Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury
Abstract
1. Pathophysiology of AKI and CKD
2. Current Treatments for Kidney Failure
3. Regenerative Properties of Mesenchymal Stem Cells
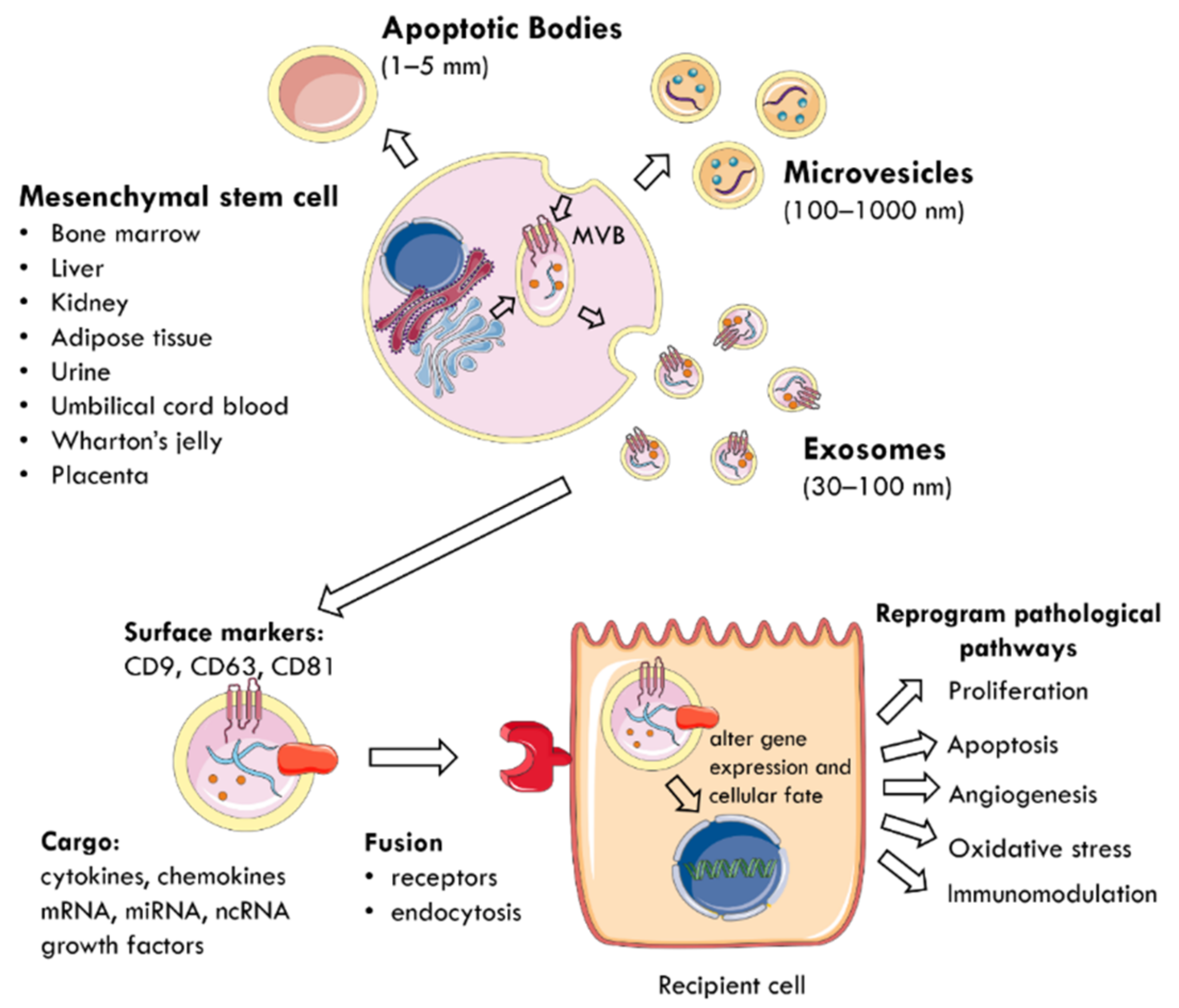
4. Mesenchymal Stem Cell-Derived Extracellular Vesicles
5. Methodologies of Exosome Isolation
6. Therapeutic Applications of MSC-EVs
6.1. Macular Degeneration
6.2. Cancer
6.3. Alzheimer’s Disease
6.4. Ischaemic Stroke
6.5. ARDS and COVID-19
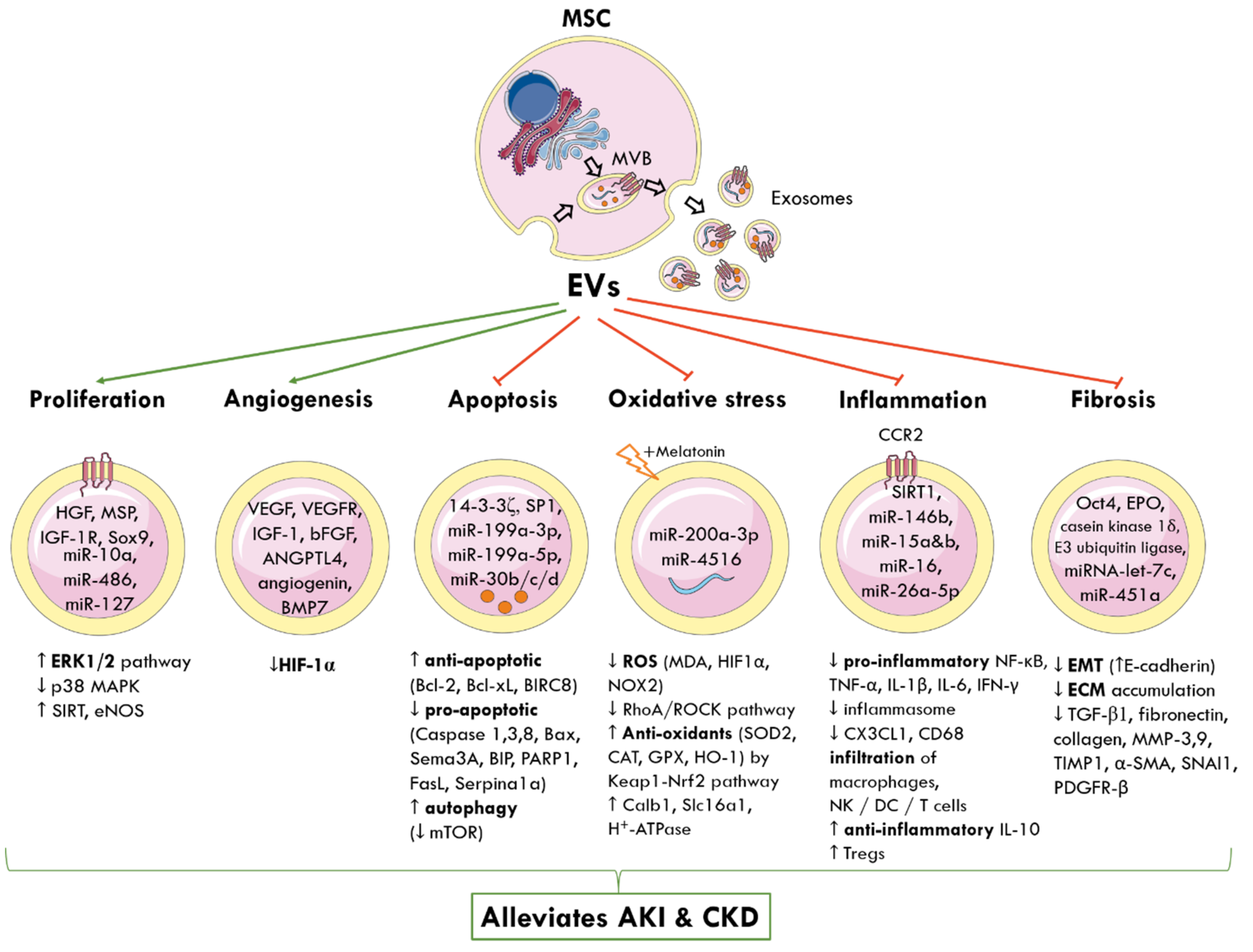
7. Nephroprotective Role of MSC-EVs in AKI
7.1. Tubular Proliferation and Dedifferentiation
7.2. Inhibition of Apoptosis
7.3. Angiogenesis
7.4. Anti-Oxidation
7.5. Immunomodulation
| MSC Source | In Vivo Model | EV Subtype | Dose | Administration | Pathophysiological Effects | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Bone marrow | Glycerol | EVs | Single: 200 μg | Intravenous | EVs accumulate specifically in injured kidneys | [100] | |
| Bone marrow | Glycerol | MVs | Single: 15 μg | Caudal vein | MVs accumulated within lumen of injured tubules ↑ proliferation ↓ apoptosis ↑ tubuloepithelial regeneration | Delivery of HGF, MSP | [101] |
| Bone marrow | Glycerol | EVs | Single: 16.5 × 107 or 8.25 × 107 | Intravenous | Pro-regenerative miRNA-enriched EVs are superior to naïve EVs at lower doses ↓ BUN, creatinine ↓ necrosis | Pro-regenerative miRNA: miR-10a, miR-486, miR-127 | [105] |
| Bone marrow | Cisplatin | MVs | Multiple: 100 μg, then 50 μg days 2, 6, 10, 14, 18 | Intravenous | ↓ apoptosis, necrosis ↑ proliferation ↓ mortality Did not prevent chronic tubular injury at 3 weeks | ↓ Caspase-1,8, lymphotoxin-α ↑ Bcl-2, Bcl-xL, BIRC8 | [109] |
| Bone marrow | Cisplatin | EVs | Single: 150 μg | Intra-arterial kidney | ↓ BUN, creatinine ↓ tubular cast formation ↑ proliferation ↓ inflammation | ↓ IL-6, TNF-α, NF-κB | [107] |
| Bone marrow | Cisplatin | EVs | Single: 200 μg/100 g body weight on day 3 | Intraperitoneal | Combined pre-treatment with pulsed focused ultrasound on d2 ↓ BUN, creatinine ↓ tissue damage (KIM-1, NGAL) ↓ inflammation | ↓ HSP70, HSP90 activation of NLRP3 inflammasome ↓ IL-1β, IL-18 | [136] |
| Bone marrow | Cisplatin | EVs | Single: 150 μg/100 g body weight on day 3 | Caudal vein | Pulsed focused ultrasound pre-treatment ↓ tissue damage (KIM-1, TIMP-1) ↑ proliferation ↑ angiogenesis ↓ apoptosis ↓ inflammation | ↑ ERK signalling ↑ PI3K/Akt ↑ VEGF, PCNA, survivin ↑ SIRT3, eNOS ↓ Caspase-3, Bax ↓ TNF-α, IL-6, IL-1β | [108] |
| Bone marrow | Gentamicin | Exosomes | Multiple: 100 μg | Caudal vein | ↓ apoptosis, necrosis ↑ proliferation ↓ inflammation | Unknown RNA ↓ IL-6, IFN-γ, TNF-α; ↑ IL-10 | [98] |
| Bone marrow | IRI | Exosomes | Single: 200 μg | Renal capsule | ↓ macrophage infiltration ↓ inflammation | CCR2 expression on exosomes suppress CCL2 activity | [134] |
| Bone marrow | IRI | Exosomes preconditioned with 5 μM melatonin | Single: 250 μg | Perfusion | ↓ BUN, creatinine ↓ apoptosis ↓ oxidative stress ↓ inflammation ↑ regeneration ↑ angiogenesis | Melatonin: ↓ Caspase-3, Bax, PARP1; ↑ Bcl-2 ↓ ROS: MDA, HIF-1α, NOX2 ↑ anti-oxidants (HO-1, SOD, CAT, GPX) ↓ MPO activity, ICAM-1, IL-1β, NF-κB; ↑ IL-10 ↑ bFGF, HGF, Sox9, VEGF | [113] |
| Bone marrow | IRI | Exosomes enriched with miR-199a-3p | Single: 5 × 105 | Caudal vein | ↓ apoptosis | ↓ Sema3A and reactivate Akt and ERK pathways ↓ Caspase-3 | [112] |
| Bone marrow | IRI | Exosomes enriched with miR-199a-5p | Single: 5 × 105 | Caudal vein | ↓ endoplasmic reticulum stress at 8–16 h after reperfusion ↓ apoptosis | Targets BIP | [123] |
| Bone marrow | IRI, nephrectomy | EVs | Single: released from 3 × 106 MSCs | Perfusion | ↓ ischaemic damage | ↑ Expression of proteins in membrane transport and homeostasis (Calb1, Slc16a1, vaculor H+-ATPase d2 subunit) | [74] |
| Bone marrow | UUO | EVs | Single: 0.5 mg/kg | Intravenous | ↓ inflammation ↓ macrophage infiltration (ED-1+) ↓ mitochondrial damage ↓ oxidative stress ↓ apoptosis ↓ fibrosis | Delivered MFG-E8 to inhibit RhoA/ROCK pathway ↓ IL-1β, TNF-α, IL-6 ↓ MDA; ↑ anti-oxidants (SOD, CAT) ↓ Caspase-3, PARP1 ↓ α-SMA, ↓fibronectin, ↑E-cadherin | [135] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↓ apoptosis, necrosis ↓ oxidative stress ↑ proliferation | ↓ Caspase 3 ↓ p38 MAPK pathway | [106] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↑ autophagy: ↑LC3B ↓ BUN, creatinine after 3d ↓ apoptosis ↓ inflammation | ↓ mTOR activity ↓ Bax, ↓ Caspase-3; ↑ Bcl-2, Bcl-XL ↓ IL-1β, IL-6, TNF-α | [114] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↑ autophagy ↓ BUN, creatinine after 3d ↓ apoptosis | Delivered 14–3-3ζ to ↑ autophagy via promoting the localisation of ATG16L ↓ Caspase 3 | [115] |
| Umbilical cord | IRI | EVs overexpressing Oct4 | Single: 100 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↑ proliferation ↓ fibrosis | Oct4 inhibited fibrosis (↓ SNAIl, α-SMA) | [117] |
| Umbilical cord | Sepsis (caecal ligation and puncture) | Exosomes | Single: 120 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↓ inflammation ↑ survival (45% vs. 28% control) | Upregulation of miR-146b ↓ IRAK1 and ↓ NF-κB expression ↓ IL-1β, TNF-α | [137] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↑ tubular cell proliferation ↓ inflammation ↓ CD68+ macrophage infiltration ↓ fibrosis | Delivery of miRN-15a/-15b/-16 reduced CX3CL ↓ α-SMA | [110] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Caudal vein | ↓ oxidative stress ↓ apoptosis ↑ proliferation ↓ fibrosis | ↓ NOX2 expression, ↓ ROS levels ↓ α-SMA | [119] |
| Wharton’s jelly | IRI | MVs | Single: 30 μg | Caudal vein | ↑ tubular cell dedifferentiation and growth | ↑ HGF RNA | [103] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Intravenous | ↑ survival ↓ BUN, creatinine ↓ apoptosis ↑ proliferation ↓ inflammation ↓ CD68+ macrophage infiltration ↓ fibrosis | ↓ TNF-α; ↑ IL-10 ↓ α-SMA, TGF-β1 ↑ HGF | [118] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Intravenous | ↓ BUN, creatinine after 24 h ↓ NK cells in kidney without the involvement of the spleen | ↓ CX3CL1, TLR2 | [138] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↑ angiogenesis ↓ fibrosis | Delivery of VEGF and its RNA; ↓ HIF-1α, α-SMA | [124] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↓ mitochondrial fission ↓ apoptosis | Delivery of miR-30b/c/d | [120] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↓ oxidative stress↓ renal cell injury (↓NGAL) ↓ apoptosis | ↑ Nrf2/ARE activation ↑ ROS scavenging enzymes (HO-1) | [130] |
| Renal | IRI | EVs | Single: 4 × 108 | Intravenous | EVs detected in ischaemic kidneys within 1 h ↓ BUN, creatinine ↑tubular cell proliferation | Identified 62 miRNAs | [53] |
| Renal | IRI | EVs | Single: 2 × 107 | Caudal vein | ↓ apoptosis ↑ peritubular capillary endothelial cell proliferation ↑ angiogenesis | Selective engraftment in ischaemic kidneys Delivery of VEGF-A, bFGF, IGF-1 | [111] |
| Adipose | IRI | Exosomes | Single: 100 μg | Intravenous | Combined ADMSC and exosome therapy is superior to monotherapy: ↓ proteinuria ↓ kidney injury score | [140] | |
| Adipose | Sepsis (caecal ligation and puncture) | Exosomes | Single: 100 μg | Caudal vein | ↓ inflammation ↓ inflammatory cell infiltration ↓ apoptosis ↓ mortality | ↑ SIRT1 inhibited NF-κB and its inflammatory activity ↓ TNF-α, IL-6, MCP-1 ↓ Bax, ↓ Caspase-3; ↑ Bcl-2 | [141] |
| Human induced pluripotent stem cells | IRI | EVs | Single: 1 × 1012 | Intravenous | ↓ necroptosis | Delivery of SP1 to renal cells | [122] |
| Human placenta-derived | IRI | EVs | Single: 80 μg | Intravenous | EVs specifically accumulated in ischaemic kidney and taken up by proximal TECs ↑ mitochondrial antioxidant defence ↓ mitochondrial fragmentation | Keap1-Nrf2 pathway- ↑ SOD2, ↑ATP production | [132] |
| Human placenta-derived | IRI | EVs | Multiple: 100 μg daily for 3 days | EVs travelled to injured kidneys ↑ proliferation and regeneration ↓ BUN, creatinine ↓ apoptosis ↓ fibrosis d28 | ↑ Sox9+ expression in tubular epithelial cells ↓ α-SMA, fibronectin, collagen I, TGF-β1 | [142] |
8. Anti-Fibrotic Effect of MSC-EVs in CKD
8.1. Downregulate Pro-Fibrotic Gene Expression and the EMT
8.2. Reduce Tubular Atrophy
8.3. Vascular Regeneration
8.4. Anti-Inflammatory
| MSC Source | In Vivo Model | EV Subtypes | Dose | Administration | Pathophysiological Effects | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Bone marrow | IRI | MVs | Single: 30 μg | Intravenous | ↓ BUN, creatinine, proteinuria ↓ fibrosis, ↓glomerular matrix accumulation ↓ interstitial lymphocyte infiltrate ↓ tubular atrophy | Dependent on RNA cargo | [104] |
| Bone marrow | Chronic CsA | EVs | Multiple: 100 μg Preventive: 24 h after CsA, weekly for 4 weeks Curative: 2 weeks after CsA, weekly for 4 weeks | Intraperitoneal | Greater improvement when administered after damage (curative regime), rather than prophylactically ↓ tubular casts | ↓ PAI-1, TIMP-1, IFN-γ | [154] |
| Bone marrow | Aristolochic acid | EVs | Single: 1 × 1010 on day 3 | Intravenous | ↓ BUN, creatinine ↓ necrosis ↓ CD45+ immune cells, fibroblast, pericyte infiltration ↓ interstitial fibrosis | Downregulation of hsa-miR-21-5p, 34a-5p, 34c-5p, 132-3p, 214-3p, 342-3p; and mmu-miR-212-3p Upregulation of hsa-miR-194-5p, 192-5p; and mmu-miR-378-3p ↓ α-SMA, TGF-β1, collagen Iα1 | [151] |
| Bone marrow | 5/6 subtotal nephrectomy | MVs | Multiple: 30 μg, days 2, 3, 5 | Caudal vein | ↓ BUN, creatinine, uric acid, proteinuria prevent fibrosis ↓ tubular atrophy ↓ interstitial lymphocyte infiltrate | [153] | |
| Bone marrow | UUO | MVs | Single: 30 μg | Caudal vein | ↓ BUN, creatinine ↓ fibrosis | ↓ TGF-β1, α-SMA ↑ E-cadherin | [152] |
| Bone marrow | UUO | Exosomes enriched with miR-let7c | Single: released from 1 × 106 MSCs | Intravenous | Exosomes home to injured kidneys ↓ fibrosis | Delivery of miRNA-let7c ↓ collagen, MMP-9, α-SMA, TGF-βR1 | [149] |
| Bone marrow | Type 2 diabetes, STZ Type 1 diabetes | Exosomes | Single: 5.3 × 107 | Renal subcapsular | ↓ degeneration, vacuolation and tubular atrophy ↓ EMT ↓ ICAM-1-mediated interstitial inflammatory infiltration | ↓ TGF-β ↓ TNF-α | [16] |
| Bone marrow | STZ Type 1 diabetes | Exosomes | Single: 100 μg | Intravenous | ↑ Autophagy: ↑ LC3-II, Beclin-1 ↓ BUN, creatinine, blood glucose, proteinuria at 10 and 12 weeks ↓ fibrosis | ↓ mTOR activity ↓ collagen, TGF-β | [165] |
| Bone marrow, Liver | STZ Type 1 diabetes | EVs | Multiple: 1 × 1010 | Intravenous | ↓ BUN, creatinine ↓ fibrosis, ↓ EMT ↓ inflammatory cell recruitment | ↓ collagen I, MMP3, TIMP1, FasL, Serpina1a, SNAI1 ↓ CCL3 | [54] |
| Umbilical cord | STZ-induced DN with hyperuricaemia | MVs enriched with miR-451a | Single: 1.5 mg/kg | Caudal vein | ↓ BUN, creatinine ↓ fibrosis, ↓ EMT ↑ proliferation and removed arrest on cell cycle | ↓ α-SMA, ↑ E-cadherin miR-451a targeted 3′UTR sites of cell cycle inhibitors (P15INK4b, P19INK4d) | [150] |
| Umbilical cord | UUO | Exosomes | Single: 200 μg | Intravenous | ↓ tubulointerstitial fibrosis | Exosomes delivered casein kinase 1δ and E3 ubiquitin ligase β-TRCP to degrade YAP | [166] |
| Umbilical cord | UUO | Exosomes | Single: 200 μg | Intra-arterial kidney | ↓ BUN, creatinine ↓ apoptosis ↓ oxidative stress ↓ tubulointerstitial fibrosis | ↓ ROS-mediated p38 MAPK/ERK signalling pathway ↓ ROS: MDA ↑ anti-oxidants: GSH | [145] |
| Wharton’s jelly | CsA | EVs | Multiple: 100 μg at day 7, 21 | Intravenous | ↓ creatinine ↓ fibrosis, ↓ EMT ↓ oxidative stress | ↓ α-SMA ↓ ROS: MDA ↑ anti-oxidants: SOD | [146] |
| Renal | UUO | MPs | Single: 2 × 107 | Caudal vein | ↓ EndoMT of PTC endothelial cells ↓ PTC rarefaction ↓ F4/80+ inflammatory cell infiltration ↓ tubulointerstitial fibrosis | ↓ α-SMA | [155] |
| Renal | UUO | EPO-enriched MPs | Single: 80 μg | Caudal vein | ↓ tubulointerstitial fibrosis, ↓ EMT ↓ myofibroblast and F4/80+ macrophage infiltration | ↓ phosphorylated Smad2, Smad3, MAPK 38 expression to inhibit EMT ↓ α-SMA, fibronectin, collagen | [144] |
| Adipose (transfected with GDNF) | UUO | Exosomes | Single: 200 μg | Caudal vein | ↓ PTC rarefaction ↓ tubulointerstitial fibrosis ↑ endothelial function and angiogenesis | GDNF: ↑ SIRT1/p-eNOS pathway ↓ α-SMA ↑ VEGF, ↓ HIF-1α | [167] |
| Adipose | IRI | Exosomes | Single: 100 μg | Caudal vein | ↑ tubular proliferation, regeneration ↓ TGF-β1-induced transformation of TECs to pro-fibrotic phenotype ↓ AKI to CKD transition | ↑ Sox9 ↓ α-SMA, PDGFR-β | [168] |
| Adipose | Type 1 diabetes | Exosomes | Single: not stated, 12-week therapy | Caudal vein | ↓ BUN, creatinine, proteinuria ↑ autophagy, ↓ apoptosis podocytes | miR-486 reduced Smad1 expression, leading to ↓ mTOR activation | [169] |
| Adipose | Hindlimb Ischaemia | Melatonin-stimulated exosomes | CKD-MSCs treated with 30 μg exosomes, and 1 × 106 cells injected | Injection into ischaemic site | CKD-MSCs were treated with melatonin-stimulated exosomes and injected into mice ↑ neovascularisation ↑ functional recovery | Upregulation of miR-4516 ↑ PrPc in exosomes | [170] |
| Adipose | DN (C57BL/KsJ db/db) | EVs | Single | Caudal vein | ↓ histopathology of DN, ↓ BUN, creatinine ↓ VEGFA leads to ↓ podocyte apoptosis | miR-26a-5p inhibited TLR4 and inactivated NF-κB/VEGFA pathway (↓ IKKβ, IκBα, p65) ↓ Caspase-3, Bax, ↑ Bcl-2 | [161] |
| Adipose | Unilateral renovascular disease on background of Metabolic Syndrome | EVs | Single: 1 × 107 | Intra-renal vein | ↑ cortical microvascular, PTC density ↑ RBF, GFR ↓ glomerular, tubulointerstitial fibrosis ↓ apoptosis ↓ oxidative stress | Delivered proangiogenic factors: VEGF-A,C, VEGF receptor, angiopoietin like 4, HGF ↓ Caspase-3 ↓ ROS: superoxides, CD31, nitro tyrosine | [158] |
| Adipose | Unilateral renal artery stenosis on background of Metabolic Syndrome | EVs | Single: 1 × 1010 | Intrarenal artery | EVs derived from lean pigs were injected into pigs with Metabolic Syndrome | ↑ TGF-β induction of Tregs ↓ IL-1β | [163] |
| ↑ anti-inflammatory M2 macrophages | |||||||
| ↓ pro-inflammatory M1 macrophages | |||||||
| ↓ CD8+ T cells | |||||||
| Adipose | Unilateral renal artery stenosis on background of Metabolic Syndrome | EVs | Single: 1 × 1010 | Intrarenal artery | Metabolic Syndrome alters the cargo of 19 mitochondria-related miRNA, impairing regenerative capacity | ↑ miR-196a, 132 ↓ miR-192, 320 | [164] |
| Adipose | Unilateral renal artery stenosis | MVs, exosomes | Single: 100 μg | Caudal vein | ↓ HIF-1α Stabilised systolic blood pressure ↓ proteinuria (MVs only) ↑ natriuresis (exosomes only) ↓ fibrosis ↓ inflammation | ↓ collagen I, TGF-β ↑ IL-10 | [147] |
| Urine | STZ Type 1 diabetes | Exosomes | Multiple: 100 μg weekly x × 12 | Intravenous | ↓ apoptosis of podocyte and tubular cells ↑ glomerular endothelial cell proliferation ↑ angiogenesis | ↓ Caspase-3 Delivery of VEGF, TGF-β1, angiogenin, BMP7 | [121] |
9. Biological Cargo Carried by MSC-EVs to Alleviate AKI and CKD
9.1. mTOR
9.2. 14-3-3ζ
9.3. YAP
9.4. Oct-4
9.5. SP1
9.6. Sox-9
9.7. SIRT1
9.8. MFG-E8
9.9. Melatonin and PrPc
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, Y.; Bowe, B.; Mokdad, A.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem cell-derived extracellular vesicles and kidney regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Westenfelder, C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, K.; Luo, J.; Dong, Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 2010, 176, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E., II; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Wu, V.-C.; Wu, C.-H.; Huang, T.-M.; Wang, C.-Y.; Lai, C.-F.; Shiao, C.-C.; Chang, C.-H.; Lin, S.-L.; Chen, Y.-Y.; Chen, Y.-M.; et al. Long-term risk of coronary events after AKI. Clin. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef]
- Tuttle, K. A turning point for chronic kidney disease in diabetes. Lancet 2019, 393, 1913–1914. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. The role of TGF-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar]
- Kida, Y.; Tchao, B.N.; Yamaguchi, I. Peritubular capillary rarefaction: A new therapeutic target in chronic kidney disease. Pediatr. Nephrol. 2014, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D. Renal tubulointerstitial fibrosis: Common but never simple. Am. J. Physiol. Ren. Physiol. 2009, 296, F1239–F1244. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of tubulointerstitial fibrosis and the chronic kidney disease phenotype—Role of risk factors and epigenetics. Front. Pharm. 2017, 8, 520–528. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2015, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of global kidney health care status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Admitted Patient Care 2014–15: Australian Hospital Statistics; Australian Institute of Health and Welfare: Canberra, ACT, Australia, 2018.
- Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care, 4th ed.; Kidney Health Australia: Melbourne, VIC, Australia, 2020. [Google Scholar]
- Chadban, S.; Cass, A.; Gallagher, M.; Howard, K.; Jones, A.; McDonald, S.; Snelling, P.; White, S. The Economic Impact of End-Stage Kidney Disease in Australia: Projections to 2020; Kidney Health Australia: Melbourne, VIC, Australia, 2010. [Google Scholar]
- Wyld, M.; Morton, R.L.; Hayen, A.; Howard, K.; Webster, A.C. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012, 9, e1001307. [Google Scholar] [CrossRef]
- Wright, J.; Narayan, S. Analysis of Kidney Allocation during 2015; National Organ Matching Service: Adelaide, SA, Australia, 2016.
- ANZDATA Registry. The 40th Annual ANZDATA Report; Australia and New Zealand Dialysis and Transplant Registry: Adelaide, SA, Australia, 2017. [Google Scholar]
- Wong, G.; Turner, R.M.; Chapman, J.R.; Howell, M.; Lim, W.H.; Webster, A.C.; Craig, J.C. Time on dialysis and cancer risk after kidney transplantation. Transplantation 2013, 95, 114–121. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Chronic Kidney Disease; Australian Institute of Health Welfare: Canberra, ACT, Australia, 2019.
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on mesenchymal stem cell-derived exosomes: Opportunities and challenges in cell-free therapy. Stem. Cells Int. 2017, 2017, 6305295–6305305. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular vesicles: Evolving factors in stem cell biology. Stem. Cells Int. 2016, 2016, 1073140. [Google Scholar] [CrossRef]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef]
- Bochon, B.; Kozubska, M.; Surygala, G.; Witkowska, A.; Kuzniewicz, R.; Grzeszczak, W.; Wystrychowski, G. Mesenchymal stem cells—Potential applications in kidney diseases. Int. J. Mol. Sci. 2019, 20, 2462. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef]
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.C.; Borlongan, C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015, 17, 18–24. [Google Scholar] [CrossRef]
- Kamal, M.M.; Kassem, D.H. Therapeutic potential of Wharton’s jelly mesenchymal stem cells for diabetes: Achievements and challenges. Front. Cell Dev. Biol. 2020, 8, 16. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem. Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.; Wu, Y. Excess integrins cause lung entrapment of mesenchymal stem cells. Stem. Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef]
- Tögel, F.; Isaac, J.; Westenfelder, C. Hematopoietic stem cell mobilization–associated granulocytosis severely worsens acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1261–1267. [Google Scholar] [CrossRef]
- Fennema, E.M.; Tchang, L.A.H.; Yuan, H.; van Blitterswijk, C.A.; Martin, I.; Scherberich, A.; de Boer, J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J. Tissue Eng. Regen. 2018, 12, e150–e158. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Katsuda, T.; Gailhouste, L.; Nakagama, H.; Ochiya, T. Mesenchymal stem cell-derived extracellular vesicles: A glimmer of hope in treating Alzheimer’s disease. Int. Immunol. 2017, 29, 11–19. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Deng, Y.; Yang, C. An update on isolation methods for proteomic studies of extracellular vesicles in biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017, 7, 17095–17104. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087–30118. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wang, K.J.Z.; Webster, K.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; Ni, C.; et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem. Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark Res. 2019, 7, 8–16. [Google Scholar] [CrossRef]
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: Deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front. Genet. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem. Cell Res. 2017, 8, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468–4481. [Google Scholar] [CrossRef]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell Vesicles 2016, 5, 32945–32950. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014, 3, 26913–26918. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.d.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335–5345. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorum, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319–17335. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-L.; Zhu, J.; Liu, J.-X.; Jiang, F.; Ni, W.-K.; Qu, L.-S.; Ni, R.-Z.; Lu, C.-H.; Xiao, M.-B. A comparison of traditional and novel methods for the separation of exosomes from human samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Morón-Font, M.; Gámez-Valero, A.; Carreras-Planella, L.; Borràs, F.E.; Franquesa, M. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr. Protoc. Cell Biol. 2019, 49, e82. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J. Extracell Vesicles 2018, 7, 1490145–1490158. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.M.; Kuhnell, D.; Orr-Asman, M.A.; Biesiada, J.; Zhang, X.; Medvedovic, M.; Thomas, H.E. Balancing yield, purity and practicality: A modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019, 16, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V.; Nagaeva, O.; Dehlin, E. Isolation and characterization of exosomes from cultures of tissue explants and cell lines. Curr. Protoc. Immunol. 2016, 115, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W. Effect of Microvesicles and Exosomes Therapy on β-cell Mass in Type I Diabetes Mellitus (T1DM). Available online: https://clinicaltrials.gov/ct2/show/NCT02138331 (accessed on 18 May 2021).
- Ezquer, F.E.M.; Contador, D.; Ricca, M.; Simon, V.; Conget, P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem. Cells 2012, 30, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Sherman, L.; Munoz, J.; Rameshwar, P. Immunological properties of mesenchymal stem cells and clinical implications. Arch. Immunol. Exp. 2008, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bank, J.R.; Rabelink, T.J.; de Fijter, J.W.; Reinders, M.E.J. Safety and efficacy endpoints for mesenchymal stromal cell therapy in renal transplant recipients. J. Immunol. Res. 2015, 2015, 391797–391811. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with mesenchymal stromal Cells/extracellular vesicles prevents ischaemic injury. J. Cell Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.-u.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, 63–73. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562–34574. [Google Scholar] [CrossRef]
- Zhang, X.; Tianjin Medical University. MSC-Exos Promote Healing of MHs (MSCs). Available online: https://clinicaltrials.gov/ct2/show/NCT03437759 (accessed on 18 May 2021).
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Jt. Res. 2018, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Istituto Ortopedico Galeazzi. Effects of ASC Secretome on Human Osteochondral Explants (ASC-OA). Available online: https://clinicaltrials.gov/ct2/show/NCT04223622 (accessed on 18 May 2021).
- Takahashi, R.H.; Milner, T.A.; Li, F.; Nam, E.E.; Edgar, M.A.; Yamaguchi, H.; Beal, M.F.; Xu, H.; Greengard, P.; Gouras, G.K. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002, 161, 1869–1879. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef]
- Wang, G.; Gao, X.; Ruijin Hospital. Safety and Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04388982 (accessed on 18 May 2021).
- Dehghani, L.; Soleimani, M.; Isfahan University of Medical Sciences. Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke. Available online: https://clinicaltrials.gov/ct2/show/NCT03384433 (accessed on 18 May 2021).
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front. Behav. Neurosci. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- State-Financed Health Facility “Samara Regional Medical Center Dinasty”. Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia (COVID-19EXO). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04491240 (accessed on 18 May 2021).
- Qu, J.-M.; Ruijin Hospital. A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04276987 (accessed on 18 May 2021).
- Qu, J.-M.; Ruijin Hospital. A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS. Available online: https://clinicaltrials.gov/ct2/show/NCT04602104 (accessed on 18 May 2021).
- Luttrell, T.C.; Chawla, S.P.; Mission Community Hospital. The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Cuased by COVID-19 (ARDOXSO). Available online: https://clinicaltrials.gov/ct2/show/NCT04798716 (accessed on 18 May 2021).
- Li, I.; Nabet, B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol. Cancer 2019, 18, 32. [Google Scholar] [CrossRef]
- Bouché, O.; de Reims, C.H.U. Identification of New Diagnostic Protein Markers for Colorectal Cancer in Circulating Tumor Exosomes. Available online: https://clinicaltrials.gov/ct2/show/NCT04394572 (accessed on 18 May 2021).
- McDonald, L.; King’s College London. A Study to Measure the Expression of HER2-HER3 Dimer in Tumour and Blood (Exosomes) Samples from Patients with HER2 Positive Breast Cancer Receiving HER2 Targeted Therapies (HERdi PREDICT). Available online: https://clinicaltrials.gov/ct2/show/NCT04288141 (accessed on 18 May 2021).
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.M.D.; Anderson Cancer Center. iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT03608631 (accessed on 18 May 2021).
- Leissring, M.A.; Farris, W.; Chang, A.Y.; Walsh, D.M.; Wu, X.; Sun, X.; Frosch, M.P.; Selkoe, D.J. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003, 40, 1087–1093. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Börger, V.; Weiss, D.J.; Anderson, J.D.; Borràs, F.E.; Bussolati, B.; Carter, D.R.F.; Dominici, M.; Falcón-Pérez, J.M.; Gimona, M.; Hill, A.F.; et al. International society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: Considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy 2020, 22, 482–485. [Google Scholar] [PubMed]
- Reis, L.A.; Borges, F.T.; Simões, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Bruno, S.; Chatterjee, D.; Quesenberry, P.J.; Tetta, C.; Camussi, G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014, 33, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Clin. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Tomasoni, S.L.L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem. Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.-Q.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.-Y.; Zhang, G.-Y.; Gu, D.; Miao, S.; Zhu, Y.-J.; Sun, J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion–induced acute and chronic kidney injury. Nephrol. Dial. Transpl. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Tapparo, M.; Bruno, S.; Collino, F.; Togliatto, G.; Deregibus, M.C.; Provero, P.; Wen, S.; Quesenberry, P.J.; Camussi, G. Renal regenerative potential of extracellular vesicles derived from miRNA-engineered mesenchymal stromal cells. Int. J. Mol. Sci. 2019, 20, 2381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem. Cell Res. 2013, 4, 34–47. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Choi, J.; Wang, J.; Concepcion, W.; Thakor, A.S. A novel approach to deliver therapeutic extracellular vesicles directly into the mouse kidney via its arterial blood supply. Cells 2020, 9, 937. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Concepcion, W.; Thakor, A.S. Pulsed focused ultrasound enhances the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles in acute kidney injury. Stem. Cell Res. 2020, 11, 398. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem. Cell Res. 2014, 5, 40–53. [Google Scholar] [CrossRef]
- Choi, H.Y.; Moon, S.J.; Ratliff, B.B.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, S.; Lim, B.J.; Kim, B.S.; Plotkin, M.D.; et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS ONE 2014, 9, e87853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Lin, F.; Yin, H.; Li, X.; He, W.; Liu, N.; Gou, X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell Physiol. 2019, 234, 23736–23749. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A. Melatonin improves therapeutic potential of mesenchymal stem cells-derived exosomes against renal ischemia-reperfusion injury in rats. Am. J. Transl. Res. 2019, 11, 2887–2907. [Google Scholar] [PubMed]
- Wang, B.Y.; Jia, H.Y.; Zhang, B.; Wang, J.J.; Ji, C.; Zhu, X.M.; Yan, Y.M.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem. Cell Res. 2017, 8, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Liu, W.Z.; Zhang, B.; Wang, J.J.; Wu, P.P.; Tandra, N.; Liang, Z.F.; Ji, C.; Yin, L.; Hu, X.Y.; et al. HucMSC exosomes-delivered 14-3-3 zeta enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am. J. Transl. Res. 2018, 10, 101–113. [Google Scholar]
- Zhang, J.; Zhang, H.; Liu, J.; Tu, X.; Zang, Y.; Zhu, J.; Chen, J.; Dong, L.; Zhang, J. miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem. Biophys. Res. Commun. 2012, 417, 1100–1105. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Hou, Y.P.; Zou, X.Y.; Xing, X.Y.; Ju, G.Q.; Zhong, L.; Sun, J. Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press. Res. 2020, 45, 95–108. [Google Scholar] [CrossRef]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C.; Tian, X.; Wang, J. Micro-vesicles derived from human Wharton’s Jelly mesenchymal stromal cells mitigate renal ischemia-reperfusion injury in rats after cardiac death renal transplantation. J. Cell Biochem. 2018, 119, 1879–1888. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem. Cells Int. 2016, 2016, 2093940–2093952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N.-S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem. Cell Res. 2016, 7, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200–3218. [Google Scholar] [PubMed]
- Wang, C.Y.; Zhu, G.M.; He, W.Y.; Yin, H.B.; Lin, F.; Gou, X.; Li, X.Y. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019, 33, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Gu, D.; Xing, X.; Cheng, Z.; Gong, D.; Zhang, G.; Zhu, Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am. J. Transl. Res. 2016, 8, 4289–4299. [Google Scholar]
- Devarajan, P. Update on mechanisms of ischemic acute kidney injury. Clin. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M.; Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 2014, 25, 998–1012. [Google Scholar] [CrossRef]
- Baniene, R.; Trumbeckas, D.; Kincius, M.; Pauziene, N.; Raudone, L.; Jievaltas, M.; Trumbeckaite, S. Short ischemia induces rat kidney mitochondria dysfunction. J. Bioenerg. Biomembr. 2016, 48, 77–85. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez, S.J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Borthwick, K.J.; Karet, F.E. Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H(+)—ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene 2002, 297, 169–177. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res. 2016, 41, 119–128. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.C.; Peng, H.; Ye, Z.; Zhang, J.; Liu, X.; Lou, T. Activation of the Nrf2-ARE pathway attenuates hyperglycemia-mediated injuries in mouse podocytes. Cell Physiol. Biochem. 2014, 34, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.M.; Cheng, Y.Q.; Gao, H.Q.; Zhuang, J.; Zhang, W.G.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.J.; Kong, D.L.; et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem. Cells Int. 2016, 2016, 1240301–1240311. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Zhang, Y.; Jiang, D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem. Cell Res. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Rai, S.; Concepcion, W.; Thakor, A.S. HSP70-mediated NLRP3 inflammasome suppression underlies reversal of acute kidney injury following extracellular vesicle and focused ultrasound combination therapy. Int. J. Mol. Sci. 2020, 21, 4085. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhu, Y.; Li, Y.; Liu, W.Z.; Yin, L.; Yin, S.Q.; Ji, C.; Hu, Y.Y.; Wang, Q.N.; Zhou, X.R.; et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol. Lett. 2020, 42, 669–679. [Google Scholar] [CrossRef]
- Zou, X.; Gu, D.; Zhang, G.; Zhong, L.; Cheng, Z.; Liu, G.; Zhu, T. NK Cell regulatory property is involved in the protective role of MSC-derived extracellular vesicles in renal ischemic reperfusion injury. Hum. Gene 2016, 27, 926–935. [Google Scholar] [CrossRef]
- Bruno, S.; Chiabotto, G.; Favaro, E.; Deregibus, M.C.; Camussi, G. Role of extracellular vesicles in stem cell biology. Am. J. Physiol. Cell Physiol. 2019, 317, C303–C313. [Google Scholar] [CrossRef]
- Lin, K.C.; Yip, H.K.; Shao, P.L.; Wu, S.C.; Chen, K.H.; Chen, Y.T.; Yang, C.C.; Sun, C.K.; Kao, G.S.; Chen, S.Y.; et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int. J. Cardiol. 2016, 216, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020, 255, 117719. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, S.; Huimin, S.; Wang, L.; Li, H.; Zhao, J.; Zhang, C.; Li, N.; Guo, Z.; Han, Z.; et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J Biol. Chem. 2020, 295, 12203–12213. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, K.; Umezono, T.; Abe, M.; Kobayashi, T.; Kato, M.; Miyauchi, M.; Yamamoto, N.; Kimura, M.; Toyoda, M.; Suzuki, D. Expression of transcription factor Snai1 and tubulointerstitial fibrosis in progressive nephropathy. J. Nephrol. 2011, 25, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.-h.; Jhee, J.H.; Kim, T.Y.; Choi, H.Y.; Kim, H.J.; Park, H.C. Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis. Stem. Cell Res. 2020, 11, 422. [Google Scholar] [CrossRef]
- Liu, B.; Hu, D.; Zhou, Y.; Yu, Y.; Shen, L.; Long, C.; Butnaru, D.; Timashev, P.; He, D.; Lin, T.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am. J. Transl. Res. 2020, 12, 4998–5014. [Google Scholar] [PubMed]
- Zhang, G.Y.; Yu, S.Y.; Sun, S.; Zhang, L.; Zhang, G.L.; Xu, K.; Zheng, Y.X.; Xue, Q.; Chen, M. Extracellular-vesicles derived from human Wharton-Jelly mesenchymal stromal cells ameliorated cyclosporin A-induced renal fibrosis in rats. Int. J. Clin. Exp. Med. 2019, 12, 8943–8949. [Google Scholar]
- Ishiy, C.S.R.A.; Ormanji, M.S.; Maquigussa, E.; Ribeiro, R.S.; da Silva Novaes, A.; Boim, M.A. Comparison of the effects of mesenchymal stem cells with their extracellular vesicles on the treatment of kidney damage induced by chronic renal artery stenosis. Stem. Cells Int. 2020, 2020, 8814574. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.-H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, G.; Wang, X.; Li, L.; Zhang, J.; Chen, Y.; Liu, J.; Liu, S.; Wei, L.; Zhang, W.; et al. Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp. Biol. Med. 2019, 243, 1233–1242. [Google Scholar] [CrossRef]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Bruno, S.; Antico, F.; Brizzi, M.F.; Quesenberry, P.J.; et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front. Cell Dev. Biol. 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- Ramirez-Bajo, M.J.; Martin-Ramirez, J.; Bruno, S.; Pasquino, C.; Banon-Maneus, E.; Rovira, J.; Moya-Rull, D.; Lazo-Rodriguez, M.; Campistol, J.M.; Camussi, G.; et al. Nephroprotective potential of mesenchymal stromal cells and their extracellular vesicles in a murine model of chronic cyclosporine nephrotoxicity. Front. Cell Dev. Biol. 2020, 8, 296–310. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, H.G.; Kim, B.S.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, J.E.; Kim, H.J.; Ha, S.K.; Park, H.C. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem. Cell Res. 2015, 6, 18. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Lupo, A.; Yabarek, T.; Graziani, M.S.; Bonfante, L.; Abaterusso, C.; Gambaro, G. Metabolic syndrome, cardiovascular disease, and risk for chronic kidney disease in an Italian cohort: Analysis of the INCIPE study. Metab. Syndr. Relat. Disord. 2011, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and Type 2 Diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem. Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transpl. 2018, 27, 1080–1095. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Leung, J.C.K.; Chan, L.Y.Y.; Tsang, A.W.L.; Lai, K.N. Activation of tubular epithelial cells in diabetic nephropathy and the role of the peroxisome proliferator–activated receptor-γ agonist. J. Am. Soc. Nephrol. 2006, 17, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.K.; Lan, H.Y. Chemokines in renal injury. Clin. J. Am. Soc. Nephrol. 2011, 22, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25 highforkhead box P3+ regulatory T cells. J. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef]
- Song, T.R.; Eirin, A.; Zhu, X.Y.; Zhao, Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Woollard, J.R.; Taner, T.; Lerman, A.; et al. Mesenchymal stem cell-derived extracellular vesicles induce regulatory T cells to ameliorate chronic kidney injury. Hypertension 2020, 75, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Farahani, R.A.; Zhu, X.-Y.; Tang, H.; Jordan, K.L.; Lerman, A.; Lerman, L.O.; Eirin, A. Metabolic syndrome alters the cargo of mitochondria-related microRNAs in swine mesenchymal stem cell-derived extracellular vesicles, impairing their capacity to repair the stenotic kidney. Stem. Cells Int. 2020, 2020, 8845635. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; Gazzar, W.B.E.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, J.H.; Zhu, Y.; Shi, H.; Yin, S.Q.; Sun, F.T.; Wang, Q.N.; Zhang, L.L.; Yan, Y.M.; Zhang, X.; et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1 delta/beta-TRCP-mediated YAP degradation. Cell Death Dis. 2020, 11, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Li, S.; Zuo, B.; Zhang, X.; Wang, F.; Sun, D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 2020, 10, 9425–9442. [Google Scholar] [CrossRef]
- Zhu, F.; Chong Lee Shin, O.L.S.; Pei, G.; Hu, Z.; Yang, J.; Zhu, H.; Wang, M.; Mou, J.; Sun, J.; Wang, Y.; et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget 2017, 8, 70707–70726. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem. Cell Res. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Lee, J.H.; Song, K.H.; Noh, H.; Lee, S.H. Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J. Pineal Res. 2020, 68, e12632. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, Y.; Cao, H.; Wen, P.; Jiang, L.; He, W.; Dai, C.; Yang, J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE 2013, 8, e60546. [Google Scholar] [CrossRef] [PubMed]
- Gödel, M.; Hartleben, B.; Herbach, N.; Liu, S.; Zschiedrich, S.; Lu, S.; Debreczeni-Mór, A.; Lindenmeyer, M.T.; Rastaldi, M.-P.; Hartleben, G.; et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Investig. 2011, 121, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Portt, L.; Khoury, C.; Sheibani, S.; Norman, G.; Ebner, P.; Eid, R.; Vali, H.; Mandato, C.A.; Madeo, F.; et al. 14-3-3 protects against stress-induced apoptosis. Cell Death Dis. 2012, 3, e348. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2–mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem. Cell 2010, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Huang, W.; Li, X.; Gao, L.; Su, T.; Li, X.; Ma, S.; Liu, T.; Li, C.; Chen, J.; et al. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J. Pineal Res. 2016, 60, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem. Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Wang, Z.; Gutkind, J.S.; Wang, Z.; Wang, F.; Lu, J.; Niu, G.; Teng, G.; Chen, X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem. Cells 2015, 33, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, J.; Geng, Y.; Qian, H.; Wang, F.; Liu, X.; Shang, M.; Nie, S.; Liu, N.; Du, X.; et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS ONE 2015, 10, e0129164. [Google Scholar] [CrossRef]
- Heldring, N.; Mäger, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21–32. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
| Method | Differential Ultracentrifugation | Density Gradient | Size Exclusion Chromatography | Invitrogen Precipitation | Affinity-Based |
|---|---|---|---|---|---|
| Principle | Based on size and sedimentation rate by successive centrifugation at increasing speed and duration [61,62] | Based on density upon flotation or pelleting [45,63] | Based on separating sample molecules relative to pore size of chromatography gel column [57,64] | Compound polymer-based precipitation [63] | Affinity interaction between surface protein, sugar, or lipids, with antibodies coated on magnetic beads [48,57,63,65] |
| Yield | Intermediate | Low | Intermediate | High | Low |
| Purity | Low | Intermediate | High | Low | Highest |
| Advantages |
|
| |||
| Disadvantages |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birtwistle, L.; Chen, X.-M.; Pollock, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021, 22, 6596. https://doi.org/10.3390/ijms22126596
Birtwistle L, Chen X-M, Pollock C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. International Journal of Molecular Sciences. 2021; 22(12):6596. https://doi.org/10.3390/ijms22126596
Chicago/Turabian StyleBirtwistle, Lucy, Xin-Ming Chen, and Carol Pollock. 2021. "Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury" International Journal of Molecular Sciences 22, no. 12: 6596. https://doi.org/10.3390/ijms22126596
APA StyleBirtwistle, L., Chen, X.-M., & Pollock, C. (2021). Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. International Journal of Molecular Sciences, 22(12), 6596. https://doi.org/10.3390/ijms22126596






