Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression
Abstract
1. Introduction
2. Results
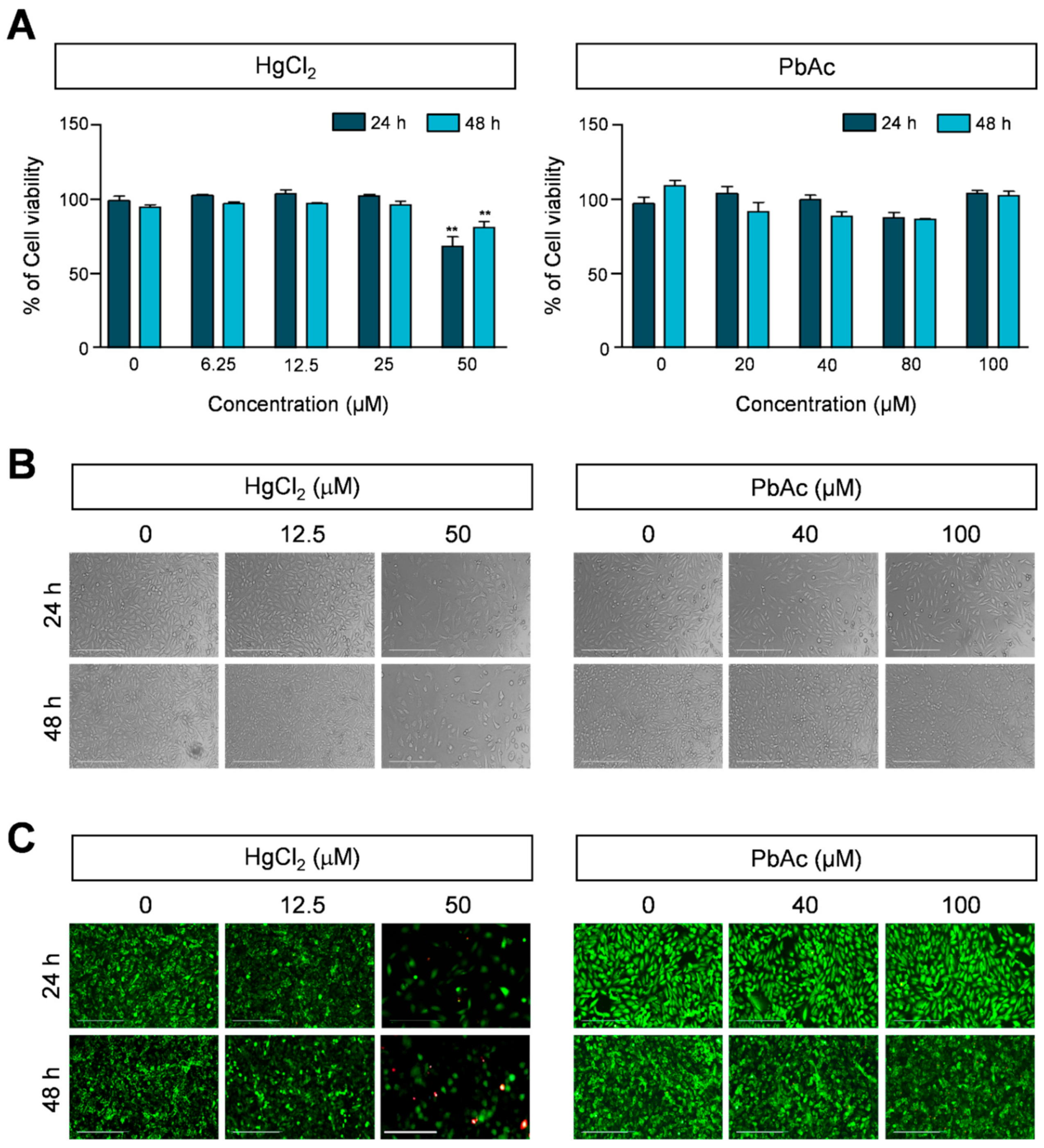
2.1. Effect of Heavy Metals on Viability of Human Lung Fibroblast Cells
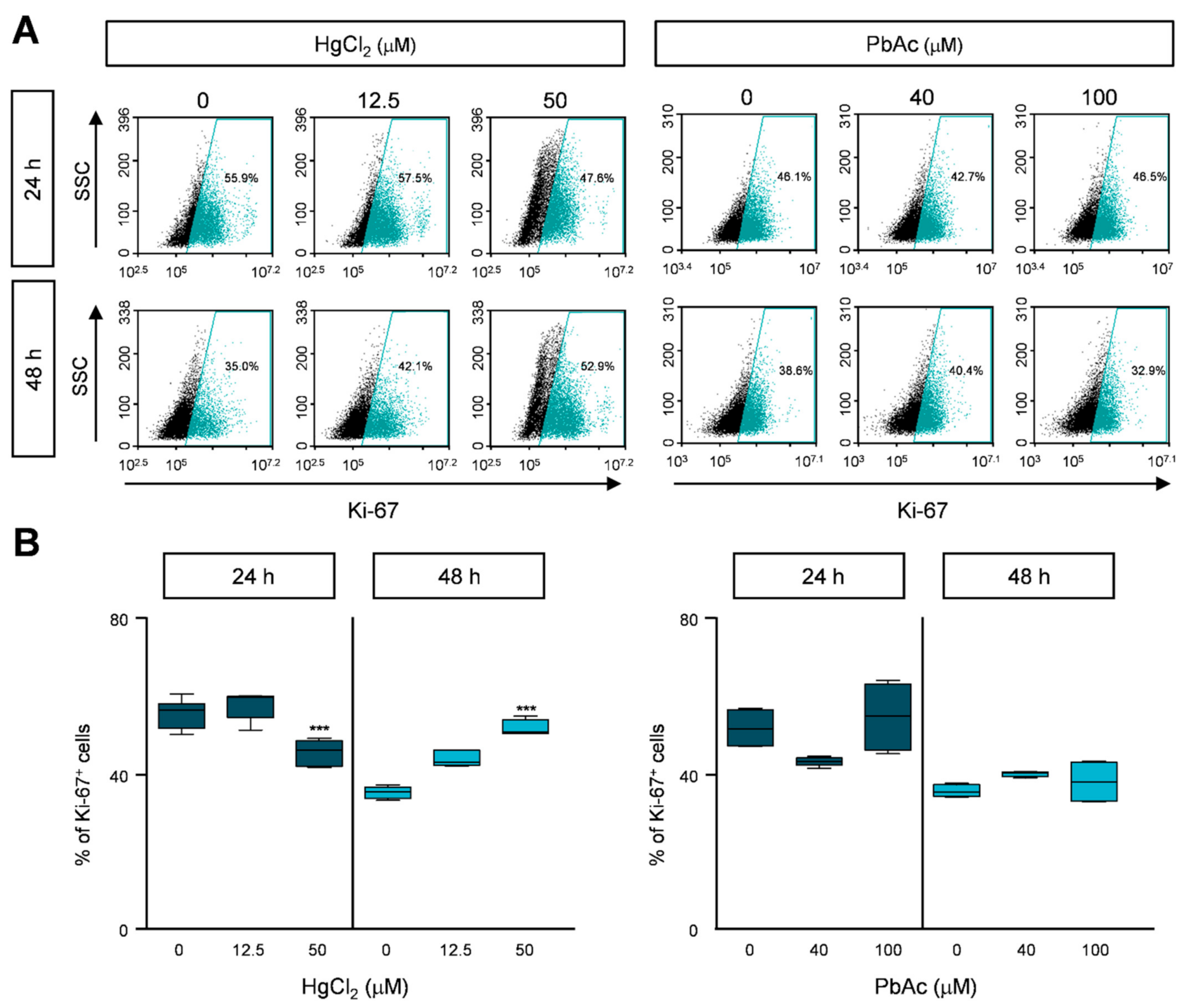
2.2. HgCl2 Treatment, but Not PbAc Treatment, Decreased MRC5 Cell Proliferation
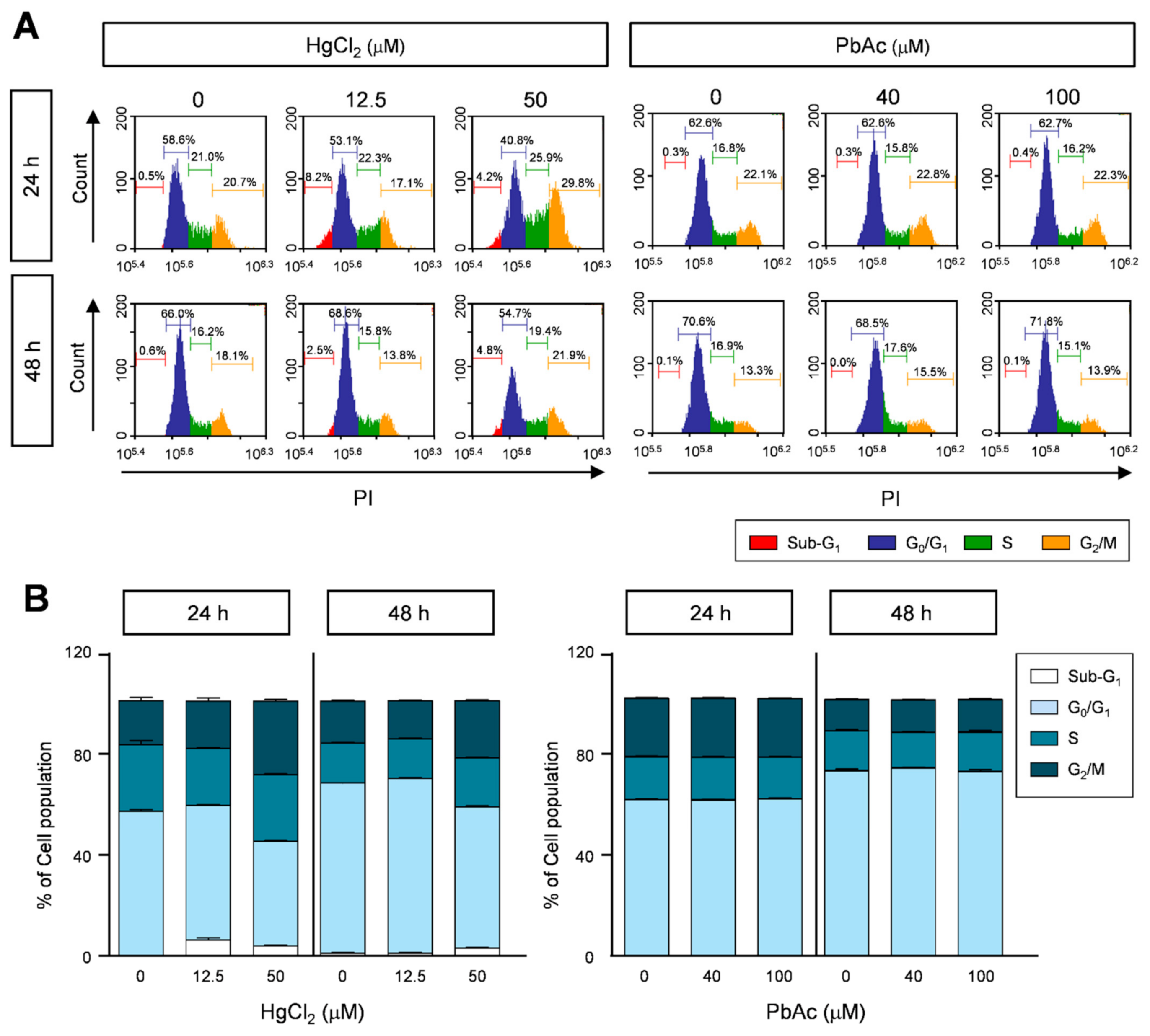
2.3. Effects of Heavy Metals on Cell Cycle Progression in Human Lung Fibroblast Cells
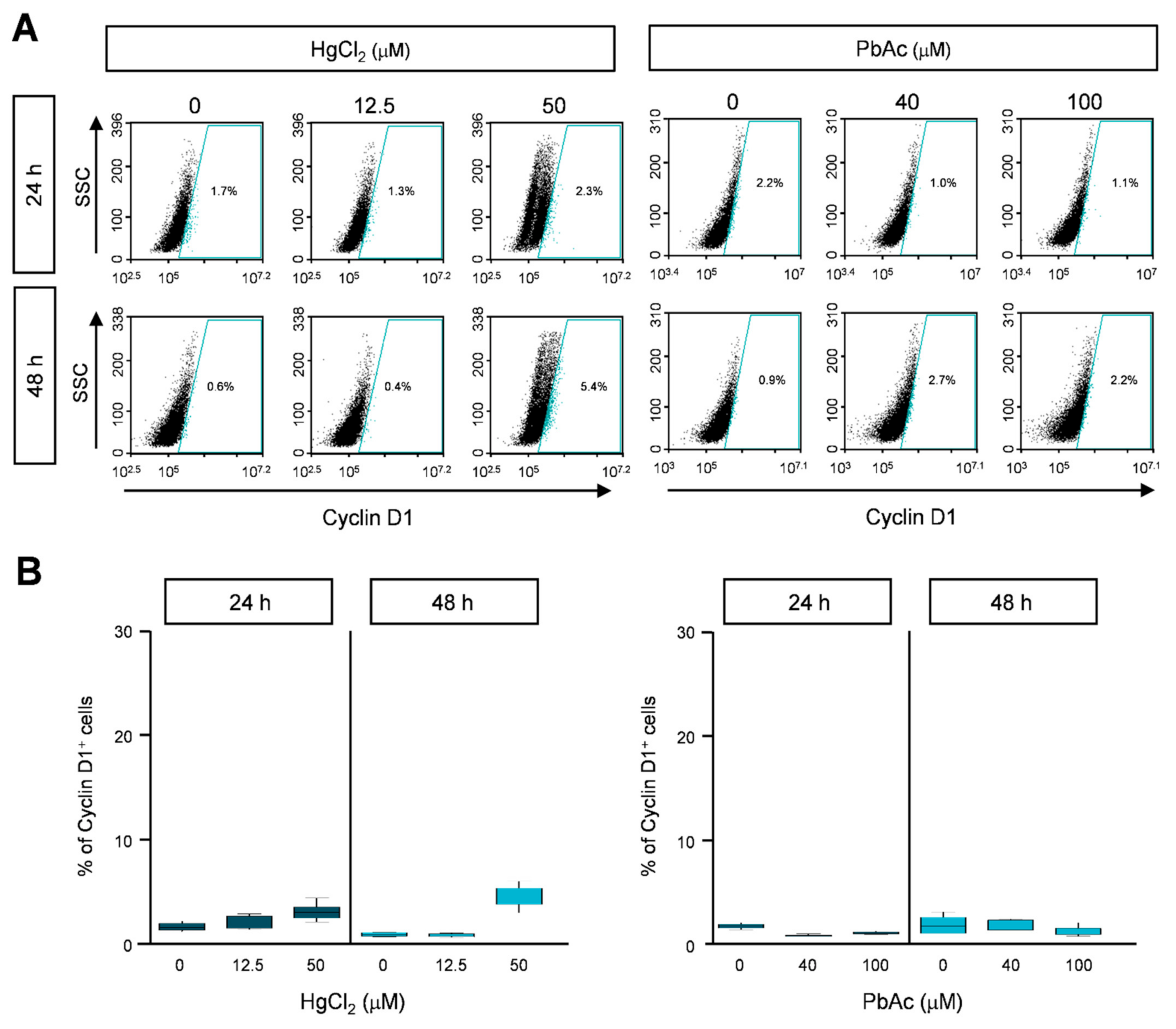
2.4. Cyclin B1 and Cyclin D1 Expression in Heavy Metal-Treated MRC5 Cells
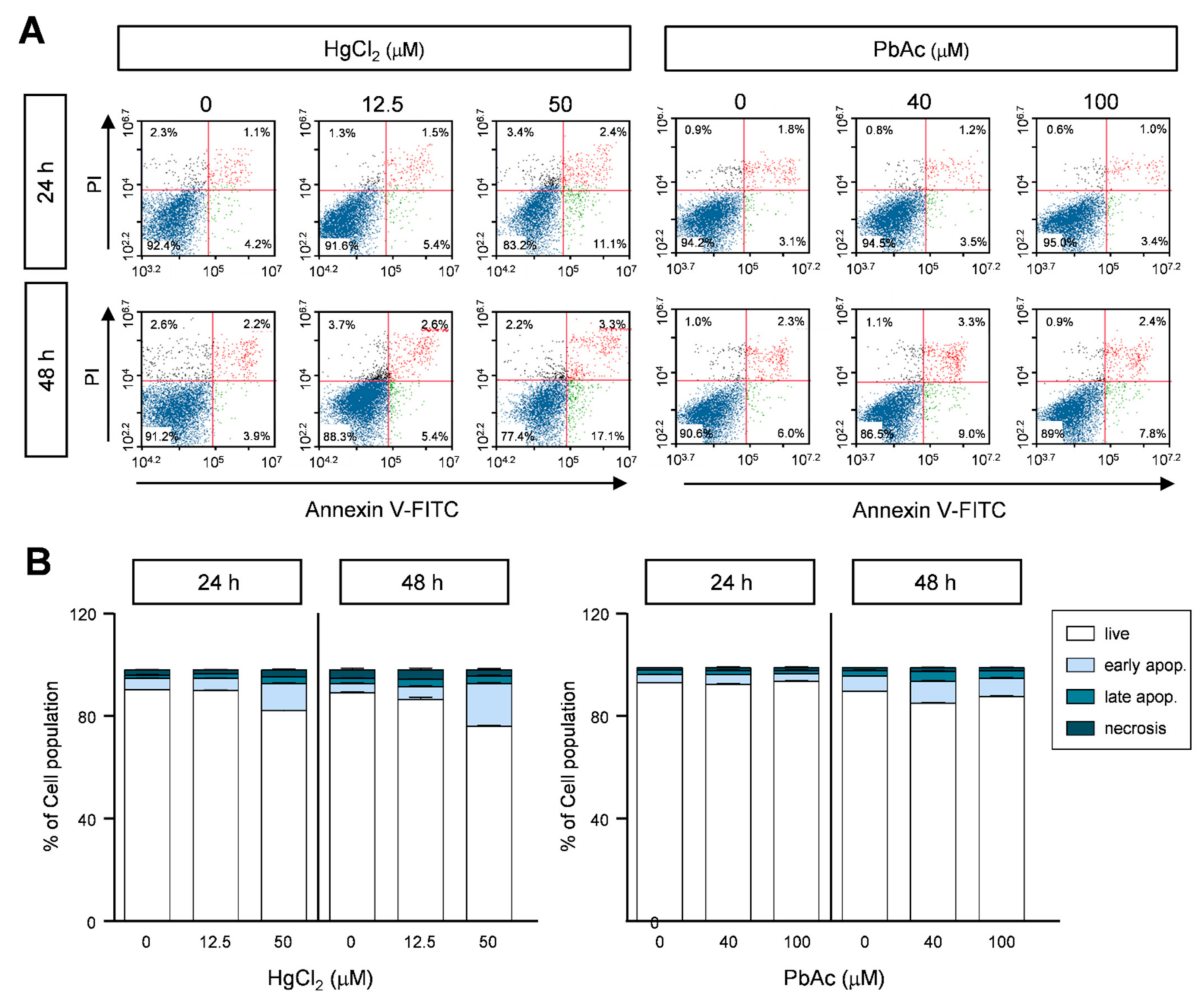
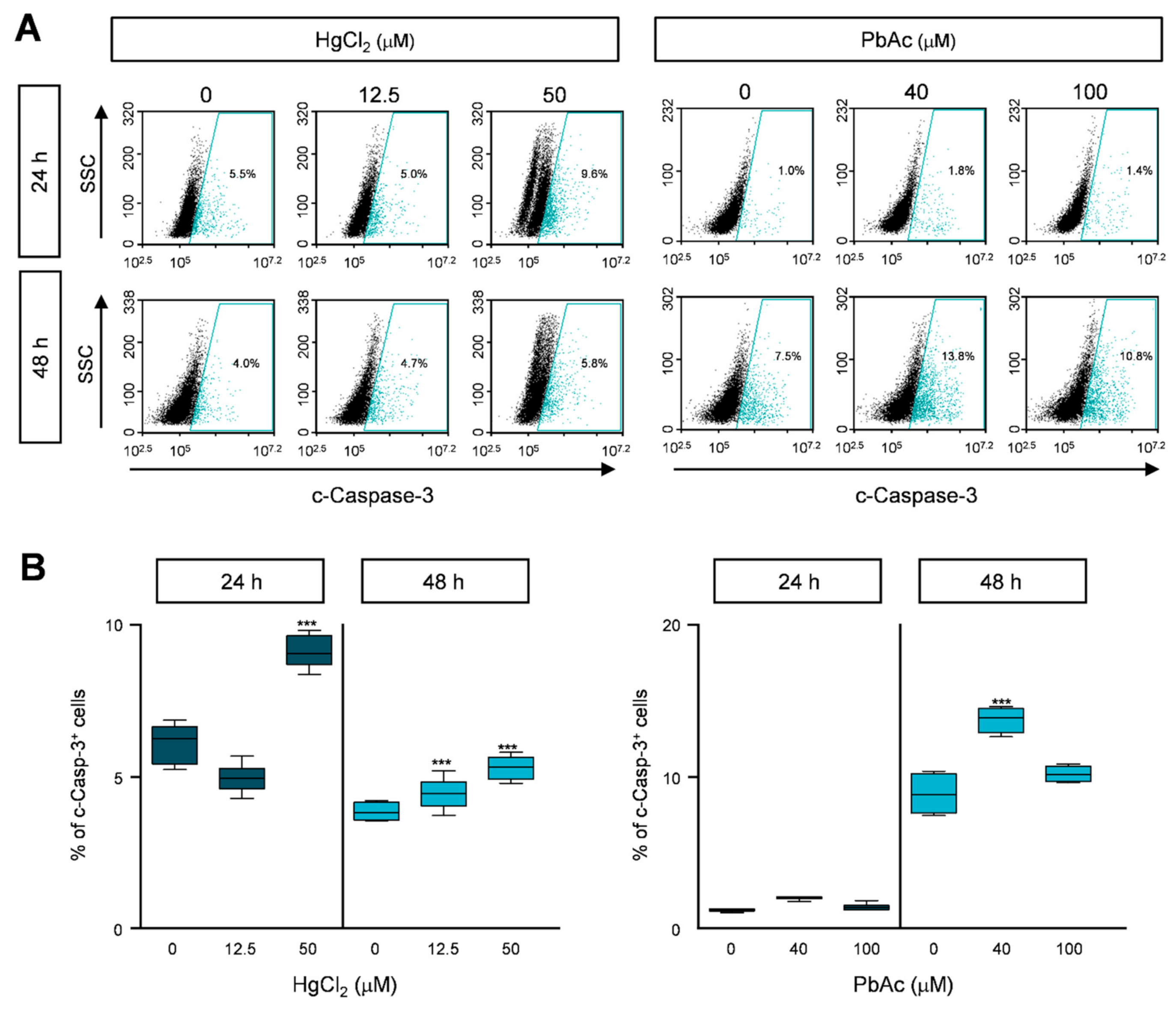
2.5. Effect of Heavy Metals on Apoptotic Cell Death in MRC5 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. MTS Assay
4.4. Morphological Change and Live/Dead Cell Assay
4.5. Cell Cycle Analysis
4.6. Annexin V-FITC/Propidium Iodide (PI) Apoptosis Assay
4.7. Immunostaining for Fluorescence-Activated Cell Sorting (FACS) Analysis
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HgCl2 | Mercury chloride |
| PbAc | Lead acetate |
| EtOH | Ethanol |
| FBS | Fetal bovine serum |
| EthD-1 | Ethidium homodimer-1 |
| PI | Propidium iodide |
References
- Marchetti, C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013, 2013, 184360. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D. Environmental pollution and the global burden of disease. Br. Med. Bull. 2003, 68, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Guney, M.; Zagury, G.J. Heavy metals in toys and low-cost jewelry: Critical review of U.S. and Canadian legislations and recommendations for testing. Environ. Sci. Technol. 2012, 46, 4265–4274. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.; Valiente, M. Determination of pollution trends in an abandoned mining site by application of a multivariate statistical analysis to heavy metals fractionation using SM&T-SES. J. Environ. Monit. 2005, 7, 29–36. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Jang, D.H.; Hoffman, R.S. Heavy metal chelation in neurotoxic exposures. Neurol. Clin. 2011, 29, 607–622. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Tellez-Rojo, M.M.; Hernandez-Avila, M.; Gonzalez-Cossio, T.; Romieu, I.; Aro, A.; Palazuelos, E.; Schwartz, J.; Hu, H. Impact of breastfeeding on the mobilization of lead from bone. Am. J. Epidemiol. 2002, 155, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B. Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention; Center for Disease Control and Prevention: Atlanta, GA, USA, 2002.
- Silbergeld, E.K.; Waalkes, M.; Rice, J.M. Lead as a carcinogen: Experimental evidence and mechanisms of action. Am. J. Ind. Med. 2000, 38, 316–323. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003, 18, 149–175. [Google Scholar] [CrossRef]
- Bhan, A.; Sarkar, N.N. Mercury in the environment: Effect on health and reproduction. Rev. Environ. Health 2005, 20, 39–56. [Google Scholar] [CrossRef]
- Guzzi, G.; La Porta, C.A. Molecular mechanisms triggered by mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Jiang, A.; Li, X.; Chang, W.; Chen, J.; Ye, F. Metformin alleviates lead-induced mitochondrial fragmentation via AMPK/Nrf2 activation in SH-SY5Y cells. Redox Biol. 2020, 36, 101626. [Google Scholar] [CrossRef]
- Barbosa, F., Jr.; Farina, M.; Viegas, S.; Kempinas Wde, G. Toxicology of metals and metalloids. Biomed. Res. Int. 2014, 2014, 253738. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Kim, S.H.; Sharma, R.P. Mercury-induced apoptosis and necrosis in murine macrophages: Role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol. Appl. Pharmacol. 2004, 196, 47–57. [Google Scholar] [CrossRef]
- Thurmer, K.; Williams, E.; Reutt-Robey, J. Autocatalytic oxidation of lead crystallite surfaces. Science 2002, 297, 2033–2035. [Google Scholar] [CrossRef]
- World Health Organization. Childhood Lead Poisoning; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. Children’s Exposure to Mercury Compounds; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Huel, G.; Tubert, P.; Frery, N.; Moreau, T.; Dreyfus, J. Joint effect of gestational age and maternal lead exposure on psychomotor development of the child at six years. Neurotoxicology 1992, 13, 249. [Google Scholar]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Macedo, G.L.; Pereira, S.I.; Arrifano, G.P.; Picanco-Diniz, D.L.; do Nascimento, J.L.; Herculano, A.M. Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef]
- Menon, S.S.; Guruvayoorappan, C.; Sakthivel, K.M.; Rasmi, R.R. Ki-67 protein as a tumour proliferation marker. Clin. Chim. Acta. 2019, 491, 39–45. [Google Scholar] [CrossRef]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. Caspase-3 mediated cell death in the normal development of the mammalian cerebellum. Int. J. Mol. Sci. 2018, 19, 3999. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Diff. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Fonfria, E.; Vilaro, M.T.; Babot, Z.; Rodriguez-Farre, E.; Sunol, C. Mercury compounds disrupt neuronal glutamate transport in cultured mouse cerebellar granule cells. J. Neurosci. Res. 2005, 79, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Lohren, H.; Blagojevic, L.; Fitkau, R.; Ebert, F.; Schildknecht, S.; Leist, M.; Schwerdtle, T. Toxicity of organic and inorganic mercury species in differentiated human neurons and human astrocytes. J. Trace Elem. Med. Biol. 2015, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; de Oliveira, A.C.A.; Leao, L.K.R.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; da Silva, M.C.F.; Amado, L.L.; Sagica, F.E.S.; de Oliveira, E.H.C.; et al. Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front. Mol. Neurosci. 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Tchounwou, H.M.; Tchounwou, P.B. DNA Damage, Cell cycle arrest, and apoptosis induction caused by lead in human leukemia cells. Int. J. Environ. Res. Public Health 2015, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.; Kumar, V.; Ramos, D.; Oliveira, E.; Schuhmacher, M. An in vitro cytotoxic approach to assess the toxicity of heavy metals and their binary mixtures on hippocampal HT-22 cell line. Toxicol. Lett. 2018, 282, 25–36. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; An, M.-J.; Shin, G.-S.; Lee, H.-M.; Kim, M.J.; Kim, C.-H.; Kim, J.-W. Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression. Int. J. Mol. Sci. 2021, 22, 2494. https://doi.org/10.3390/ijms22052494
Kim J-Y, An M-J, Shin G-S, Lee H-M, Kim MJ, Kim C-H, Kim J-W. Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression. International Journal of Molecular Sciences. 2021; 22(5):2494. https://doi.org/10.3390/ijms22052494
Chicago/Turabian StyleKim, Ji-Young, Mi-Jin An, Geun-Seup Shin, Hyun-Min Lee, Mi Jin Kim, Chul-Hong Kim, and Jung-Woong Kim. 2021. "Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression" International Journal of Molecular Sciences 22, no. 5: 2494. https://doi.org/10.3390/ijms22052494
APA StyleKim, J.-Y., An, M.-J., Shin, G.-S., Lee, H.-M., Kim, M. J., Kim, C.-H., & Kim, J.-W. (2021). Mercury Chloride but Not Lead Acetate Causes Apoptotic Cell Death in Human Lung Fibroblast MRC5 Cells via Regulation of Cell Cycle Progression. International Journal of Molecular Sciences, 22(5), 2494. https://doi.org/10.3390/ijms22052494





