Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model
Abstract
1. Introduction
2. Results
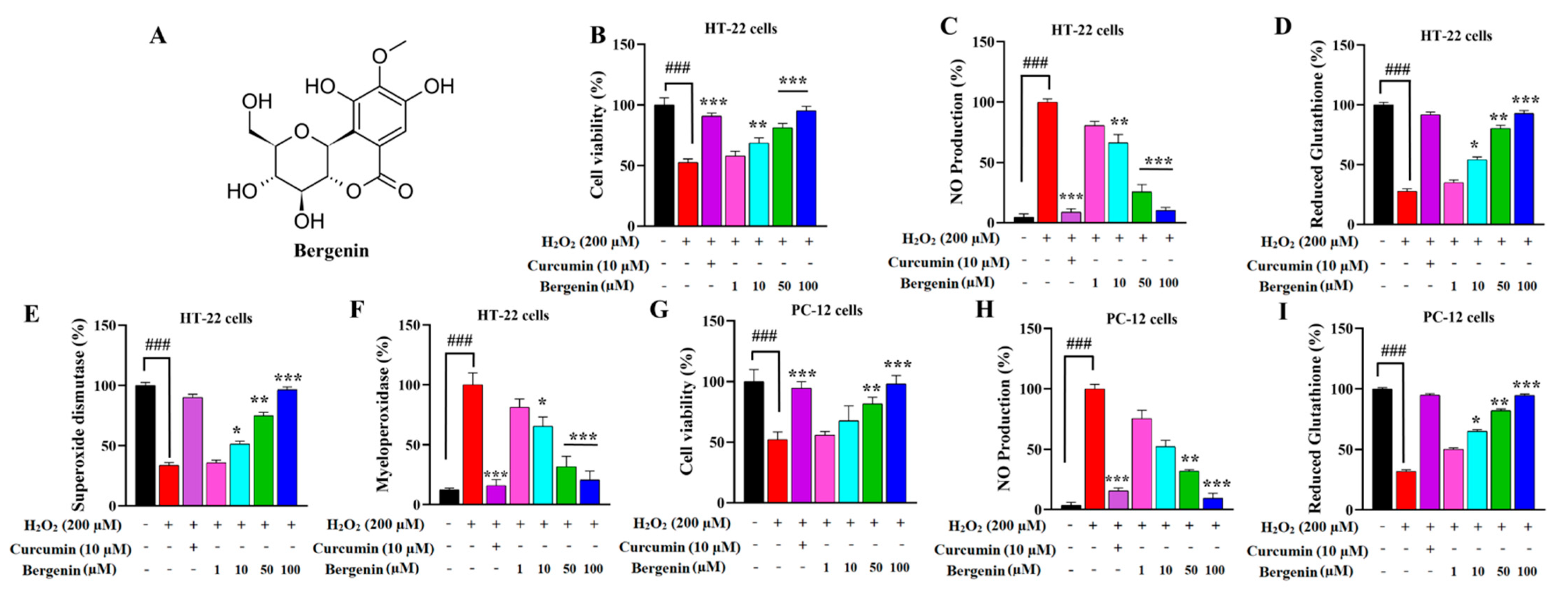
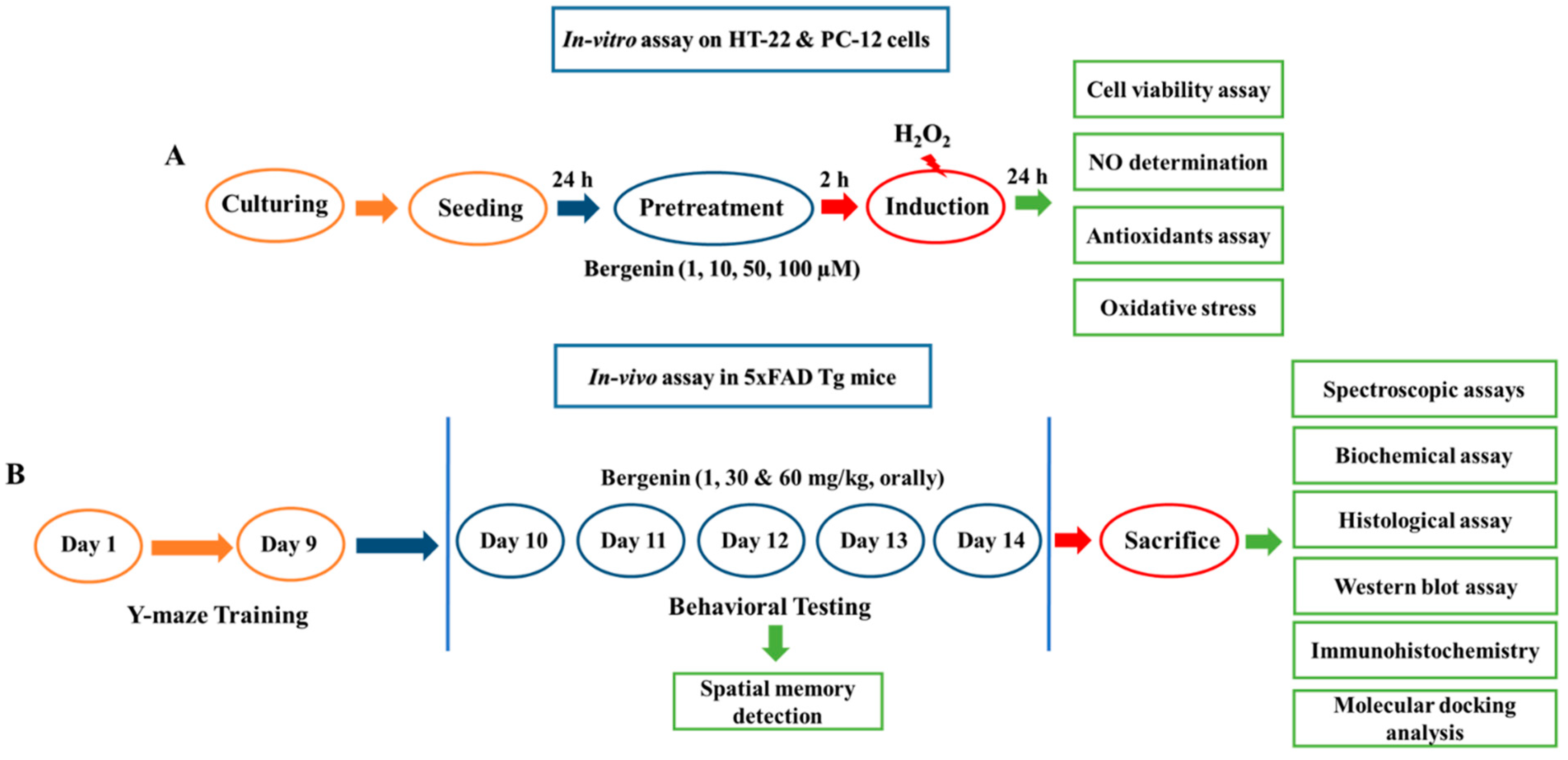
2.1. Bergenin Exerts Neuro-Protective Effect in HT-22 and PC-12 Cells
2.2. Bergenin Reduces Nitrite Production in HT-22 and PC-12 Cells
2.3. Bergenin Enhances Anti-Oxidants and Decreases Oxidative Stress in Cells
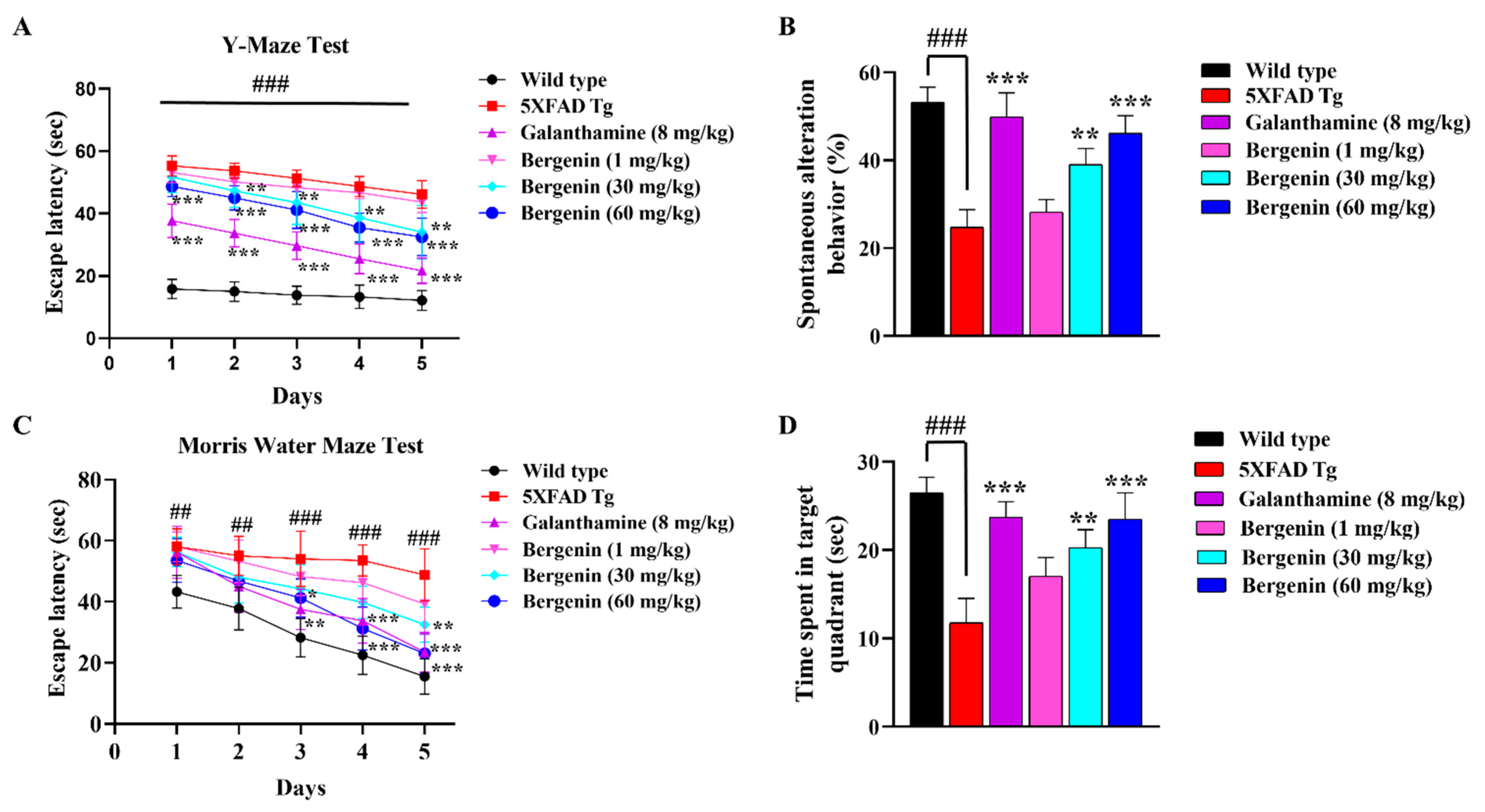
2.4. Bergenin Enhances Spatial Memory in 5xFAD Tg Mice
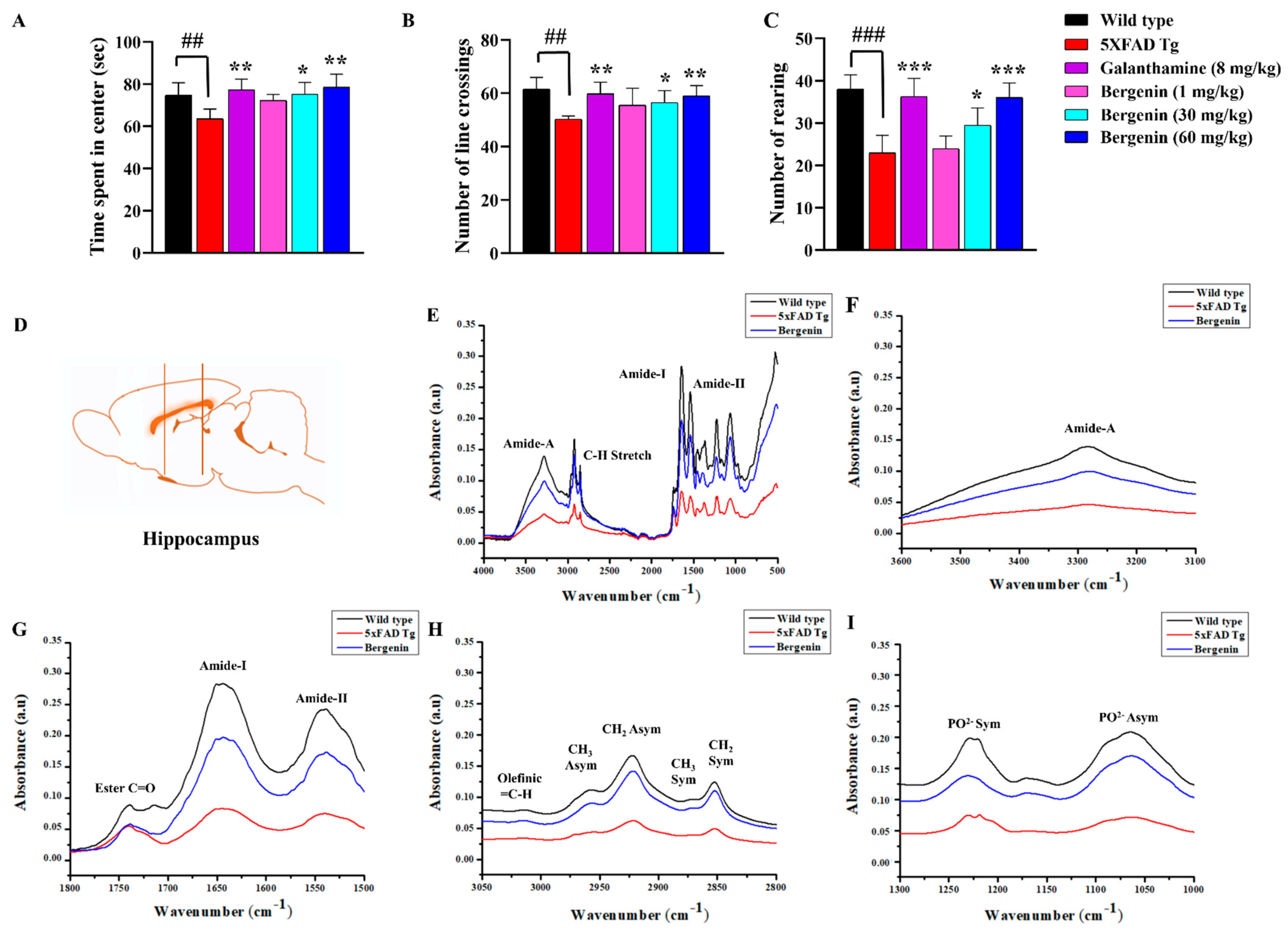
2.5. Effect of Bergenin on Locomotor and Anxiety-Like Behavior in 5xFAD Tg Mice
2.6. Bergenin Protects against Decrease in Proteins, Lipids and Their Secondary Derivatives
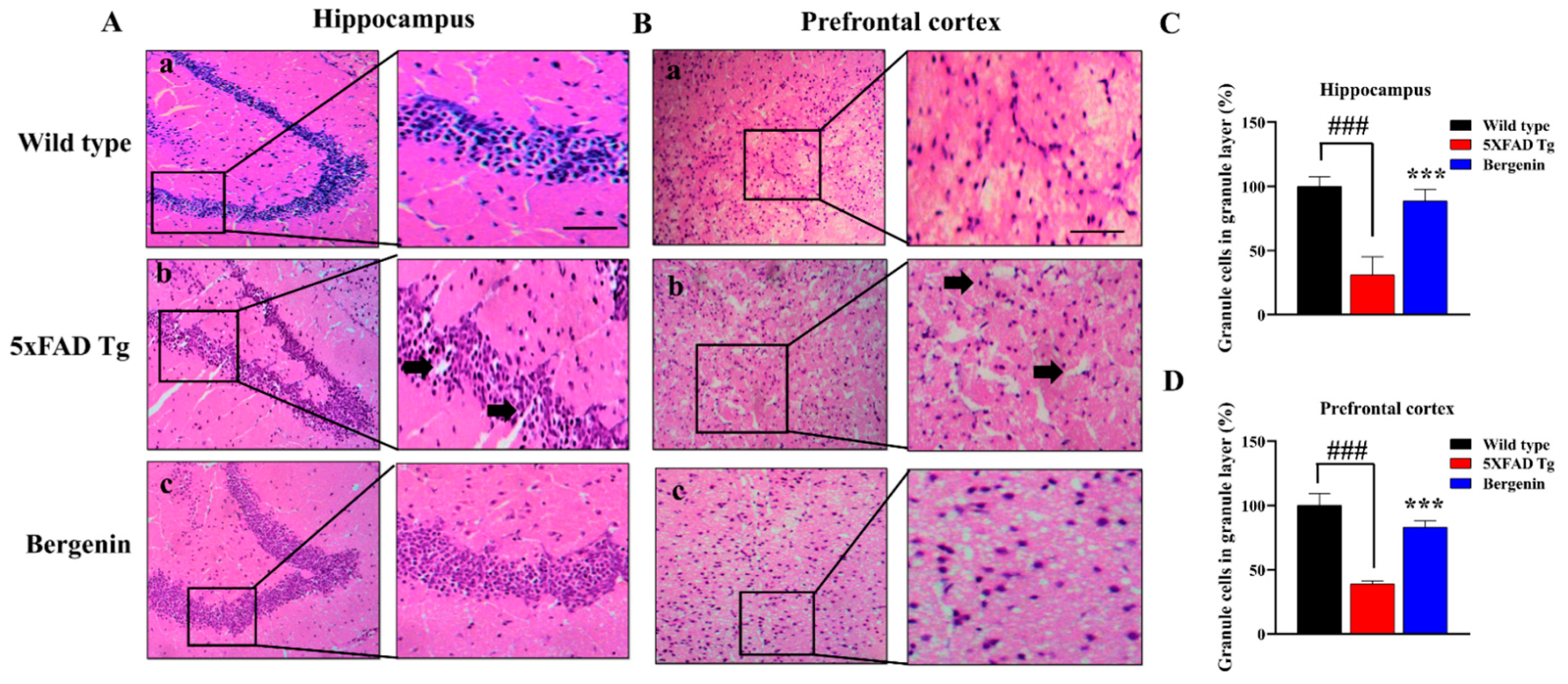
2.7. Bergenin Improves Histopathology in H&E Stained 5xFAD Tg Mice Brain Tissues
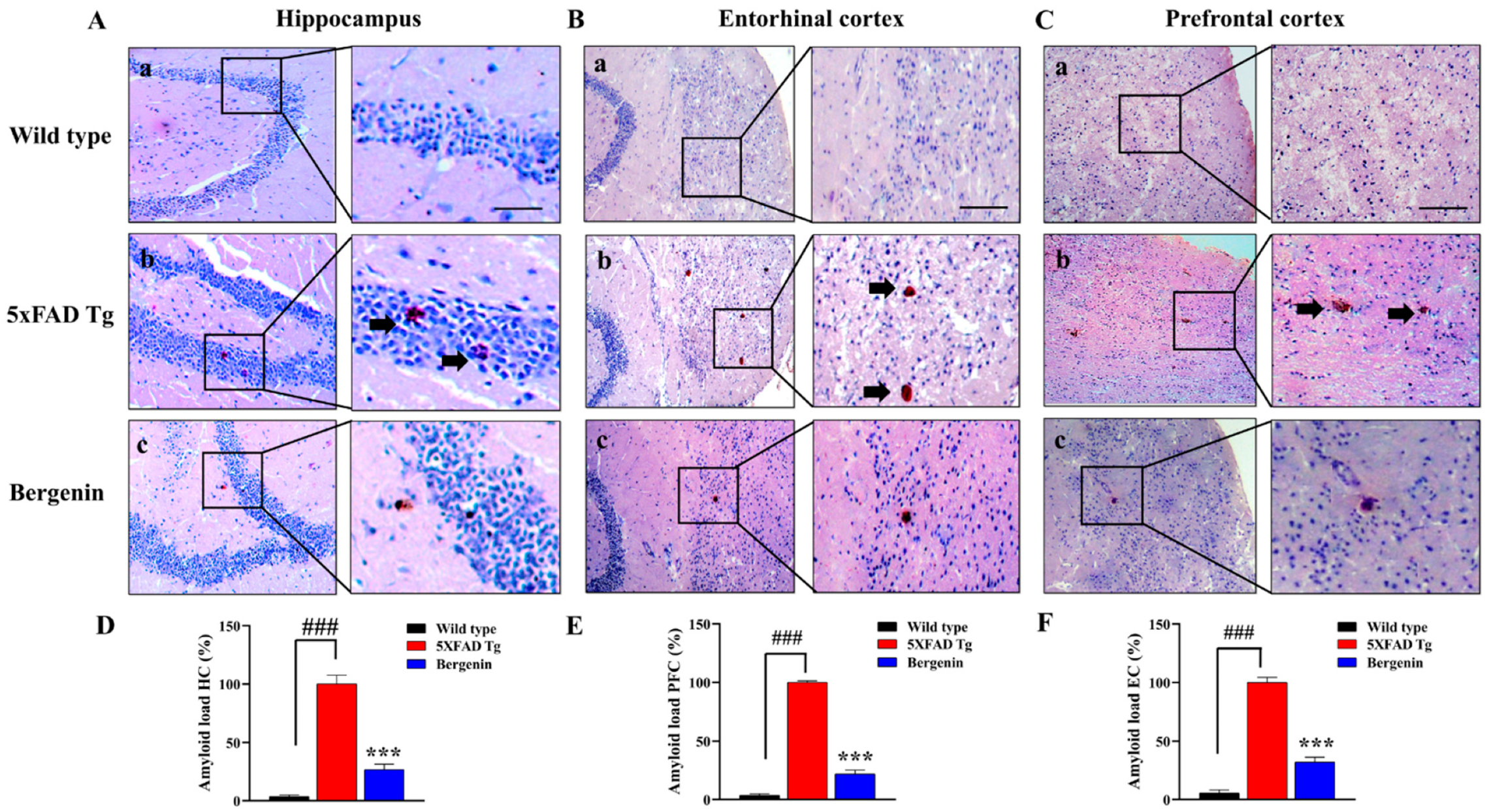
2.8. Bergenin Attenuates Amyloid Plaque Deposits in 5xFAD Tg Mice Brain Tissues
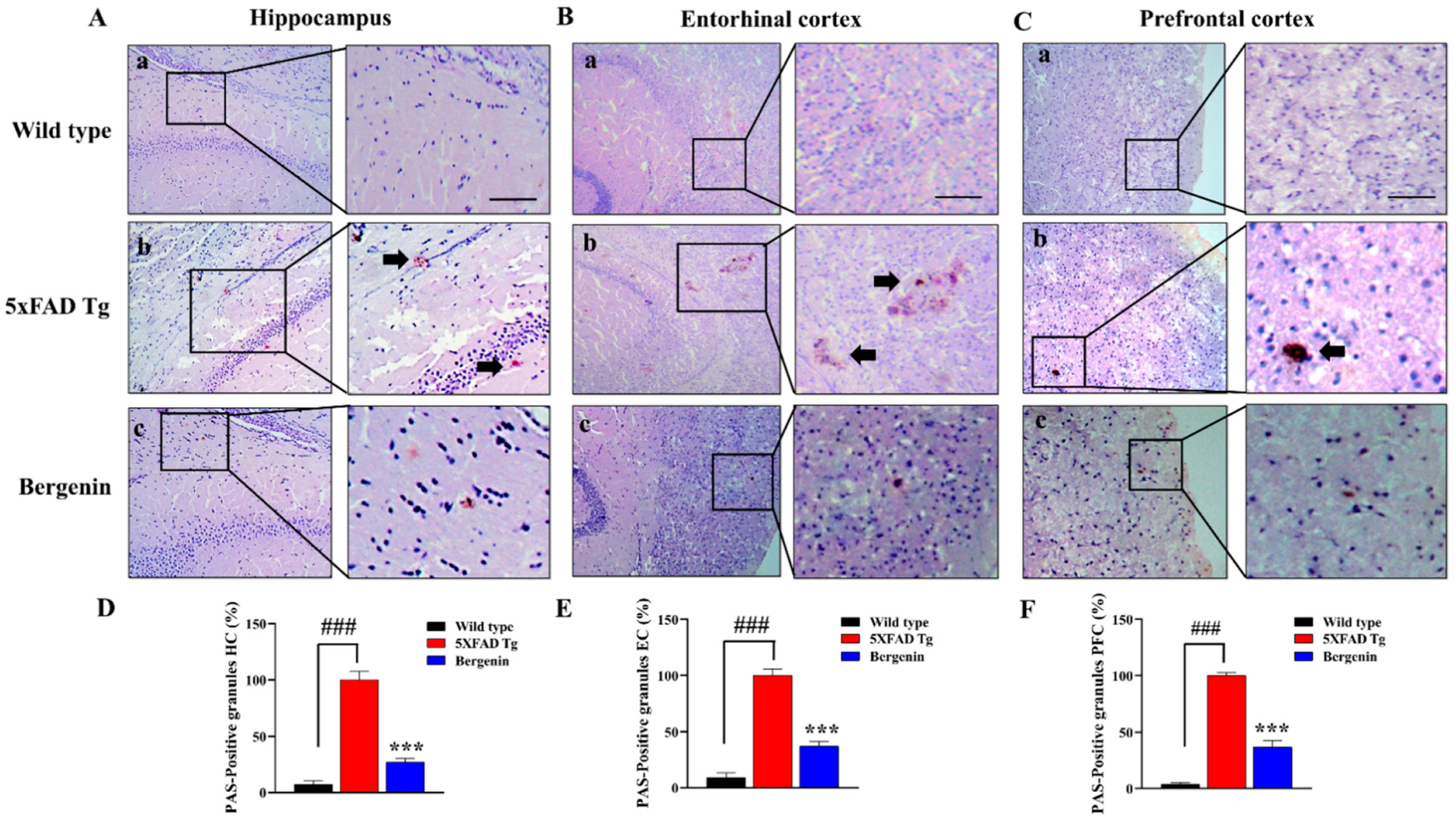
2.9. Bergenin Decreases Clusters of PAS-Positive Granules in 5xFAD Tg Mice Brain Tissues
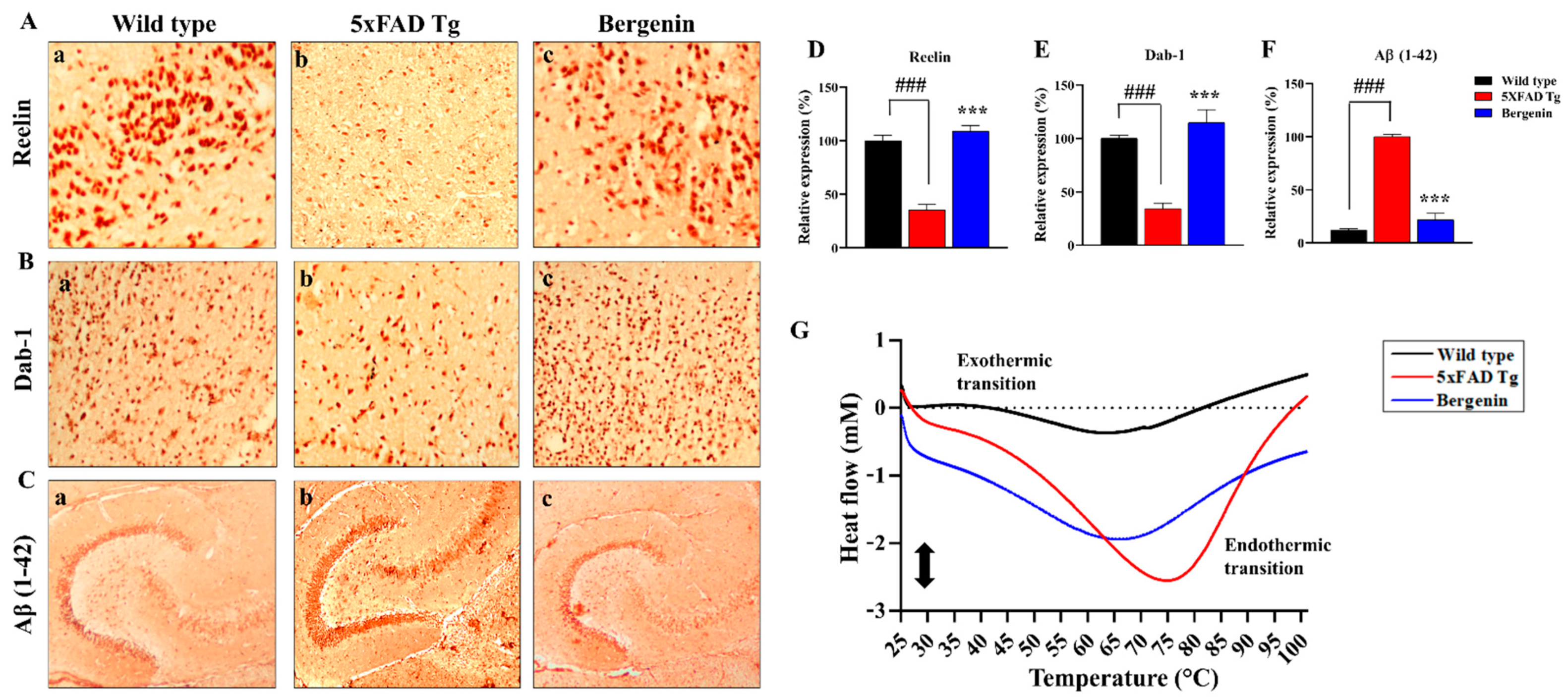
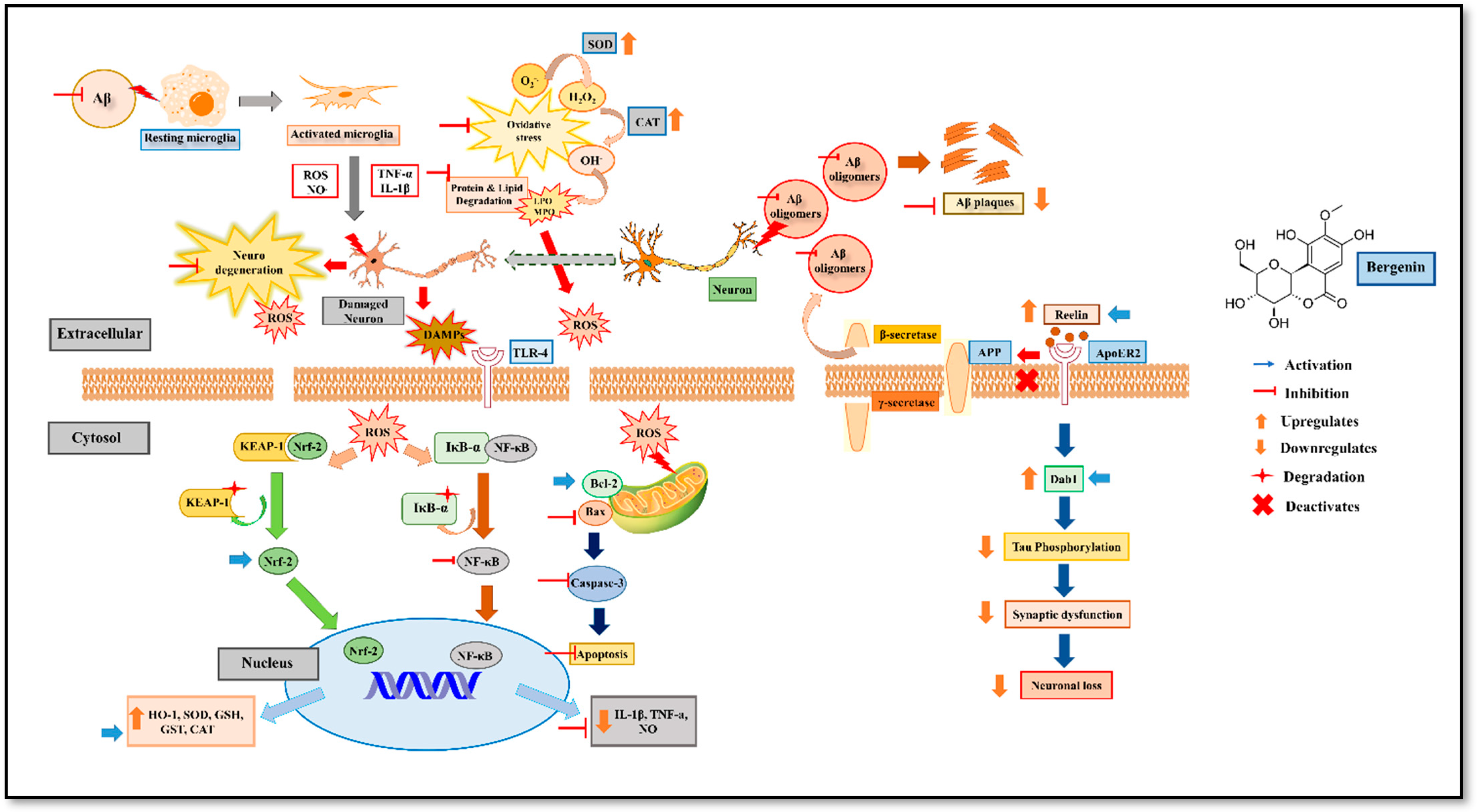
2.10. Bergenin Increases Reelin Signaling Pathway and Attenuates Aβ (1-42)
2.11. Effect of Bergenin on DSC Analysis
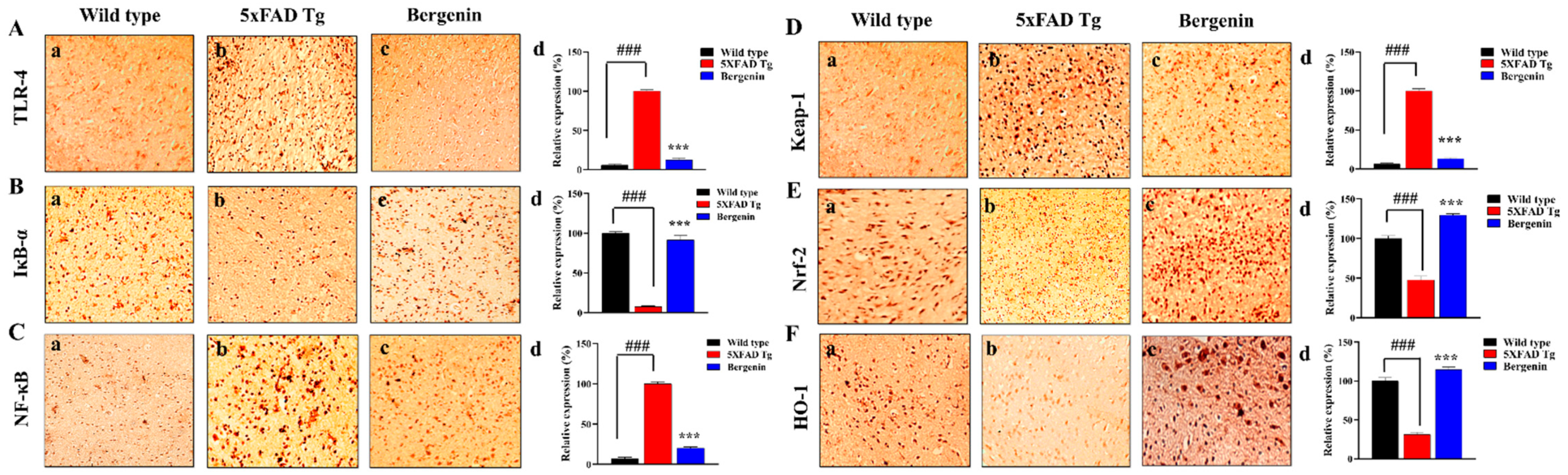
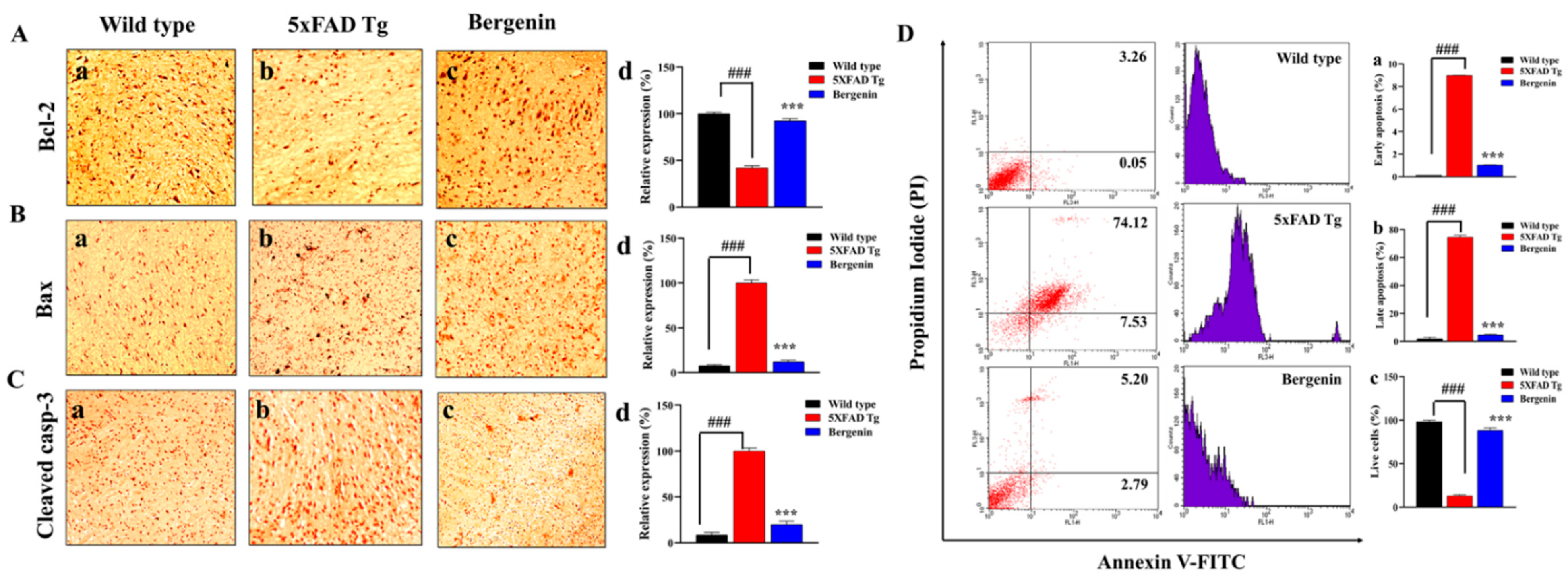
2.12. Effect of Bergenin on Nrf-2, NF-κB and Bax Signaling Proteins
2.13. Bergenin Decreases Inflammatory and Oxidative Stress Pathway
2.14. Bergenin Reduces Apoptotic Pathway
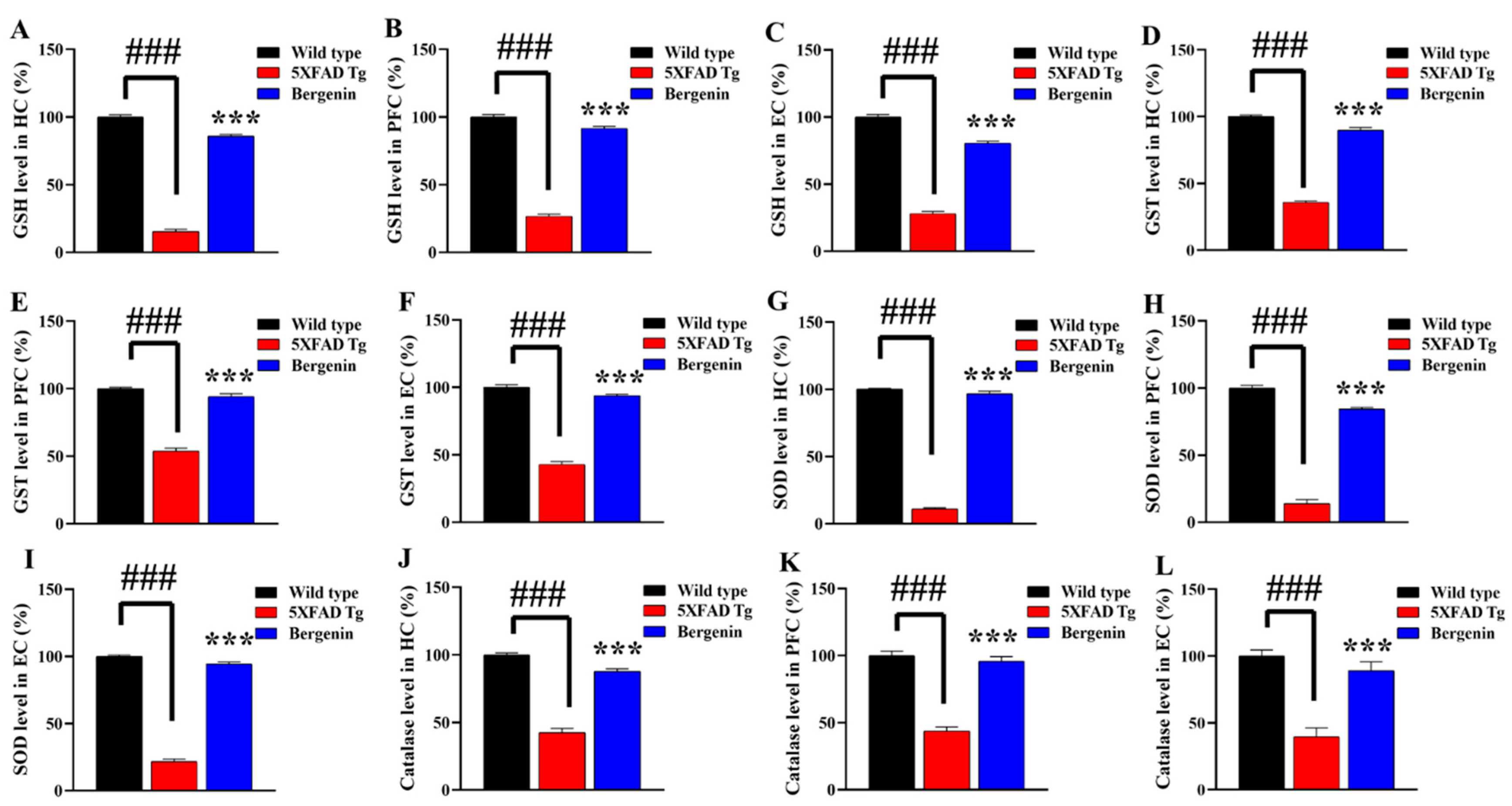
2.15. Bergenin Enhances Antioxidant Levels in 5xFAD Tg Mice Brain
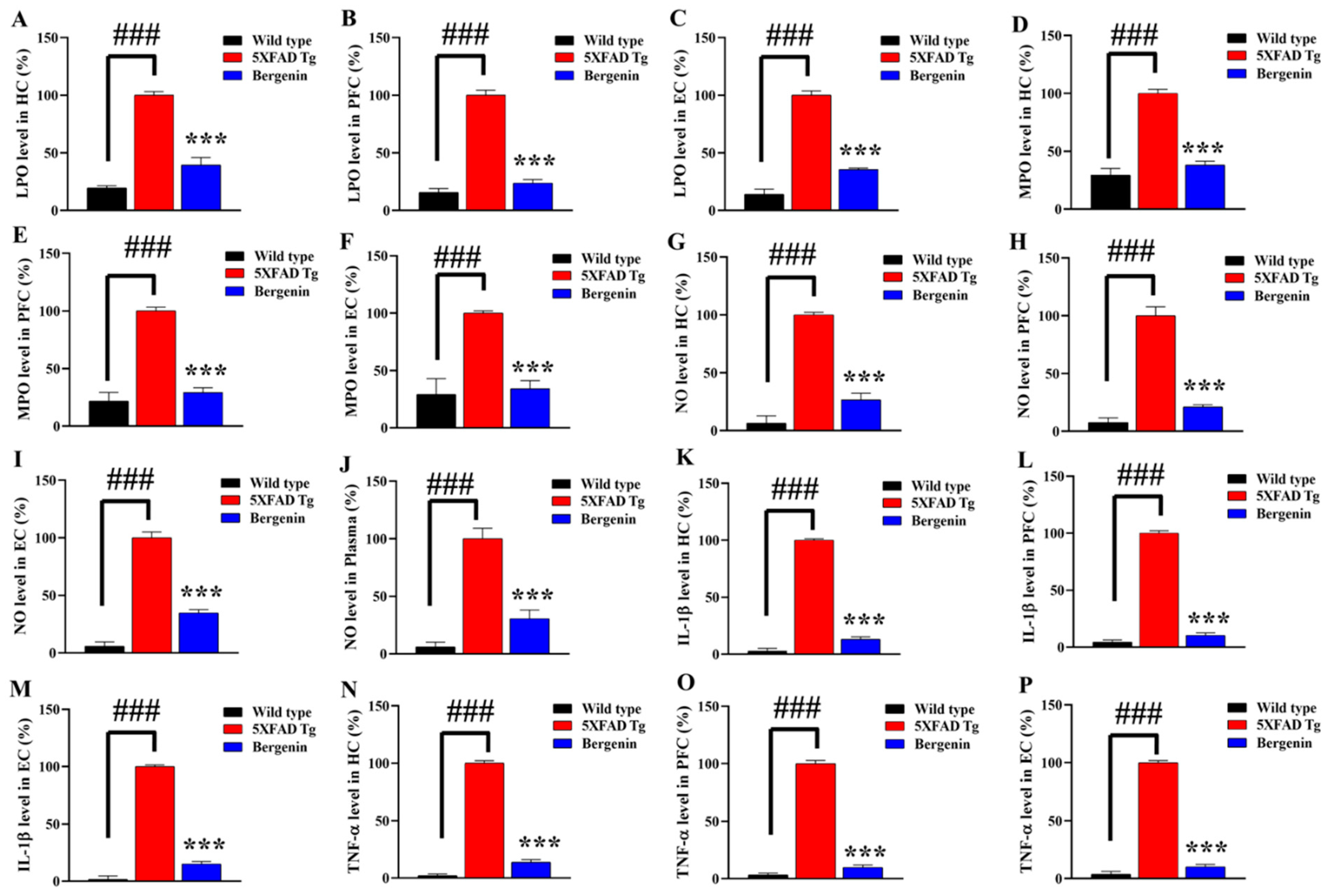
2.16. Bergenin Reduces Oxidative Stress and Nitrite Production in 5xFAD Tg Mice Brain
2.17. Bergenin Reduces Pro-Inflammatory Cytokine Production in Mice Brain
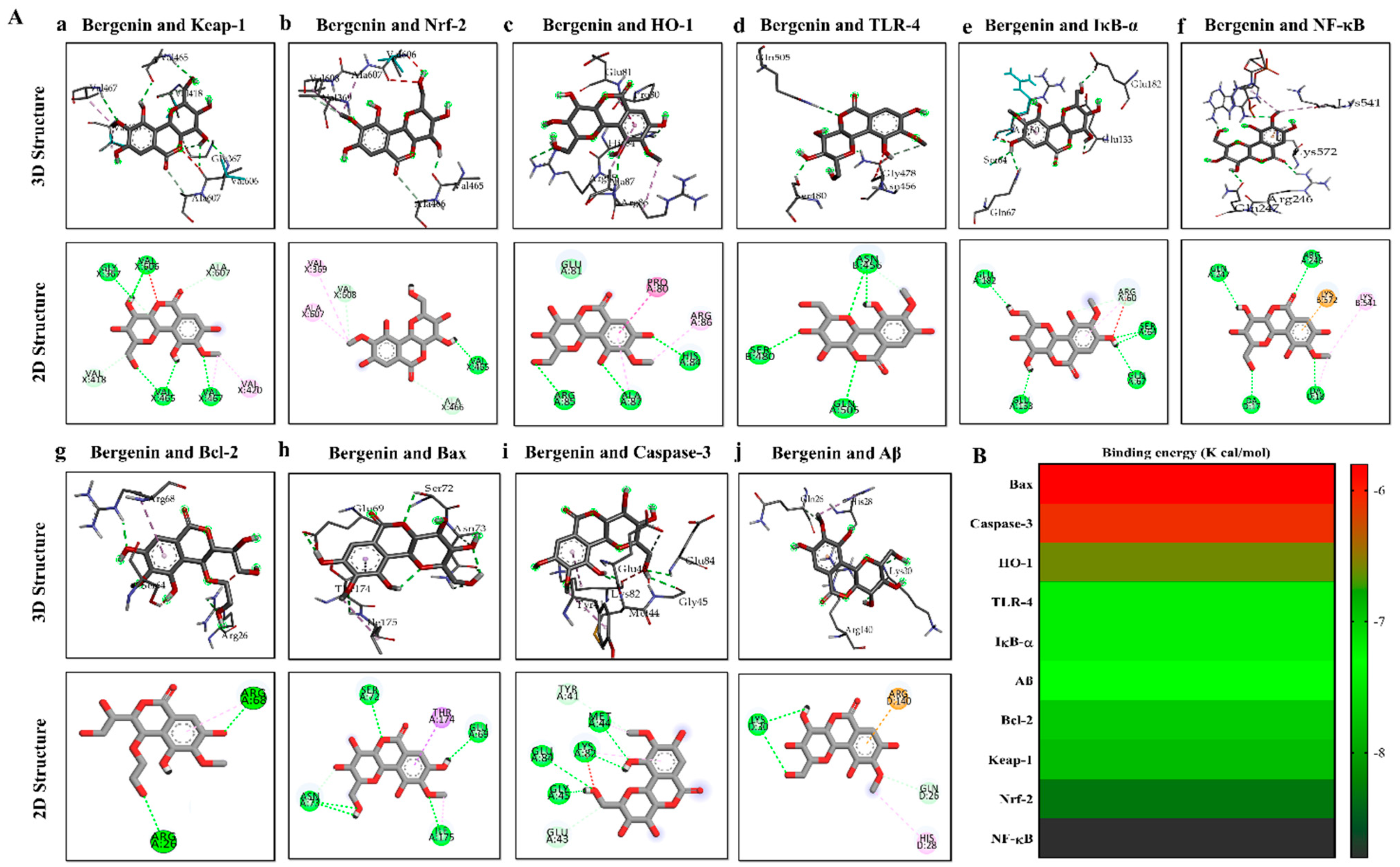
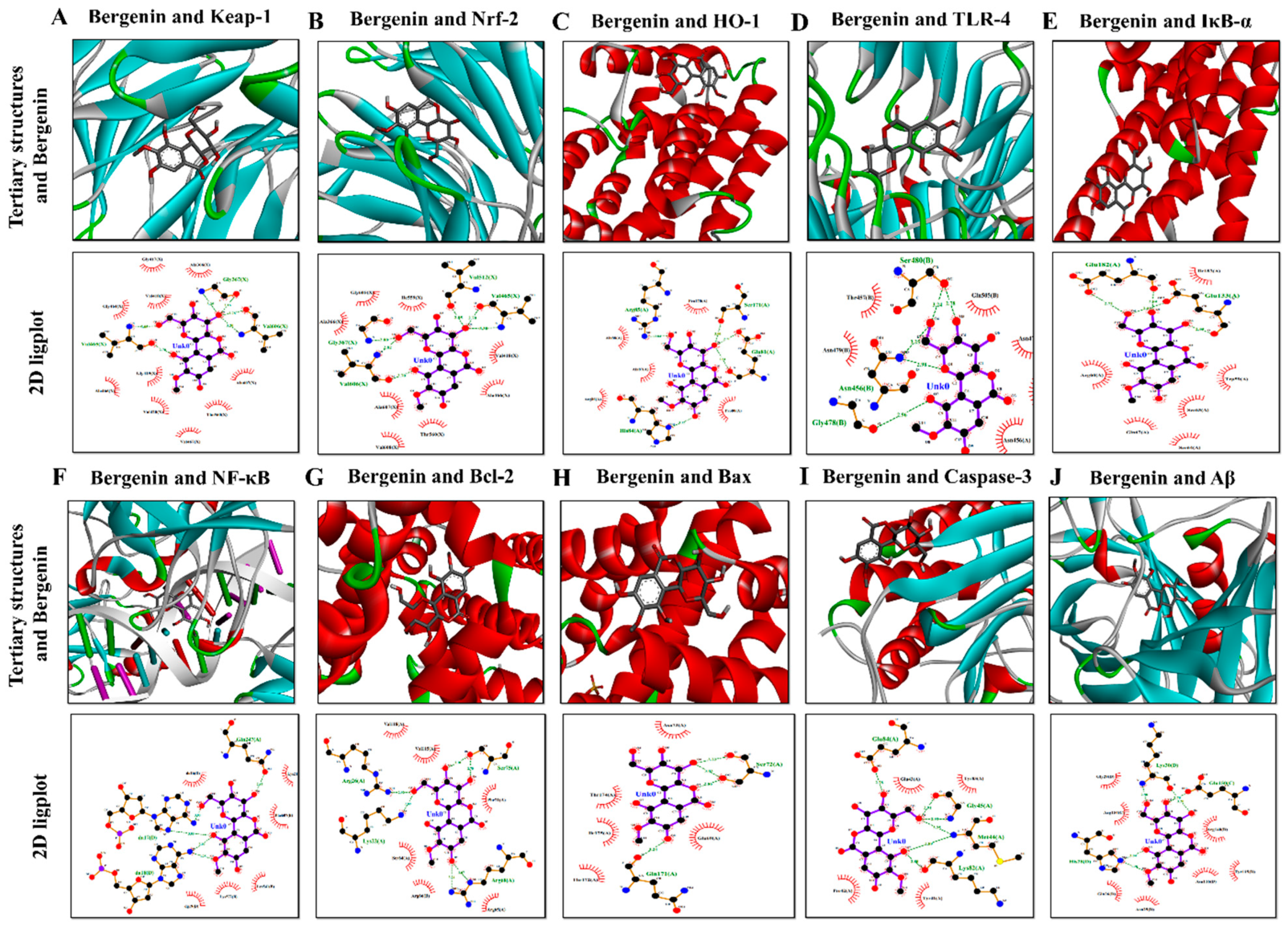
2.18. Molecular Docking Analysis with Bergenin
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Cells and Culture Media
4.4. Cell Viability Assay
4.5. In-Vitro Determination of Nitric Oxide (NO) Production
4.6. In-Vitro Determination of Antioxidants and Oxidative Stress Level
4.7. Animals
4.8. Experimental Groups
4.9. Dose Preparation and Administration
4.10. Experimental Design and Sample Collection
4.11. Preparation of Brain Tissues for Histological Analysis
4.12. Behavioral Analysis
4.12.1. Y-Maze Test
4.12.2. Morris Water Maze (MWM) Test
4.12.3. Open Field Test (OFT)
4.13. Biochemical Analysis
4.13.1. Fourier Transform-Infrared (FT-IR) Analysis
4.13.2. Differential Scanning Calorimeter (DSC)
4.13.3. Evaluation of Histopathological Changes in Hematoxylin & Eosin (H&E) Stained Tissues
4.13.4. Assessment of Amyloid Beta Deposits in Brain Tissue
4.13.5. Assessment of Periodic Acid-Schiff (PAS) Staining in Brain Tissue
4.13.6. Western Blot Analysis
4.13.7. Immunohistochemical Analysis
4.13.8. Detection of Apoptotic Cells by Annexin V Staining
4.13.9. Determination of Anti-Oxidant Level
4.13.10. Evaluation of Oxidative Stress Level
4.13.11. Determination of Nitrite Content
4.13.12. Evaluation of Pro-Inflammatory Cytokines Level in Mice Brain Tissue
4.13.13. Molecular Docking Analysis
4.13.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Chin, J.; Massaro, C.M.; Palop, J.J.; Thwin, M.T.; Yu, G.Q.; Bien-Ly, N.; Bender, A.; Mucke, L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2727–2733. [Google Scholar] [CrossRef]
- Pujadas, L.; Rossi, D.; Andrés, R.; Teixeira, C.M.; Serra-Vidal, B.; Parcerisas, A.; Maldonado, R.; Giralt, E.; Carulla, N.; Soriano, E. Reelin delays amyloid-beta fibril formation and rescues cognitive deficits in a model of Alzheimer’s disease. Nat. Commun. 2014, 5, 3443. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Koenigsknecht, J.; Landreth, G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 9838–9846. [Google Scholar] [CrossRef]
- Walter, S.; Letiembre, M.; Liu, Y.; Heine, H.; Penke, B.; Hao, W.; Bode, B.; Manietta, N.; Walter, J.; Schulz-Schuffer, W.; et al. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2007, 20, 947–956. [Google Scholar] [CrossRef]
- Marin, D.P.; Bolin, A.P.; Campoio, T.R.; Guerra, B.A.; Otton, R. Oxidative stress and antioxidant status response of handball athletes: Implications for sport training monitoring. Int. Immunopharmacol. 2013, 17, 462–470. [Google Scholar] [CrossRef]
- Lüth, H.J.; Münch, G.; Arendt, T. Aberrant expression of NOS isoforms in Alzheimer’s disease is structurally related to nitrotyrosine formation. Brain Res. 2002, 953, 135–143. [Google Scholar] [CrossRef]
- Calabrese, V.; Sultana, R.; Scapagnini, G.; Guagliano, E.; Sapienza, M.; Bella, R.; Kanski, J.; Pennisi, G.; Mancuso, C.; Stella, A.M.; et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wulij, O.; Li, W.; Jiang, Z.G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.B.; Khan, A.U.; Ullah, R.; Baig, M.W.; ul Haq, I.; Seo, E.K.; Khan, S. Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain. Int. J. Mol. Sci. 2021, 22, 6084. [Google Scholar] [CrossRef]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Bajracharya, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia 2015, 101, 133–152. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, F.; Williamson, N.; Jian, J.; Zhang, L.; Liang, Z.; Wang, J.; An, L.; Tunnacliffe, A.; Zheng, Y. Effects of secondary metabolite extract from Phomopsis occulta on β-amyloid aggregation. PLoS ONE 2014, 9, e109438. [Google Scholar] [CrossRef][Green Version]
- Jia, J.; Ma, L.; Wu, M.; Zhang, L.; Zhang, X.; Zhai, Q.; Jiang, T.; Wang, Q.; Xiong, L. Anandamide Protects HT22 Cells Exposed to Hydrogen Peroxide by Inhibiting CB1 Receptor-Mediated Type 2 NADPH Oxidase. Oxid. Med. Cell. Longev. 2014, 2014, 893516. [Google Scholar] [CrossRef]
- Van Dam, D.; de Deyn, P.P. Drug discovery in dementia: The role of rodent models. Nature reviews. Drug Discov. 2006, 5, 956–970. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Nelson, P.T.; Schmitt, F.A.; Van Eldik, L.J. Using mice to model Alzheimer’s dementia: An overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front. Genet. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.; Eriksen, J.; Hutton, M. A decade of modeling Alzheimer’s disease in transgenic mice. Trends Genet. TIG 2006, 22, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr. Protoc. Neurosci. 2004, 26, 8.5A.1–8.5A.12. [Google Scholar] [CrossRef] [PubMed]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Sawmiller, D.; Li, S.; Mori, T.; Habib, A.; Rongo, D.; Delic, V.; Bradshaw, P.C.; Shytle, R.D.; Sanberg, C.; Bickford, P. Beneficial effects of a pyrroloquinolinequinone-containing dietary formulation on motor deficiency, cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer’s disease. Heliyon 2017, 3, e00279. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.P.; Robertson, A.; Chipman, P.H.; Rafuse, V.F.; Brown, R.E. Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 337, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Canevari, L.; Abramov, A.Y.; Duchen, M.R. Toxicity of amyloid beta peptide: Tales of calcium, mitochondria, and oxidative stress. Neurochem. Res. 2004, 29, 637–650. [Google Scholar] [CrossRef]
- Brown, H.A.; Murphy, R.C. Working towards an exegesis for lipids in biology. Nat. Chem. Biol. 2009, 5, 602–606. [Google Scholar] [CrossRef]
- Depciuch, J.; Sowa-Kućma, M.; Misztak, P.; Szewczyk, B.; Nowak, G.; Pankiewicz, P.; Parlińska-Wojtan, M. Olfactory bulbectomy-induced changes in phospholipids and protein profiles in the hippocampus and prefrontal cortex of rats. A preliminary study using a FTIR spectroscopy. Pharmacol. Rep. PR 2016, 68, 521–528. [Google Scholar] [CrossRef]
- Cakmak, G.; Togan, I.; Severcan, F. 17Beta-estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. Amst. Neth. 2006, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Jung, C. Insight into protein structure and protein–ligand recognition by Fourier transform infrared spectroscopy. J. Mol. Recognit. 2000, 13, 325–351. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Khalid, S.; Shal, B.; Kang, E.; Lee, H.; Laumet, G.; Seo, E.K.; Khan, S. 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions. Molecules 2021, 26, 181. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Toyran, N.; Zorlu, F.; Dönmez, G.; Oğe, K.; Severcan, F. Chronic hypoperfusion alters the content and structure of proteins and lipids of rat brain homogenates: A Fourier transform infrared spectroscopy study. Eur. Biophys. J. EBJ 2004, 33, 549–554. [Google Scholar] [CrossRef]
- Miller, L.M.; Bourassa, M.W.; Smith, R.J. FTIR spectroscopic imaging of protein aggregation in living cells. Biochim. Biophys. Acta 2013, 1828, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Blout, E. The infrared spectra of polypeptides in various conformations: Amide I and II bands1. J. Am. Chem. Soc. 1961, 83, 712–719. [Google Scholar] [CrossRef]
- Cerf, E.; Sarroukh, R.; Tamamizu-Kato, S.; Breydo, L.; Derclaye, S.; Dufrêne, Y.F.; Narayanaswami, V.; Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Antiparallel β-sheet: A signature structure of the oligomeric amyloid β-peptide. Biochem. J. 2009, 421, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.M.; Rakib, F.; Abdelalim, E.M.; Limbeck, A.; Mall, R.; Ullah, E.; Mesaeli, N.; McNaughton, D.; Ahmed, T.; Al-Saad, K. Fourier-Transform Infrared Imaging Spectroscopy and Laser Ablation -ICPMS New Vistas for Biochemical Analyses of Ischemic Stroke in Rat Brain. Front. Neurosci. 2018, 12, 647. [Google Scholar] [CrossRef]
- Tissir, F.; Goffinet, A.M. Reelin and brain development. Nature reviews. Neuroscience 2003, 4, 496–505. [Google Scholar] [PubMed]
- Pujadas, L.; Gruart, A.; Bosch, C.; Delgado, L.; Teixeira, C.M.; Rossi, D.; de Lecea, L.; Martínez, A.; Delgado-García, J.M.; Soriano, E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 4636–4649. [Google Scholar] [CrossRef]
- Kocherhans, S.; Madhusudan, A.; Doehner, J.; Breu, K.S.; Nitsch, R.M.; Fritschy, J.M.; Knuesel, I. Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer’s disease mice. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9228–9240. [Google Scholar] [CrossRef]
- Beffert, U.; Morfini, G.; Bock, H.H.; Reyna, H.; Brady, S.T.; Herz, J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J. Biol. Chem. 2002, 277, 49958–49964. [Google Scholar] [CrossRef]
- Jakob, U.; Kriwacki, R.; Uversky, V.N. Conditionally and transiently disordered proteins: Awakening cryptic disorder to regulate protein function. Chem. Rev. 2014, 114, 6779–6805. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. Oligomers on the brain: The emerging role of soluble protein aggregates in neurodegeneration. Protein Pept. Lett. 2004, 11, 213–228. [Google Scholar] [CrossRef]
- Stępkowski, T.M.; Kruszewski, M.K. Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free. Radic. Biol. Med. 2011, 50, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-Y.; Kim, J.-H.; He, X.; Chen, P.; Farooqui, A.A.; Jenner, A.M. Changes in Brain Cholesterol Metabolome After Excitotoxicity. Mol. Neurobiol. 2010, 41, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef]
- Chen, G.-f.; Xu, T.-h.; Yan, Y.; Zhou, Y.-r.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Olmez, I.; Ozyurt, H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem. Int. 2012, 60, 208–212. [Google Scholar] [CrossRef]
- Varadarajan, S.; Yatin, S.; Aksenova, M.; Butterfield, D.A. Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Manich, G.; Cabezón, I.; Augé, E.; Pelegrí, C.; Vilaplana, J. Periodic acid-Schiff granules in the brain of aged mice: From amyloid aggregates to degenerative structures containing neo-epitopes. Ageing Res. Rev. 2016, 27, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Doehner, J.; Genoud, C.; Imhof, C.; Krstic, D.; Knuesel, I. Extrusion of misfolded and aggregated proteins—A protective strategy of aging neurons? Eur. J. Neurosci. 2012, 35, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shin, E.M.; Choi, R.J.; Jung, Y.H.; Kim, J.; Tosun, A.; Kim, Y.S. Suppression of LPS-induced inflammatory and NF-kappaB responses by anomalin in RAW 264.7 macrophages. J. Cell. Biochem. 2011, 112, 2179–2188. [Google Scholar] [CrossRef]
- Khan, S.; Choi, R.J.; Lee, J.; Kim, Y.S. Attenuation of neuropathic pain and neuroinflammatory responses by a pyranocoumarin derivative, anomalin in animal and cellular models. Eur. J. Pharmacol. 2016, 774, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef]
- Robertson, T.; Dutton, N.; Martins, R.; Taddei, K.; Papadimitriou, J. Comparison of astrocytic and myocytic metabolic dysregulation in apolipoprotein E deficient and human apolipoprotein E transgenic mice. Neuroscience 2000, 98, 353–359. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Shallow water (paddling) variants of water maze tests in mice. J. Vis. Exp. 2013, 2608. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Ahmad, N.; Akram, M.; Kumari, G.; Amin, M.U.; Umar, M.N. Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals 2020, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Lamprea, M.R.; Cardenas, F.P.; Setem, J.; Morato, S. Thigmotactic responses in an open-field. Braz. J. Med. Biol. Res. 2008, 41, 135–140. [Google Scholar] [CrossRef]
- Belovicova, K.; Bogi, E.; Csatlosova, K.; Dubovicky, M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017, 10, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Miller, L.M.; Zorlu, F.; Severcan, F. Amifostine, a radioprotectant agent, protects rat brain tissue lipids against ionizing radiation induced damage: An FTIR microspectroscopic imaging study. Arch. Biochem. Biophys. 2012, 520, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shivanoor, S.M.; David, M. Reversal of deltamethrin-induced oxidative damage in rat neural tissues by turmeric-diet: Fourier transform-infrared and biochemical investigation. J. Basic Appl. Zool. 2016, 77, 56–68. [Google Scholar] [CrossRef][Green Version]
- Akkas, S.B.; Severcan, M.; Yilmaz, O.; Severcan, F. Effects of lipoic acid supplementation on rat brain tissue: An FTIR spectroscopic and neural network study. Food Chem. 2007, 105, 1281–1288. [Google Scholar] [CrossRef]
- Götz, M.E.; Freyberger, A.; Hauer, E.; Burger, R.; Sofic, E.; Gsell, W.; Heckers, S.; Jellinger, K.; Hebenstreit, G.; Frölich, L.; et al. Susceptibility of Brains from Patients with Alzheimer’s Disease to Oxygen-Stimulated Lipid Peroxidation and Differential Scanning Calorimetry. Dement. Geriatr. Cogn. Disord. 1992, 3, 213–222. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008. [Google Scholar] [CrossRef]
- Le Cudennec, C.; Faure, A.; Ly, M.; Delatour, B. One-year longitudinal evaluation of sensorimotor functions in APP751SL transgenic mice. Genes Brain Behav. 2008, 7 (Suppl. 1), 83–91. [Google Scholar] [CrossRef]
- Cana, A.; Herder, V.; Hansmann, F.; Beineke, A.; Baumgärtner, W.; Spitzbarth, I. Characterization of Periodic Acid-Schiff-Positive Granular Deposits in the Hippocampus of SJL/J Mice. Toxicol. Pathol. 2015, 43, 737–742. [Google Scholar] [CrossRef]
- Khan, A.M.; Khan, A.U.; Ali, H.; Islam, S.U.; Seo, E.K.; Khan, S. Continentalic acid exhibited nephroprotective activity against the LPS and E. coli-induced kidney injury through inhibition of the oxidative stress and inflammation. Int. Immunopharmacol. 2020, 80, 106209. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Chun, J.; Choi, R.J.; Park, S.; Islam, M.N.; Choi, J.S.; Kim, Y.S. Anti-hyperalgesic and anti-allodynic activities of capillarisin via suppression of inflammatory signaling in animal model. J. Ethnopharmacol. 2014, 152, 478–486. [Google Scholar] [CrossRef]
- Shal, B.; Khan, A.; Naveed, M.; Ali, H.; Seo, E.K.; Choi, H.; Khan, S. Neuroprotective effect of 25-Methoxyhispidol A against CCl(4)-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci. 2020, 253, 117684. [Google Scholar] [CrossRef]
- Naseer, M.I.; Ullah, I.; Narasimhan, M.L.; Lee, H.Y.; Bressan, R.A.; Yoon, G.H.; Yun, D.J.; Kim, M.O. Neuroprotective effect of osmotin against ethanol-induced apoptotic neurodegeneration in the developing rat brain. Cell Death Dis. 2014, 5, e1150. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- MARKLUND, S.; MARKLUND, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Warholm, M.; Guthenberg, C.; von Bahr, C.; Mannervik, B. [62] Glutathione transferases from human liver. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 499–504. [Google Scholar]
- Hamsa, T.P.; Kuttan, G. Protective role of Ipomoea obscura (L.) on cyclophosphamide-induced uro- and nephrotoxicities by modulating antioxidant status and pro-inflammatory cytokine levels. Inflammopharmacology 2011, 19, 155–167. [Google Scholar] [CrossRef]
- Shal, B.; Khan, A.; Naveed, M.; Ullah Khan, N.; Ihsan-Ul-Haq; AlSharari, S.D.; Kim, Y.S.; Khan, S. Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 111, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shal, B.; Naveed, M.; Nasir, B.; Irshad, N.; Ali, H.; Khan, S. Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology 2020, 237, 2327–2343. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Manktelow, A.; Meyer, A.A. Lack of correlation between decreased chemotaxis and susceptibility to infection in burned rats. J. Trauma 1986, 26, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Muhammad Khan, A.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Khan, S. The newly synthesized compounds (NCHDH and NTHDH) attenuates LPS-induced septicemia and multi-organ failure via Nrf2/HO1 and HSP/TRVP1 signaling in mice. Chem. Biol. Interact. 2020, 329, 109220. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shehzad, O.; Chun, J.; Kim, Y.S. Mechanism underlying anti-hyperalgesic and anti-allodynic properties of anomalin in both acute and chronic inflammatory pain models in mice through inhibition of NF-κB, MAPKs and CREB signaling cascades. Eur. J. Pharmacol. 2013, 718, 448–458. [Google Scholar] [CrossRef]
- Afridi, R.; Khan, A.U.; Khalid, S.; Shal, B.; Rasheed, H.; Ullah, M.Z.; Shehzad, O.; Kim, Y.S.; Khan, S. Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol. Toxicol. 2019, 20, 57. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Naveed, M.; Shah, F.A.; Atiq, A.; Khan, N.U.; Kim, Y.S.; Khan, S. Matrine ameliorates anxiety and depression-like behaviour by targeting hyperammonemia-induced neuroinflammation and oxidative stress in CCl4 model of liver injury. Neuro Toxicol. 2019, 72, 38–50. [Google Scholar] [CrossRef]
- Naveed, M.; Khan, S.Z.; Zeeshan, S.; Khan, A.; Shal, B.; Atiq, A.; Ali, H.; Ullah, R.; Zia Ur, R.; Khan, S. A new cationic palladium (II) dithiocarbamate exhibits anti-inflammatory, analgesic, and antipyretic activities through inhibition of inflammatory mediators in in vivo models. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 961–977. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Islam, S.U.; Ali, H.; Shehzad, A.; Khan, S. Inhibition of NF-κB signaling and HSP70/HSP90 proteins by newly synthesized hydrazide derivatives in arthritis model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Londhe, A.M.; Gadhe, C.G.; Lim, S.M.; Pae, A.N. Investigation of Molecular Details of Keap1-Nrf2 Inhibitors Using Molecular Dynamics and Umbrella Sampling Techniques. Molecules 2019, 24, 4085. [Google Scholar] [CrossRef]

| Wild Type (a.u) | 5xFAD Tg (a.u) | Bergenin (a.u) | ||
|---|---|---|---|---|
| Proteins | Amide-A | 0.142 ± 0.005 | 0.047 ± 0.005 ### | 0.107 ± 0.01 *** |
| Amide-I | 0.273 ± 0.01 | 0.083 ± 0.002 ### | 0.213 ± 0.005 *** | |
| Amide-II | 0.24 ± 0.002 | 0.073 ± 0.002 ### | 0.174 ± 0.002 *** | |
| CH3 symmetric stretching | 0.103 ± 0.02 | 0.03 ± 0.01 ### | 0.081 ± 0.001 *** | |
| Lipids | Olefinic=C-H stretching | 0.075 ± 0.001 | 0.035 ± 0.003 ### | 0.065 ± 0.05 *** |
| CH3 asymmetric stretching | 0.123 ± 0.005 | 0.046 ± 0.003 ### | 0.084 ± 0.001 *** | |
| CH2 asymmetric stretching | 0.16 ± 0.017 | 0.052 ± 0.01 ### | 0.127 ± 0.02 *** | |
| CH2 symmetric stretching | 0.156 ± 0.01 | 0.056 ± 0.01 ### | 0.125 ± 0.021 *** | |
| Phospholipids | PO2− asymmetric stretch | 0.21 ± 0.01 | 0.042 ± 0.002 ### | 0.073 ± 0.02 *** |
| Nucleic acids | PO2− symmetric stretch | 0.226 ± 0.02 | 0.076 ± 0.002 ### | 0.172 ± 0.002 *** |
| Lipid/Protein Ratio | Wild Type (a.u) | 5XFAD Tg (a.u) | Bergenin (a.u) |
|---|---|---|---|
| Antiparallel β-sheet (I1744/I1695) | 0.53 ± 0.02 | 0.77 ± 0.005 ### | 0.67 ± 0.005 *** |
| Parallel β-sheet (I1744/I1629) | 0.23 ± 0.05 | 0.44 ± 0.002 ### | 0.36 ± 0.008 *** |
| Proteins | PDB ID | Binding Energy (Kcal/mol) | Hydrogen Bonds | Hydrogen Bond Amino Acids | Hydrophobic Interactions |
|---|---|---|---|---|---|
| Keap-1 | 1U6D | −7.8 | 6 | Val606, Gly367, Ala607, Val418, Val465, Val467 | Val420 |
| Nrf-2 | 2FLU | −8.3 | 3 | Val465, Val608, Ala466 | Val369, Ala607 |
| HO-1 | 1irm | −6.6 | 3 | Arg85, Ala87, His84 | Arg86, Pro80 |
| TLR-4 | 3vq2 | −7.2 | 3 | Gln505, Ser480, Asn456 | --- |
| IκB-α | 6y1j | −7.2 | 4 | Glu182, Ser64,Glu133, Gln67 | Arg60 |
| NF-κB | 1vkx | −8.8 | 4 | Gln247, Arg246, DA18, DA17 | Lys541, Lys572 |
| Bcl-2 | 2W3L | −7.7 | 2 | Arg26, Arg68 | --- |
| Bax | 5W62 | −5.8 | 4 | Ile175, Asn73, Ser72, Glu69 | --- |
| Casp-3 | 2XYG | −6.1 | 6 | Met44, Lys82, Gly45, Glu84, Tyr41, Tyr83, Glu43 | --- |
| Aβ | 4mvl | −7.3 | 2 | Lys30, GLN26 | His28 |
| Wavenumbers (cm−1) | Definition of the Assignment |
|---|---|
| 3304 | Amide A: mainly N–H stretching of proteins |
| 3012 | Olefinic=C-H stretching: lipids |
| 2958 | CH3 asymmetric stretching: mainly lipids |
| 2922 | CH2 asymmetric stretching: mainly lipids |
| 2872 | CH3 symmetric stretching: mainly proteins |
| 2851 | CH2 symmetric stretching: mainly lipids |
| 1739 | Ester C=O stretch: lipids |
| 1653 | Amide 1: C=O stretching of proteins |
| 1548 | Amide II: N-H bending and C-N stretching of proteins |
| 1249 | PO2− asymmetric stretch: mainly phospholipids |
| 1057 | PO2− symmetric stretch: mainly nucleic acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shal, B.; Khan, A.; Khan, A.U.; Ullah, R.; Ali, G.; Islam, S.U.; Haq, I.u.; Ali, H.; Seo, E.-K.; Khan, S. Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model. Int. J. Mol. Sci. 2021, 22, 6603. https://doi.org/10.3390/ijms22126603
Shal B, Khan A, Khan AU, Ullah R, Ali G, Islam SU, Haq Iu, Ali H, Seo E-K, Khan S. Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model. International Journal of Molecular Sciences. 2021; 22(12):6603. https://doi.org/10.3390/ijms22126603
Chicago/Turabian StyleShal, Bushra, Adnan Khan, Ashraf Ullah Khan, Rahim Ullah, Gowhar Ali, Salman Ul Islam, Ihsan ul Haq, Hussain Ali, Eun-Kyoung Seo, and Salman Khan. 2021. "Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model" International Journal of Molecular Sciences 22, no. 12: 6603. https://doi.org/10.3390/ijms22126603
APA StyleShal, B., Khan, A., Khan, A. U., Ullah, R., Ali, G., Islam, S. U., Haq, I. u., Ali, H., Seo, E.-K., & Khan, S. (2021). Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model. International Journal of Molecular Sciences, 22(12), 6603. https://doi.org/10.3390/ijms22126603







