; von-Kries, J.P.; Birchmeier, W. A Small-molecule antagonist of the β-Catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res. 2016, 76, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Q.; Wang, C.; Zhang, Y.; Xue, X.; Song, M.; Zhang, C.; Li, C.; Wu, C.; Li, K.; Hui, X.; et al. Discovery and optimization of 1-(1H-indol-1-yl)ethanone derivatives as CBP/EP300 bromodomain inhibitors for the treatment of castration-resistant prostate cancer. Eur. J. Med. Chem. 2018, 147, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Chiarini, F.; Cappellini, A.; Paganelli, F.; Fini, M.; Santi, S.; Martelli, A.M.; Neri, L.M.; Evangelisti, C. Targeting Wnt/beta-catenin and PI3K/Akt/ mTOR pathways in T-cell acute lymphoblastic leukemia. J. Cell. Physiol. 2020, 235, 5413–5428. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Mukai, T.; Mito, T.; Kodama, S.; Nagasu, A.; Kittaka, M.; Sone, T.; Ueki, Y.; Morita, Y. Pharmacological inhibition of tankyrase induces bone loss in mice by increasing osteoclastogenesis. Bone 2018, 106, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L.; Cardin, D.B.; Shahda, S.; Lenz, H.J.; Dotan, E.; O’Neil, B.H.; Kapoun, A.M.; Stagg, R.J.; Berlin, J.; Messersmith, W.A.; et al. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Investig. New Drugs 2020, 38, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, S.; Botchorishvili, R.; Pouly, J.L.; Canis, M. Targeting the Wnt/β-catenin pathway in endometriosis: A potentially effective approach for treatment and prevention. Mol. Cell. Ther. 2014, 2, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Jeong, W.J.; Ro, E.J.; Choi, K.Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, E.; Scotti, G.M.; Vanni, V.S.; Lazarevic, D.; Makieva, S.; Privitera, L.; Signorelli, S.; Cantone, L.; Bollati, V.; Murdica, V.; et al. Global transcriptomic changes occur in uterine fluid-derived extracellular vesicles during the endometrial window for embryo implantation. Hum. Reprod. 2021, 36, 2249–2274. [Google Scholar] [CrossRef]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- De-Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaj, K.; Miller, J.E.; Lingegowda, H.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019, 4, e128846. [Google Scholar] [CrossRef] [PubMed]
- Li, W.N.; Hsiao, K.Y.; Wang, C.A.; Chang, N.; Hsu, P.L.; Sun, C.H.; Wu, S.R.; Wu, M.H.; Tsai, S.J. Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis. Proc. Natl. Acad. Sci. USA 2020, 117, 25859–25868. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Parmar, M. Lenvatinib. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Alitalo, A.K.; Proulx, S.T.; Karaman, S.; Aebischer, D.; Martino, S.; Jost, M.; Schneider, N.; Bry, M.; Detmar, M. VEGF-C and VEGF-D block-ade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013, 73, 4212–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smaglo, B.G.; Aldeghaither, D.; Weiner, L.M. The development of immunoconjugates for targeted cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 637–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 2020, 207, 112784. [Google Scholar] [CrossRef]
- Bernardi, S.; Balbi, C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biology 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.C.; Eggermont, A.M.M.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, X.; Wu, D.; Deng, M.; Miao, J.; Jin, Z. Down-regulation of Exosomal miR-214-3p Targeting CCN2 Contributes to Endometriosis Fibrosis and the Role of Exosomes in the Horizontal Transfer of miR-214-3p. Reprod. Sci. 2021, 28, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Cancer driver mutations in endometriosis: Variations on the major theme of fibrogenesis. Reprod. Med. Biol. 2018, 17, 369–397. [Google Scholar] [CrossRef] [Green Version]
- Brigstock, D.R. Strategies for blocking the fibrogenic actions of connective tissue grow factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J. Cell Commun. Signal. 2009, 3, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.T.; Camden, J.M.; El-Sayed, F.G.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Weisman, G.A. Increased Expression of TGF-β Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation. PLoS ONE 2015, 10, e0123641. [Google Scholar]
- Wu, J.; Huang, H.; Huang, W.; Wang, L.; Xia, X.; Fang, X. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics 2020, 12, 1193–1213. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.N.; Zhou, X.B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.S.; Zhang, H.; et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Ghazal, S.; McKinnon, B.; Zhou, J.; Mueller, M.; Men, Y.; Yang, L.; Mueller, M.; Flannery, C.; Huang, Y.; Taylor, H.S. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol. Med. 2015, 7, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, D.; Yang, C.; Sun, H.; Lu, T.; Kuang, D. Overexpression of long noncodingRNA, NEAT1 promotes cell proliferation, invasion and migration in endometrial endometrioid adenocarcinoma. Biomed. Pharmacother. 2016, 84, 244–251. [Google Scholar] [CrossRef]
- Liao, W.; Du, Y.; Zhang, C.H.; Pan, F.W.; Yao, Y.; Zhang, T.; Peng, Q. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019, 86, 1–14. [Google Scholar] [CrossRef]
| Pathways | Genes | SNPs |
|---|---|---|
| Inflammation | IL1A | rs6542095 |
| VEZT | rs12320196 | |
| IL16 | rs4072111; rs1131445 | |
| SKAP1 | rs66683298 | |
| TYK2 | rs34536443 | |
| FCRL3 | rs7528684 | |
| MAP3K4 | rs144240142 | |
| WNT4 | rs16826658; rs3820282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomini, E.; Minetto, S.; Li Piani, L.; Pagliardini, L.; Somigliana, E.; Viganò, P. Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. Int. J. Mol. Sci. 2021, 22, 9033. https://doi.org/10.3390/ijms22169033
Giacomini E, Minetto S, Li Piani L, Pagliardini L, Somigliana E, Viganò P. Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. International Journal of Molecular Sciences. 2021; 22(16):9033. https://doi.org/10.3390/ijms22169033
Chicago/Turabian StyleGiacomini, Elisa, Sabrina Minetto, Letizia Li Piani, Luca Pagliardini, Edgardo Somigliana, and Paola Viganò. 2021. "Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies" International Journal of Molecular Sciences 22, no. 16: 9033. https://doi.org/10.3390/ijms22169033
APA StyleGiacomini, E., Minetto, S., Li Piani, L., Pagliardini, L., Somigliana, E., & Viganò, P. (2021). Genetics and Inflammation in Endometriosis: Improving Knowledge for Development of New Pharmacological Strategies. International Journal of Molecular Sciences, 22(16), 9033. https://doi.org/10.3390/ijms22169033







