Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components
Abstract
1. Introduction
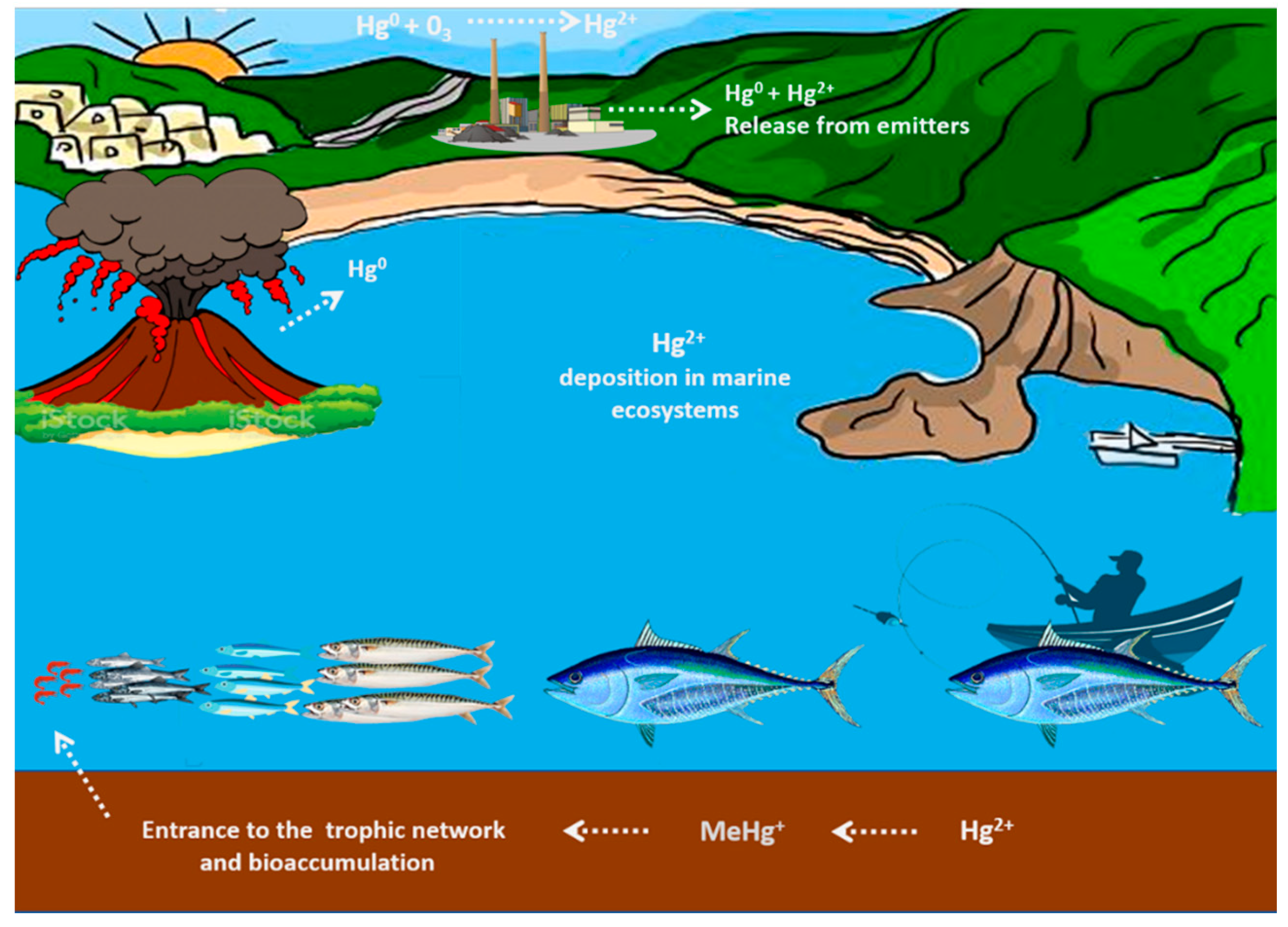
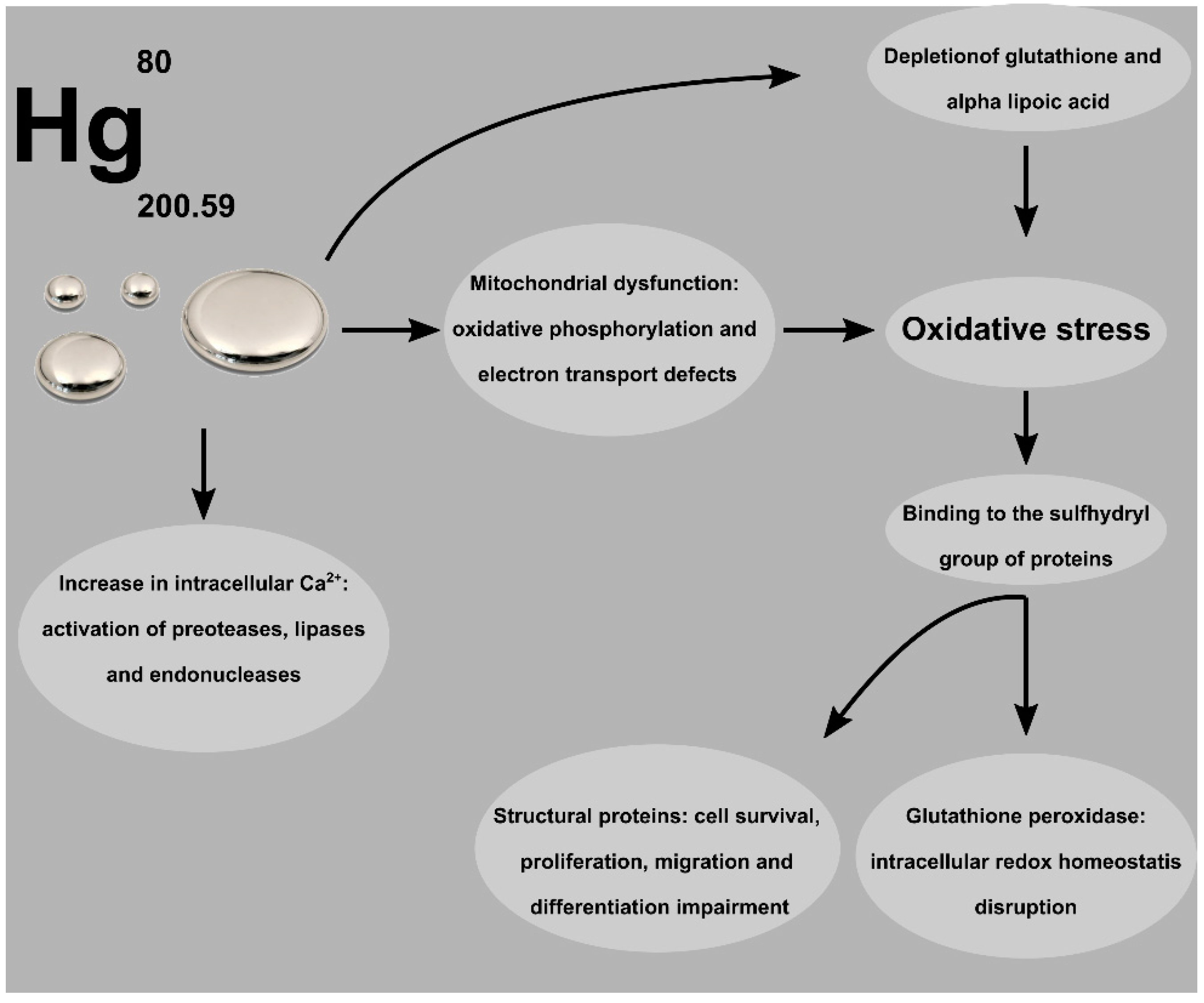
2. Mercury Exposure and Toxicity
3. Acute Poisoning and Long-Term Toxicity
4. Mercury and Endothelial Dysfunction
5. Mercury and Hypertension
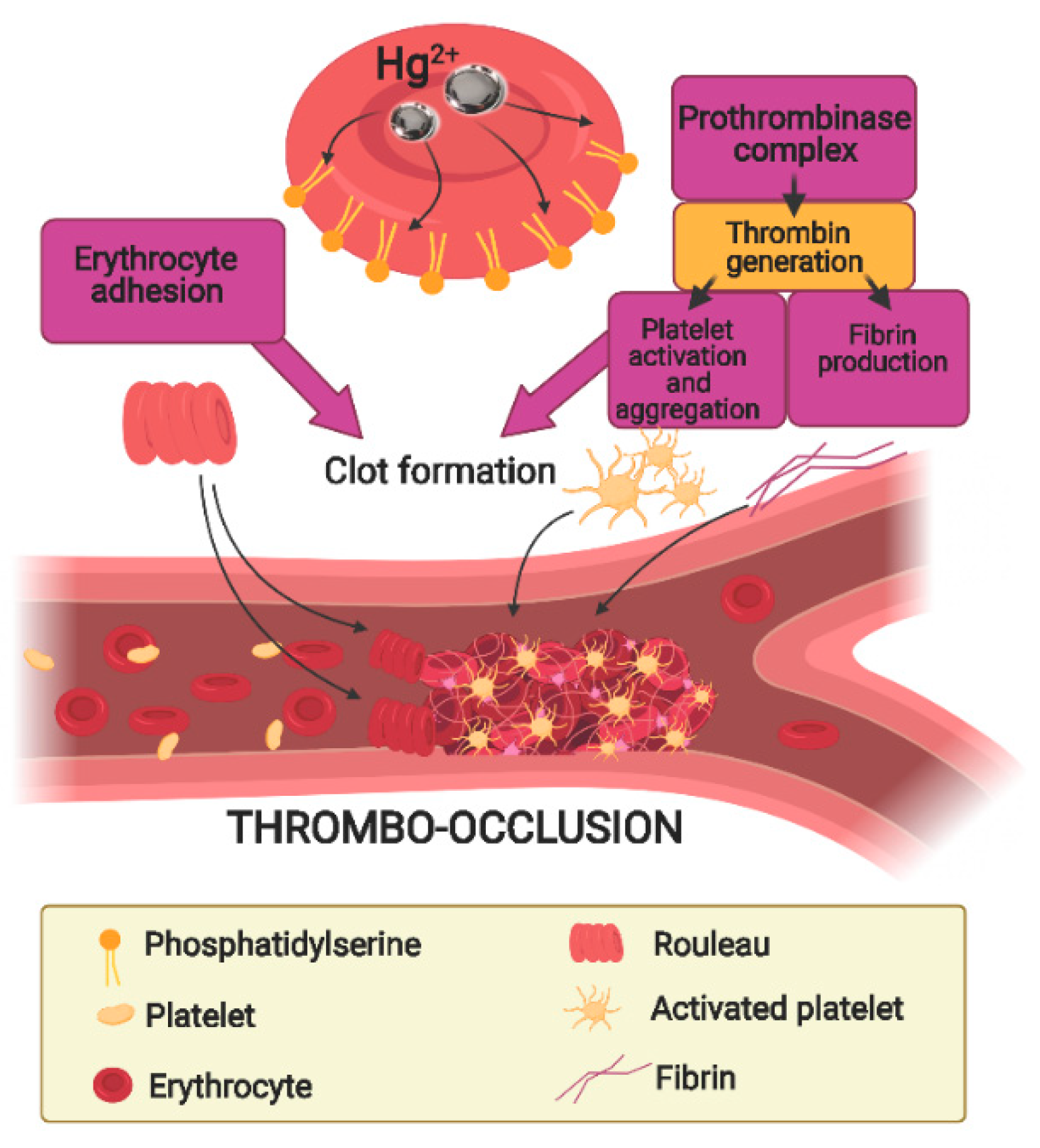
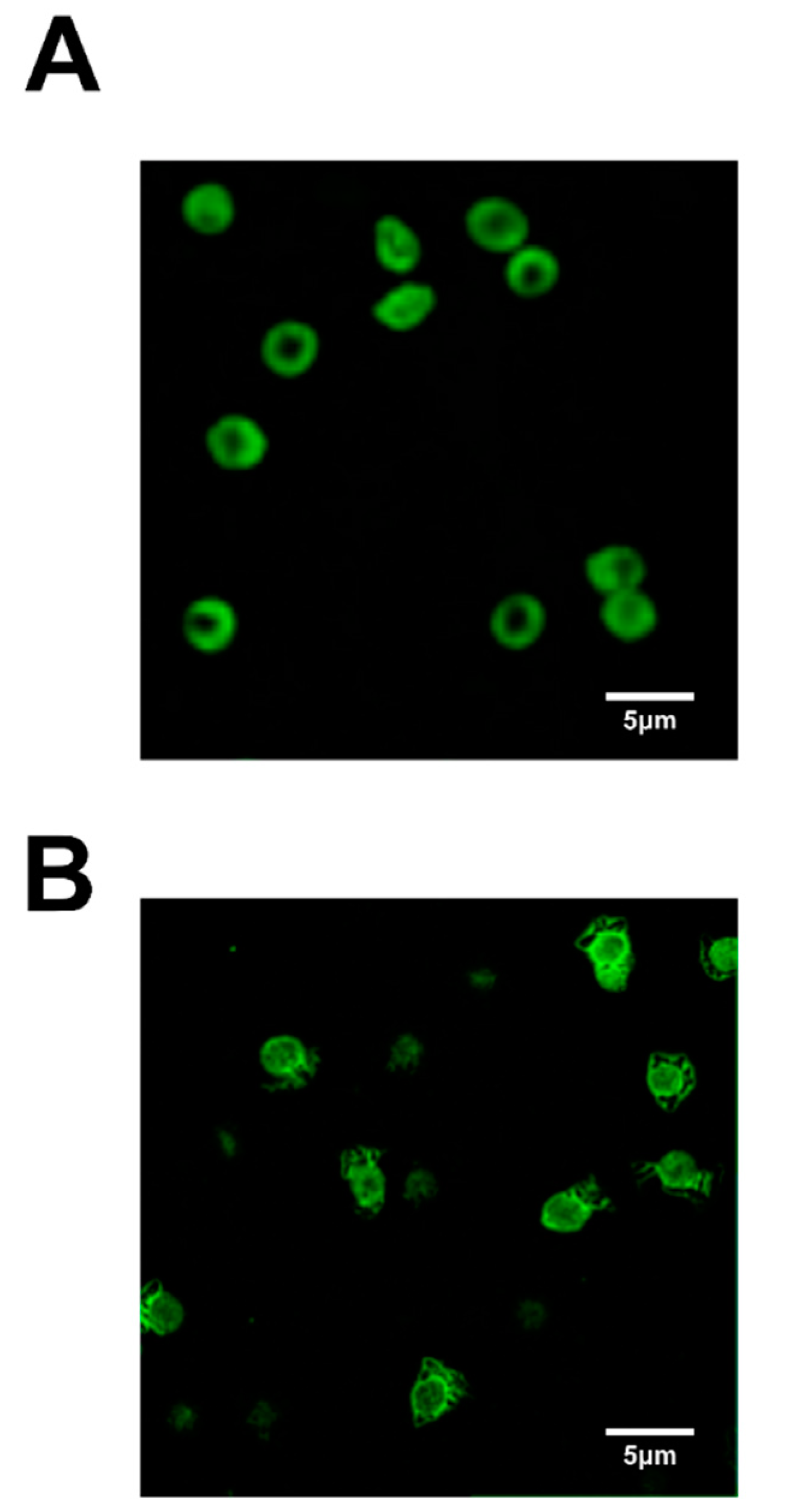
6. The Role of Erythrocytes in Hg-Induced Endothelium Dysfunction
7. Nutritional Aspects of Mercury Toxicity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| COMT | Catecholamine-O-methyl transferase |
| CVD | Cardiovascular diseases |
| Cys | Cysteine |
| GSH | Glutathione |
| Hb | Hemoglobin |
| Hg | Mercury |
| Hg0 | Elemental mercury |
| Hg2+ | Mercuric |
| HT | Hydroxythyrosol |
| MeHg | Methylmercury |
| MVs | Microvesicles |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OS | Oxidative stress |
| PS | Phosphatidylserine |
| RBC | Erythrocytes |
| ROS | Reactive oxygen species |
| SH | Sulfhydryl |
References
- Jadhav, S.H.; Sarkar, S.N.; Patil, R.D.; Tripathi, H.C. Effects of Subchronic Exposure via Drinking Water to a Mixture of Eight Water-Contaminating Metals: A Biochemical and Histopathological Study in Male Rats. Arch. Environ. Contam. Toxicol. 2007, 53, 667–677. [Google Scholar] [CrossRef]
- Jadhav, S.H.; Sarkar, S.N.; Aggarwal, M.; Tripathi, H.C. Induction of Oxidative Stress in Erythrocytes of Male Rats Subchronically Exposed to a Mixture of Eight Metals Found as Groundwater Contaminants in Different Parts of India. Arch. Environ. Contam. Toxicol. 2007, 52, 145–151. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the Human Health Effects of Chemical Mixtures. Environ. Health Perspect. 2002, 110 (Suppl. 1), 25–42. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Cooperstein, S.; Randell, H.; Filiberto, D.; Sorrentino, S.; Kaye, B.; Nicklin, C.; Yagi, J.; Brian, J.; O’Hern, J.; et al. Ecology of Increasing Diseases: Population Growth and Environmental Degradation. Hum. Ecol. Interdiscip. J. 2007, 35, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and Unknowns on Burden of Disease Due to Chemicals: A Systematic Review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Basile, J.N.; Bloch, M.J. Exposure to Air Pollution Increases the Incidence of Hypertension and Diabetes in Black Women Living in Los Angeles. J. Clin. Hypertens. 2012, 14, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular Effects of Copper on the Reproductive System of Mytilus Galloprovincialis. Mol. Reprod. Dev. 2019. [Google Scholar] [CrossRef]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Tollett, V.D.; Benvenutti, E.L.; Deer, L.A.; Rice, T.M. Differential Toxicity to Cd, Pb, and Cu in Dragonfly Larvae (Insecta: Odonata). Arch. Environ. Contam. Toxicol. 2009, 56, 77–84. [Google Scholar] [CrossRef]
- Shargorodsky, J.; Curhan, S.G.; Henderson, E.; Eavey, R.; Curhan, G.C. Heavy Metals Exposure and Hearing Loss in US Adolescents. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 1183–1189. [Google Scholar] [CrossRef]
- Wang, G.; Fowler, B.A. Roles of Biomarkers in Evaluating Interactions among Mixtures of Lead, Cadmium and Arsenic. Toxicol. Appl. Pharmacol. 2008, 233, 92–99. [Google Scholar] [CrossRef]
- Payne, R.J.; Stevens, C.J.; Dise, N.B.; Gowing, D.J.; Pilkington, M.G.; Phoenix, G.K.; Emmett, B.A.; Ashmore, M.R. Impacts of Atmospheric Pollution on the Plant Communities of British Acid Grasslands. Environ. Pollut. 2011, 159, 2602–2608. [Google Scholar] [CrossRef]
- Silva, R.L.B.; Barra, C.M.; Monteiro, T.C.d.N.; Brilhante, O.M. A study of groundwater contamination with organic fuels and potential public health impact in Itaguaí, Rio de Janeiro State, Brazil. Cad. Saude Publica 2002, 18, 1599–1607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef] [PubMed]
- Marsano, F.; Boatti, L.; Ranzato, E.; Cavaletto, M.; Magnelli, V.; Dondero, F.; Viarengo, A. Effects of Mercury on Dictyostelium Discoideum: Proteomics Reveals the Molecular Mechanisms of Physiological Adaptation and Toxicity. J. Proteome Res. 2010, 9, 2839–2854. [Google Scholar] [CrossRef] [PubMed]
- Maresca, V.; Lettieri, G.; Sorbo, S.; Piscopo, M.; Basile, A. Biological Responses to Cadmium Stress in Liverwort Conocephalum Conicum (Marchantiales). Int. J. Mol. Sci. 2020, 21, 6485. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M. Seasonal Dependence of Cadmium Molecular Effects on Mytilus Galloprovincialis (Lamarck, 1819) Protamine-like Protein Properties. Mol. Reprod. Dev. 2019, 86, 1418–1429. [Google Scholar] [CrossRef]
- Lehmann, I.; Sack, U.; Lehmann, J. Metal Ions Affecting the Immune System. Met. Ions Toxicol. Eff. Interact. Interdepend. 2011, 8, 157–185. [Google Scholar]
- Feki-Tounsi, M.; Olmedo, P.; Gil, F.; Khlifi, R.; Mhiri, M.-N.; Rebai, A.; Hamza-Chaffai, A. Cadmium in Blood of Tunisian Men and Risk of Bladder Cancer: Interactions with Arsenic Exposure and Smoking. Environ. Sci. Pollut. Res. Int. 2013, 20, 7204–7213. [Google Scholar] [CrossRef]
- Hu, L.; Greer, J.B.; Solo-Gabriele, H.; Fieber, L.A.; Cai, Y. Arsenic Toxicity in the Human Nerve Cell Line SK-N-SH in the Presence of Chromium and Copper. Chemosphere 2013, 91, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.K.; Ashok, A.; Rai, A.; Tripathi, S.; Nagar, G.K.; Mitra, K.; Bandyopadhyay, S. Exposure to As, Cd and Pb-Mixture Impairs Myelin and Axon Development in Rat Brain, Optic Nerve and Retina. Toxicol. Appl. Pharmacol. 2013, 273, 242–258. [Google Scholar] [CrossRef]
- Fathallah, S.; Medhioub, M.N.; Kraiem, M.M. Sediment Contact Assay Using the Carpet Shell Clam, Ruditapes decussatus L., Embryos and Larvae for Assessing Contamination Levels of Four Sites in Tunisian Coast. Bull. Environ. Contam. Toxicol. 2013, 90, 611–615. [Google Scholar] [CrossRef]
- Alloway, T.P. What Do We Know about the Long-Term Cognitive Effects of Iron-Deficiency Anemia in Infancy? Dev. Med. Child Neurol. 2013, 55, 401–402. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus Galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Dopp, E.; Kligerman, A.D.; Diaz-Bone, R.A. Organoarsenicals. Uptake, Metabolism, and Toxicity. Organomet. Environ. Toxicol. 2010, 7, 231–265. [Google Scholar] [CrossRef]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and Structural Biomarkers to Monitor Heavy Metal Pollution of One of the Most Contaminated Freshwater Sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.H.; Wang, G.; Chen, X.-Q.; Lipsky, M.; Smith, D.; Gwiazda, R.; Fowler, B.A. Exposure to Pb, Cd, and As Mixtures Potentiates the Production of Oxidative Stress Precursors: 30-Day, 90-Day, and 180-Day Drinking Water Studies in Rats. Toxicol. Appl. Pharmacol. 2011, 254, 154–166. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- De Guglielmo, V.; Puoti, R.; Notariale, R.; Maresca, V.; Ausió, J.; Troisi, J.; Verrillo, M.; Basile, A.; Febbraio, F.; Piscopo, M. Alterations in the Properties of Sperm Protamine-like II Protein after Exposure of Mytilus Galloprovincialis (Lamarck 1819) to Sub-Toxic Doses of Cadmium. Ecotoxicol. Environ. Saf. 2019, 169, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The Toxicology of Mercury: Current Research and Emerging Trends. Environ. Res. 2017, 159. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Maiti, S. Sources, Toxicity, and Remediation of Mercury: An Essence Review. Environ. Monit. Assess. 2019, 191. [Google Scholar] [CrossRef] [PubMed]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A Review of Global Environmental Mercury Processes in Response to Human and Natural Perturbations: Changes of Emissions, Climate, and Land Use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Baldi, F. Microbial Transformation of Mercury Species and Their Importance in the Biogeochemical Cycle of Mercury. Met. Ions Biol. Syst. 1997, 34, 213–257. [Google Scholar]
- Qiu, Y.-W.; Wang, W.-X. Comparison of Mercury Bioaccumulation between Wild and Mariculture Food Chains from a Subtropical Bay of Southern China. Environ. Geochem. Health 2016, 38, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Lind, B.; Björnberg, K.A.; Palm, B.; Einarsson, O.; Vahter, M. Inter-Individual Variations of Human Mercury Exposure Biomarkers: A Cross-Sectional Assessment. Environ. Health 2005, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J.C. Mercury Exposure and Public Health. Pediatr. Clin. N. Am. 2007, 54, 237.e1–237.e45. [Google Scholar] [CrossRef]
- Kamensky, O.L.; Horton, D.; Kingsley, D.P.; Bridges, C.C. A Case of Accidental Mercury Intoxication. J. Emerg. Med. 2019, 56, 275–278. [Google Scholar] [CrossRef]
- Jirau-Colón, H.; González-Parrilla, L.; Martinez-Jiménez, J.; Adam, W.; Jiménez-Velez, B. Rethinking the Dental Amalgam Dilemma: An Integrated Toxicological Approach. Int. J. Environ. Res. Public Health 2019, 16, 1036. [Google Scholar] [CrossRef]
- Tibau, A.V.; Grube, B.D. Mercury Contamination from Dental Amalgam. J. Health Pollut. 2019, 9, 190612. [Google Scholar] [CrossRef]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of Glutathione to the Control of Cellular Redox Homeostasis under Toxic Metal and Metalloid Stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of Mercury: Molecular Evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Fujimura, M. Decreased Plasma Thiol Antioxidant Barrier and Selenoproteins as Potential Biomarkers for Ongoing Methylmercury Intoxication and an Individual Protective Capacity. Arch. Toxicol. 2016, 90, 917–926. [Google Scholar] [CrossRef]
- Barcelos, G.R.M.; de Souza, M.F.; de Oliveira, A.Á.S.; Lengert, A.V.H.; de Oliveira, M.T.; Camargo, R.B.D.O.G.; Grotto, D.; Valentini, J.; Garcia, S.C.; Braga, G.Ú.L.; et al. Effects of Genetic Polymorphisms on Antioxidant Status and Concentrations of the Metals in the Blood of Riverside Amazonian Communities Co-Exposed to Hg and Pb. Environ. Res. 2015, 138, 224–232. [Google Scholar] [CrossRef]
- Schläwicke Engström, K.; Strömberg, U.; Lundh, T.; Johansson, I.; Vessby, B.; Hallmans, G.; Skerfving, S.; Broberg, K. Genetic Variation in Glutathione-Related Genes and Body Burden of Methylmercury. Environ. Health Perspect. 2008, 116, 734–739. [Google Scholar] [CrossRef]
- Carvalho, C.M.L.; Chew, E.-H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the Human Thioredoxin System. A Molecular Mechanism of Mercury Toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Al Bakheet, S.A.; Attafi, I.M.; Maayah, Z.H.; Abd-Allah, A.R.; Asiri, Y.A.; Korashy, H.M. Effect of Long-Term Human Exposure to Environmental Heavy Metals on the Expression of Detoxification and DNA Repair Genes. Environ. Pollut. 2013, 181, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, M.; Benoit, J.; Everett, W.; Gibson, J.; Rist, M.; Fredette, N. The Effects of Methylmercury Exposure on Behavior and Biomarkers of Oxidative Stress in Adult Mice. Neurotoxicology 2015, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ynalvez, R.; Gutierrez, J.; Gonzalez-Cantu, H. Mini-Review: Toxicity of Mercury as a Consequence of Enzyme Alteration. Biometals 2016, 29, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bajo, M.J.; de Atauri, P.; Ortega, F.; Westerhoff, H.V.; Gelpí, J.L.; Centelles, J.J.; Cascante, M. Effects of Cadmium and Mercury on the Upper Part of Skeletal Muscle Glycolysis in Mice. PLoS ONE 2014, 9, e80018. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-S.; Kim, S.-J.; Kim, J.S. Inorganic Lead (Pb)- and Mercury (Hg)-Induced Neuronal Cell Death Involves Cytoskeletal Reorganization. Lab. Anim. Res. 2011, 27, 219–225. [Google Scholar] [CrossRef]
- Piscopo, M.; Notariale, R.; Tortora, F.; Lettieri, G.; Palumbo, G.; Manna, C. Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes. Molecules 2020, 25, 3278. [Google Scholar] [CrossRef]
- Nesci, S.; Trombetti, F.; Pirini, M.; Ventrella, V.; Pagliarani, A. Mercury and Protein Thiols: Stimulation of Mitochondrial F1FO-ATPase and Inhibition of Respiration. Chem. Biol. Interact. 2016, 260, 42–49. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative Mechanisms in the Toxicity of Metal Ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Takahashi, T.; Shimohata, T. Vascular Dysfunction Induced by Mercury Exposure. Int. J. Mol. Sci. 2019, 20, 2435. [Google Scholar] [CrossRef]
- Tonazzi, A.; Giangregorio, N.; Console, L.; Scalise, M.; La Russa, D.; Notaristefano, C.; Brunelli, E.; Barca, D.; Indiveri, C. Mitochondrial Carnitine/Acylcarnitine Transporter, a Novel Target of Mercury Toxicity. Chem. Res. Toxicol. 2015, 28, 1015–1022. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Toxic Effects of Metals: Modulation of Intracellular Calcium Homeostasis. Mater. Werkst. 2005, 36, 757–760. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Lerch, M.; Bircher, A.J. Systemically Induced Allergic Exanthem from Mercury. Contact Dermat. 2004, 50, 349–353. [Google Scholar] [CrossRef]
- Oz, S.G.; Tozlu, M.; Yalcin, S.S.; Sozen, T.; Guven, G.S. Mercury Vapor Inhalation and Poisoning of a Family. Inhal. Toxicol. 2012, 24, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Eto, K. Pathology of Minamata Disease. Toxicol. Pathol. 1997, 25, 614–623. [Google Scholar] [CrossRef]
- Igata, A. Epidemiological and Clinical Features of Minamata Disease. Environ. Res. 1993, 63, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Koya, G.; Takeuchi, T. Fetal Minamata Disease: A Neuropathological Study of Two Cases of Intrauterine Intoxication by a Methyl Mercury Compound. J. Neuropathol. Exp. Neurol. 1965, 24, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central Nervous System Pericytes in Health and Disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S. Secondary Sex Ratio in Regions Severely Exposed to Methylmercury “Minamata Disease”. Int. Arch. Occup. Environ. Health 2016, 89, 659–665. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oguri, T.; Abe, M.; Kajitani, H.; Tada, M. Inhibitory Effect of Methylmercury on Migration and Tube Formation by Cultured Human Vascular Endothelial Cells. Arch. Toxicol. 1995, 69, 357–361. [Google Scholar] [CrossRef]
- Rajaee, M.; Sánchez, B.N.; Renne, E.P.; Basu, N. An Investigation of Organic and Inorganic Mercury Exposure and Blood Pressure in a Small-Scale Gold Mining Community in Ghana. Int. J. Environ. Res. Public Health 2015, 12, 10020–10038. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef]
- Robitaille, S.; Mailloux, R.J.; Chan, H.M. Methylmercury Alters Glutathione Homeostasis by Inhibiting Glutaredoxin 1 and Enhancing Glutathione Biosynthesis in Cultured Human Astrocytoma Cells. Toxicol. Lett. 2016, 256, 1–10. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on Autoimmunity and Oxidative Stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, D.V.; Wiggers, G.A.; Padilha, A.S.; Simões, M.R. Endothelium: A Target for Harmful Actions of Metals. Curr. Hypertens. Rev. 2020, 16, 201–209. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.Á.S.; de Souza, M.F.; Lengert, A.V.H.; de Oliveira, M.T.; de Camargo, R.B.O.G.; Braga, G.Ú.L.; de Cólus, I.M.S.; Barbosa, F.; Barcelos, G.R.M. Genetic Polymorphisms in Glutathione (GSH-) Related Genes Affect the Plasmatic Hg/Whole Blood Hg Partitioning and the Distribution between Inorganic and Methylmercury Levels in Plasma Collected from a Fish-Eating Population. BioMed Res. Int. 2014, 2014, e940952. [Google Scholar] [CrossRef]
- Omanwar, S.; Fahim, M. Mercury Exposure and Endothelial Dysfunction: An Interplay Between Nitric Oxide and Oxidative Stress. Int. J. Toxicol. 2015, 34, 300–307. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Salonen, J.T.; Seppänen, K.; Lakka, T.A.; Salonen, R.; Kaplan, G.A. Mercury Accumulation and Accelerated Progression of Carotid Atherosclerosis: A Population-Based Prospective 4-Year Follow-up Study in Men in Eastern Finland. Atherosclerosis 2000, 148, 265–273. [Google Scholar] [CrossRef]
- Guallar, E.; Sanz-Gallardo, M.I.; van’t Veer, P.; Bode, P.; Aro, A.; Gómez-Aracena, J.; Kark, J.D.; Riemersma, R.A.; Martín-Moreno, J.M.; Kok, F.J.; et al. Mercury, Fish Oils, and the Risk of Myocardial Infarction. N. Engl. J. Med. 2002, 347, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, G.A.; Peçanha, F.M.; Briones, A.M.; Pérez-Girón, J.V.; Miguel, M.; Vassallo, D.V.; Cachofeiro, V.; Alonso, M.J.; Salaices, M. Low Mercury Concentrations Cause Oxidative Stress and Endothelial Dysfunction in Conductance and Resistance Arteries. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1033–H1043. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide Anions and Hyperoxia Inactivate Endothelium-Derived Relaxing Factor. Am. J. Physiol. 1986, 250, H822–H827. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Vanhoutte, P.M. Superoxide Anion Is an Endothelium-Derived Contracting Factor. Am. J. Physiol. 1989, 257, H33–H37. [Google Scholar] [CrossRef]
- Lemos, N.B.; Angeli, J.K.; Faria, T.D.O.; Ribeiro Junior, R.F.; Vassallo, D.V.; Padilha, A.S.; Stefanon, I. Low Mercury Concentration Produces Vasoconstriction, Decreases Nitric Oxide Bioavailability and Increases Oxidative Stress in Rat Conductance Artery. PLoS ONE 2012, 7, e49005. [Google Scholar] [CrossRef]
- Furieri, L.B.; Fioresi, M.; Junior, R.F.R.; Bartolomé, M.V.; Fernandes, A.A.; Cachofeiro, V.; Lahera, V.; Salaices, M.; Stefanon, I.; Vassallo, D.V. Exposure to Low Mercury Concentration in Vivo Impairs Myocardial Contractile Function. Toxicol. Appl. Pharmacol. 2011, 255, 193–199. [Google Scholar] [CrossRef]
- Pecanha, F.M.; Wiggers, G.A.; Briones, A.M.; Perez-Giron, J.V.; Miguel, M.; Garcia-Redondo, A.B.; Vassallo, D.V.; Alonso, M.J.; Salaices, M. The Role of Cyclooxygenase (COX)-2 Derived Prostanoids on Vasoconstrictor Responses to Phenylephrine Is Increased by Exposure to Low Mercury Concentration. J. Physiol. Pharmacol. 2010, 61, 29–36. [Google Scholar]
- Houston, M.C. The Role of Mercury and Cadmium Heavy Metals in Vascular Disease, Hypertension, Coronary Heart Disease, and Myocardial Infarction. Altern. Ther. Health Med. 2007, 13, S128–S133. [Google Scholar]
- Magos, L.; Sparrow, S.; Snowden, R. The Comparative Renotoxicology of Phenylmercury and Mercuric Chloride. Arch. Toxicol. 1982, 50, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.D.; Rai, A.N.; Hardiek, M.L. Mercury Intoxication and Arterial Hypertension: Report of Two Patients and Review of the Literature. Pediatrics 2000, 105, E34. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Sällsten, G.; Garcia-Gómez, M.; Pompe-Kirn, V.; Zaridze, D.; Bulbulyan, M.; Caballero, J.D.; Ceccarelli, F.; Kobal, A.B.; Merler, E. Mortality from Cardiovascular Diseases and Exposure to Inorganic Mercury. Occup. Environ. Med. 2001, 58, 461–466. [Google Scholar] [CrossRef]
- Yorifuji, T.; Tsuda, T.; Kashima, S.; Takao, S.; Harada, M. Long-Term Exposure to Methylmercury and Its Effects on Hypertension in Minamata. Environ. Res. 2010, 110, 40–46. [Google Scholar] [CrossRef]
- Houston, M.C. Role of Mercury Toxicity in Hypertension, Cardiovascular Disease, and Stroke. J. Clin. Hypertens. 2011, 13, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kobal, A.B.; Horvat, M.; Prezelj, M.; Briski, A.S.; Krsnik, M.; Dizdarevic, T.; Mazej, D.; Falnoga, I.; Stibilj, V.; Arneric, N.; et al. The Impact of Long-Term Past Exposure to Elemental Mercury on Antioxidative Capacity and Lipid Peroxidation in Mercury Miners. J. Trace Elem. Med. Biol. 2004, 17, 261–274. [Google Scholar] [CrossRef]
- Fillion, M.; Mergler, D.; Sousa Passos, C.J.; Larribe, F.; Lemire, M.; Guimarães, J.R.D. A Preliminary Study of Mercury Exposure and Blood Pressure in the Brazilian Amazon. Environ. Health 2006, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E.; Stein, J.H.; Morgan, B.J.; Stanton, N.; Young, T.; Nieto, F.J. Association of Blood and Hair Mercury with Blood Pressure and Vascular Reactivity. WMJ 2009, 108, 250–252. [Google Scholar] [PubMed]
- Pedersen, E.B.; Jørgensen, M.E.; Pedersen, M.B.; Siggaard, C.; Sørensen, T.B.; Mulvad, G.; Hansen, J.C.; Asmund, G.; Skjoldborg, H. Relationship between Mercury in Blood and 24-h Ambulatory Blood Pressure in Greenlanders and Danes. Am. J. Hypertens. 2005, 18, 612–618. [Google Scholar] [CrossRef]
- Vupputuri, S.; Longnecker, M.P.; Daniels, J.L.; Guo, X.; Sandler, D.P. Blood Mercury Level and Blood Pressure among US Women: Results from the National Health and Nutrition Examination Survey 1999-2000. Environ. Res. 2005, 97, 195–200. [Google Scholar] [CrossRef]
- Valera, B.; Dewailly, E.; Poirier, P. Cardiac Autonomic Activity and Blood Pressure among Nunavik Inuit Adults Exposed to Environmental Mercury: A Cross-Sectional Study. Environ. Health 2008, 7, 29. [Google Scholar] [CrossRef]
- Kobal, A.B.; Flisar, Z.; Miklavcic, V.; Dizdarević, T.; Sesek-Briski, A. Renal Function in Miners Intermittently Exposed to Elemental Mercury Vapour. Arh. Hig. Rada Toksikol. 2000, 51, 369–380. [Google Scholar]
- Weisel, J.W.; Litvinov, R.I. Red Blood Cells: The Forgotten Player in Hemostasis and Thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zwaal, R.F.A.; Comfurius, P.; Van Deenen, L.L.M. Membrane Asymmetry and Blood Coagulation. Nature 1977, 268, 358–360. [Google Scholar] [CrossRef]
- Closse, C.; Dachary-Prigent, J.; Boisseau, M.R. Phosphatidylserine-Related Adhesion of Human Erythrocytes to Vascular Endothelium. Br. J. Haematol. 1999, 107, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, L.; Galliera, E.; Corsi Romanelli, M.M. Erythrocytes as Markers of Oxidative Stress Related Pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef]
- Hsieh, C.; Prabhu, N.C.S.; Rajashekaraiah, V. Age-Related Modulations in Erythrocytes under Blood Bank Conditions. Transfus. Med. Hemother. 2019, 46, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Minetti, G. The Potential of Erythrocytes as Cellular Aging Models. Cell Death Differ. 2017, 24, 1475–1477. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Zalups, R.K. Molecular Interactions with Mercury in the Kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar] [PubMed]
- Vianna, A.D.S.; Matos, E.P.D.; Jesus, I.M.D.; Asmus, C.I.R.F.; Câmara, V.D.M. Human Exposure to Mercury and Its Hematological Effects: A Systematic Review. Cad. Pública 2019, 35. [Google Scholar] [CrossRef] [PubMed]
- Zolla, L.; Lupidi, G.; Bellelli, A.; Amiconi, G. Effect of Mercuric Ions on Human Erythrocytes. Relationships between Hypotonic Swelling and Cell Aggregation. Biochim. Biophys. Acta 1997, 1328, 273–280. [Google Scholar] [CrossRef]
- De Palma, G.; Mariotti, O.; Lonati, D.; Goldoni, M.; Catalani, S.; Mutti, A.; Locatelli, C.; Apostoli, P. Toxicokinetics and Toxicodynamics of Elemental Mercury Following Self-Administration. Clin. Toxicol. 2008, 46, 869–876. [Google Scholar] [CrossRef][Green Version]
- Suwalsky, M.; Ungerer, B.; Villena, F.; Cuevas, F.; Sotomayor, C.P. HgCl2 Disrupts the Structure of the Human Erythrocyte Membrane and Model Phospholipid Bilayers. J. Inorg. Biochem. 2000, 81, 267–273. [Google Scholar] [CrossRef]
- Lang, F.; Lang, K.S.; Lang, P.A.; Huber, S.M.; Wieder, T. Mechanisms and Significance of Eryptosis. Antioxid. Redox Signal. 2006, 8, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Notariale, R.; Lang, F.; Manna, C. Hydroxytyrosol Decreases Phosphatidylserine Exposure and Inhibits Suicidal Death Induced by Lysophosphatidic Acid in Human Erythrocytes. Cell Physiol. Biochem. 2019, 53, 921–932. [Google Scholar] [CrossRef]
- Officioso, A.; Alzoubi, K.; Lang, F.; Manna, C. Hydroxytyrosol Inhibits Phosphatidylserine Exposure and Suicidal Death Induced by Mercury in Human Erythrocytes: Possible Involvement of the Glutathione Pathway. Food Chem. Toxicol. 2016, 89, 47–53. [Google Scholar] [CrossRef]
- Gottlieb, Y.; Topaz, O.; Cohen, L.A.; Yakov, L.D.; Haber, T.; Morgenstern, A.; Weiss, A.; Chait Berman, K.; Fibach, E.; Meyron-Holtz, E.G. Physiologically Aged Red Blood Cells Undergo Erythrophagocytosis in Vivo but Not in Vitro. Haematologica 2012, 97, 994–1002. [Google Scholar] [CrossRef]
- Arashiki, N.; Takakuwa, Y. Maintenance and Regulation of Asymmetric Phospholipid Distribution in Human Erythrocyte Membranes: Implications for Erythrocyte Functions. Curr. Opin. Hematol. 2017, 24, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, R.; Prażanowski, M.; Michalska, M.; Krajewska, U.; Mielicki, W.P. Disorders in Blood Coagulation in Humans Occupationally Exposed to Mercuric Vapors. J. Trace Elem. Exp. Med. 2002, 15, 21–29. [Google Scholar] [CrossRef]
- Lim, K.-M.; Kim, S.; Noh, J.-Y.; Kim, K.; Jang, W.-H.; Bae, O.-N.; Chung, S.-M.; Chung, J.-H. Low-Level Mercury Can Enhance Procoagulant Activity of Erythrocytes: A New Contributing Factor for Mercury-Related Thrombotic Disease. Environ. Health Perspect. 2010, 118, 928–935. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Officioso, A.; Sorbo, S.; Basile, A.; Manna, C. The Protective Role of Olive Oil Hydroxytyrosol against Oxidative Alterations Induced by Mercury in Human Erythrocytes. Food Chem. Toxicol. 2015, 82, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Eisele, K.; Lang, P.A.; Kempe, D.S.; Klarl, B.A.; Niemöller, O.; Wieder, T.; Huber, S.M.; Duranton, C.; Lang, F. Stimulation of Erythrocyte Phosphatidylserine Exposure by Mercury Ions. Toxicol. Appl. Pharmacol. 2006, 210, 116–122. [Google Scholar] [CrossRef]
- Maseko, P.B.; van Rooy, M.; Taute, H.; Venter, C.; Serem, J.C.; Oberholzer, H.M. Whole Blood Ultrastructural Alterations by Mercury, Nickel and Manganese Alone and in Combination: An Ex Vivo Investigation. Toxicol. Ind. Health 2021, 37, 98–111. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, J.L. Evidence for the Presence of L-Arginine-Nitric Oxide Pathway in Human Red Blood Cells: Relevance in the Effects of Red Blood Cells on Platelet Function. J. Cardiovasc. Pharmacol. 1998, 32, 57–61. [Google Scholar] [CrossRef]
- Kang, E.S.; Ford, K.; Grokulsky, G.; Wang, Y.B.; Chiang, T.M.; Acchiardo, S.R. Normal Circulating Adult Human Red Blood Cells Contain Inactive NOS Proteins. J. Lab. Clin. Med. 2000, 135, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chakraborty Patra, S.; Basu Roy, S.; Kahn, N.N.; Sinha, A.K. Purification and Properties of Insulin-Activated Nitric Oxide Synthase from Human Erythrocyte Membranes. Arch. Physiol. Biochem. 2001, 109, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Rassaf, T.; Schindler, A.; Picker, O.; Scheeren, T.; Gödecke, A.; Schrader, J.; Schulz, R.; et al. Plasma Nitrite Reflects Constitutive Nitric Oxide Synthase Activity in Mammals. Free Radic. Biol. Med. 2003, 35, 790–796. [Google Scholar] [CrossRef]
- Harisa, G.I.; Mariee, A.D.; Abo-Salem, O.M.; Attiaa, S.M. Erythrocyte Nitric Oxide Synthase as a Surrogate Marker for Mercury-Induced Vascular Damage: The Modulatory Effects of Naringin. Environ. Toxicol. 2014, 29, 1314–1322. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Lancaster, J.R.; Freeman, B.A.; Schechter, A.N. Nitric Oxide’s Reactions with Hemoglobin: A View through the SNO-Storm. Nat. Med. 2003, 9, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mahmood, R. Mercury Chloride Toxicity in Human Erythrocytes: Enhanced Generation of ROS and RNS, Hemoglobin Oxidation, Impaired Antioxidant Power, and Inhibition of Plasma Membrane Redox System. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Minetti, M.; Agati, L.; Malorni, W. The Microenvironment Can Shift Erythrocytes from a Friendly to a Harmful Behavior: Pathogenetic Implications for Vascular Diseases. Cardiovasc. Res. 2007, 75, 21–28. [Google Scholar] [CrossRef]
- Harisa, G.I.; Alanazi, F.K.; El-Bassat, R.A.; Malik, A.; Abdallah, G.M. Protective Effect of Pravastatin against Mercury Induced Vascular Cells Damage: Erythrocytes as Surrogate Markers. Environ. Toxicol. Pharmacol. 2012, 34, 428–435. [Google Scholar] [CrossRef]
- Saravanakumar, M.; Raja, B. Veratric Acid, a Phenolic Acid Attenuates Blood Pressure and Oxidative Stress in L-NAME Induced Hypertensive Rats. Eur. J. Pharmacol. 2011, 671, 87–94. [Google Scholar] [CrossRef]
- Nunes, E.; Cavaco, A.; Carvalho, C. Exposure Assessment of Pregnant Portuguese Women to Methylmercury through the Ingestion of Fish: Cross-Sectional Survey and Biomarker Validation. J. Toxicol. Environ. Health A 2014, 77, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Q.; Yuan, Z.; Jin, S.; Jing, M. Research Progress of Mercury Bioaccumulation in the Aquatic Food Chain, China: A Review. Bull. Environ. Contam. Toxicol. 2019, 102, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Dsikowitzky, L.; Mengesha, M.; Dadebo, E.; de Carvalho, C.E.V.; Sindern, S. Assessment of Heavy Metals in Water Samples and Tissues of Edible Fish Species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environ. Monit. Assess. 2013, 185, 3117–3131. [Google Scholar] [CrossRef]
- Barkay, T.; Poulain, A.J. Mercury (Micro)Biogeochemistry in Polar Environments. FEMS Microbiol. Ecol. 2007, 59, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Xu, X.-R.; Yu, S.; Cheng, H.; Peng, J.-X.; Hong, Y.-G.; Feng, X.-B. Mercury Contamination in Fish and Human Hair from Hainan Island, South China Sea: Implication for Human Exposure. Environ. Res. 2014, 135, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kalender, S.; Uzun, F.G.; Demir, F.; Uzunhisarcıklı, M.; Aslanturk, A. Mercuric Chloride-Induced Testicular Toxicity in Rats and the Protective Role of Sodium Selenite and Vitamin E. Food Chem. Toxicol. 2013, 55, 456–462. [Google Scholar] [CrossRef]
- Pal, R.; Rai, J.P.N. Phytochelatins: Peptides Involved in Heavy Metal Detoxification. Appl. Biochem. Biotechnol. 2010, 160, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Manini, P.; Panzella, L.; Eidenberger, T.; Giarra, A.; Cerruti, P.; Trifuoggi, M.; Napolitano, A. Efficient Binding of Heavy Metals by Black Sesame Pigment: Toward Innovative Dietary Strategies To Prevent Bioaccumulation. J. Agric. Food Chem. 2016, 64. [Google Scholar] [CrossRef]
- Rowland, I.R.; Mallett, A.K.; Flynn, J.; Hargreaves, R.J. The Effect of Various Dietary Fibres on Tissue Concentration and Chemical Form of Mercury after Methylmercury Exposure in Mice. Arch. Toxicol. 1986, 59, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Khan, R.; Shakir, H.A.; Tahir, H.M.; Mumtaz, S.; Andleeb, S. Therapeutic Role of Garlic and Vitamins C and E against Toxicity Induced by Lead on Various Organs. Environ. Sci. Pollut. Res. Int. 2020, 27, 8953–8964. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of Garlic and Its Bioactive Components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium Health Benefit Values: Updated Criteria for Mercury Risk Assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Eb, K. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15. [Google Scholar] [CrossRef]
- Rendón-Ramírez, A.; Cerbón-Solórzano, J.; Maldonado-Vega, M.; Quintanar-Escorza, M.; Calderón-Salinas, J. Vitamin-E Reduces the Oxidative Damage on δ-aminolevulinic Dehydratase Induced by Lead Intoxication in Rat Erythrocytes. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2007, 21, 1121–1126. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Al-Bideri, A.W. Histopathological Study on the Effect of Antioxidants (Vitamin E and Selenium) in Hepatotoxicity Induced by Lead Acetate in Rats. Qadisiah Med. J. 2011, 7, 142–155. [Google Scholar]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-Rich Feijoa Sellowiana (Pineapple Guava) Extracts Protect Human Red Blood Cells from Mercury-Induced Cellular Toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef]
- Mohammad Abu-Taweel, G.; Al-Fifi, Z. Protective Effects of Curcumin towards Anxiety and Depression-like Behaviors Induced Mercury Chloride. Saudi J. Biol. Sci. 2021, 28, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Xiao, Y.; Wang, Y.; Wan, Y.; Li, X.; Li, Q.; Tang, X.; Cai, D.; Ran, B.; et al. Curcumin Ameliorates Mercuric Chloride-Induced Liver Injury via Modulating Cytochrome P450 Signaling and Nrf2/HO-1 Pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111426. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary Polyphenol Intake Is Associated with HDL-Cholesterol and A Better Profile of Other Components of the Metabolic Syndrome: A PREDIMED-Plus Sub-Study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; de Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and Antioxidant Activity of Proteins from Feijoa Sellowiana Berg. Fruit before and after in Vitro Gastrointestinal Digestion. Nat. Prod. Res. 2019, 34, 2607–2611. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Martin-Calvo, N. Mediterranean Diet and Life Expectancy; beyond Olive Oil, Fruits, and Vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 401–407. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED INVESTIGATORS. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Jiang, L.; Geng, C.; Zhong, L. Protective Effect of Hydroxytyrosol against Acrylamide-Induced Cytotoxicity and DNA Damage in HepG2 Cells. Mutat. Res. 2009, 664, 64–68. [Google Scholar] [CrossRef]
- Rodríguez-Ramiro, I.; Martín, M.Á.; Ramos, S.; Bravo, L.; Goya, L. Olive Oil Hydroxytyrosol Reduces Toxicity Evoked by Acrylamide in Human Caco-2 Cells by Preventing Oxidative Stress. Toxicology 2011, 288, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Troise, A.D.; Colantuono, A.; Fiore, A. Spray-Dried Olive Mill Wastewater Reduces Maillard Reaction in Cookies Model System. Food Chem. 2020, 323, 126793. [Google Scholar] [CrossRef]
- Wu, X.; Li, C.; Mariyam, Z.; Jiang, P.; Zhou, M.; Zeb, F.; Haq, I.U.; Chen, A.; Feng, Q. Acrolein-Induced Atherogenesis by Stimulation of Hepatic Flavin Containing Monooxygenase 3 and a Protection from Hydroxytyrosol. J. Cell. Physiol. 2018, 234, 475–485. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Liu, J.; et al. Hydroxytyrosol Protects against Oxidative Damage by Simultaneous Activation of Mitochondrial Biogenesis and Phase II Detoxifying Enzyme Systems in Retinal Pigment Epithelial Cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, L.; Chen, A.; Xu, C.; Feng, Q. Protective Effects of Olive Leaf Extract on Acrolein-Exacerbated Myocardial Infarction via an Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2018, 19, 493. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol Against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In Vitro and in Vivo Study. Front. Vet. Sci. 2020, 7, 136. [Google Scholar] [CrossRef]
- Mohan, V.; Das, S.; Rao, S.B.S. Hydroxytyrosol, a Dietary Phenolic Compound Forestalls the Toxic Effects of Methylmercury-Induced Toxicity in IMR-32 Human Neuroblastoma Cells. Environ. Toxicol. 2016, 31, 1264–1275. [Google Scholar] [CrossRef]
- Officioso, A.; Panzella, L.; Tortora, F.; Alfieri, M.L.; Napolitano, A.; Manna, C. Comparative Analysis of the Effects of Olive Oil Hydroxytyrosol and Its 5-S-Lipoyl Conjugate in Protecting Human Erythrocytes from Mercury Toxicity. Oxid. Med. Cell. Longev. 2018, 2018, 9042192. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive Oil Phenolic Compounds Inhibit Homocysteine-Induced Endothelial Cell Adhesion Regardless of Their Different Antioxidant Activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.A.L.; Marcadenti, A.; Portal, V.L. Effects of Olive Oil Phenolic Compounds on Inflammation in the Prevention and Treatment of Coronary Artery Disease. Nutrients 2017, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notariale, R.; Infantino, R.; Palazzo, E.; Manna, C. Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. Int. J. Mol. Sci. 2021, 22, 6604. https://doi.org/10.3390/ijms22126604
Notariale R, Infantino R, Palazzo E, Manna C. Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. International Journal of Molecular Sciences. 2021; 22(12):6604. https://doi.org/10.3390/ijms22126604
Chicago/Turabian StyleNotariale, Rosaria, Rosmara Infantino, Enza Palazzo, and Caterina Manna. 2021. "Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components" International Journal of Molecular Sciences 22, no. 12: 6604. https://doi.org/10.3390/ijms22126604
APA StyleNotariale, R., Infantino, R., Palazzo, E., & Manna, C. (2021). Erythrocytes as a Model for Heavy Metal-Related Vascular Dysfunction: The Protective Effect of Dietary Components. International Journal of Molecular Sciences, 22(12), 6604. https://doi.org/10.3390/ijms22126604





