Abstract
Acute kidney injury (AKI) and chronic kidney disease (CKD) are rising in global prevalence and cause significant morbidity for patients. Current treatments are limited to slowing instead of stabilising or reversing disease progression. In this review, we describe mesenchymal stem cells (MSCs) and their constituents, extracellular vesicles (EVs) as being a novel therapeutic for CKD. MSC-derived EVs (MSC-EVs) are membrane-enclosed particles, including exosomes, which carry genetic information that mimics the phenotype of their cell of origin. MSC-EVs deliver their cargo of mRNA, miRNA, cytokines, and growth factors to target cells as a form of paracrine communication. This genetically reprograms pathophysiological pathways, which are upregulated in renal failure. Since the method of exosome preparation significantly affects the quality and function of MSC-exosomes, this review compares the methodologies for isolating exosomes from MSCs and their role in tissue regeneration. More specifically, it summarises the therapeutic efficacy of MSC-EVs in 60 preclinical animal models of AKI and CKD and the cargo of biomolecules they deliver. MSC-EVs promote tubular proliferation and angiogenesis, and inhibit apoptosis, oxidative stress, inflammation, the epithelial-to-mesenchymal transition, and fibrosis, to alleviate AKI and CKD. By reprogramming these pathophysiological pathways, MSC-EVs can slow or even reverse the progression of AKI to CKD, and therefore offer potential to transform clinical practice.
1. Pathophysiology of AKI and CKD
Acute kidney injury (AKI) and chronic kidney disease (CKD) are established as global health burdens, with a prevalence of one in ten adults for CKD [1]. AKI refers to a sudden deterioration in renal function, resulting in increased plasma creatinine and blood urea nitrogen (BUN) and/or declining urine output within hours to days [2,3,4]. The most common causes are pre-renal with hypovolaemia, ischaemia, toxic injury, and sepsis, leading to oxidative stress, inflammation, apoptosis, and necrosis of tubular epithelial cells (TECs) [5]. In response, the remaining TECs proliferate and differentiate so recovery occurs over a few weeks [5,6,7].
However, these nephroprotective mechanisms can be overwhelmed and there is a 25% risk of progression to CKD [8], and a 50% increase in 10-year mortality, particularly from coronary events [9,10]. The most common cause of CKD is diabetes, followed by glomerulonephritis, hypertension, and polycystic kidney disease, with smoking, a family history of kidney failure, obesity, ≥60 years old, and being of Aboriginal or Torres Strait Islander origin contributing to the overall risk [1,11]. In Type 2 diabetes mellitus (T2DM), hyperglycaemia induces oxidative stress and inflammation, which leads to maladaptive repair by fibroblasts and podocytes, and reduction of peritubular endothelial capillary (PTC) networks [5,12,13]. A notable mechanism is the epithelial-to-mesenchymal transition (EMT), whereby TECs and fibroblasts transform into myofibroblasts and deposit TGF-β1 and extracellular matrix (ECM) proteins (collagen IV, fibronectin), thereby perpetuating the cycle of glomerulosclerosis and tubulointerstitial fibrosis [5,12,14,15,16]. The fibrotic process strongly correlates with a loss of renal function and CKD is defined by elevated urinary albumin excretion ≥30 mg/g and/or a decrease in estimated glomerular filtration rate (eGFR) to <60 mL/min/1.73 m2 for greater than three months [17].
2. Current Treatments for Kidney Failure
Current treatments only slow the decline in renal function through lifestyle modifications, managing concomitant cardiovascular disease, or offering dialysis or kidney transplantation [11,18]. Dialysis is the most common reason for hospitalisation [19] and was estimated to cost the Australian government $12 billion from 2009–2020 [20,21]. Quality of life is poor on dialysis [22] and it is often considered a bridge to transplantation as people wait an average of three years on the cadaveric transplant list [23]. Transplantation from deceased or living donors increases five-year survival to 90% and 97%, respectively [24]. However, benefits are limited by donor shortages and the increased incidence of cancer and infections due to long-term immunosuppression [25,26]. Therefore, novel therapeutics slowing the progression of fibrosis and preventing or even reversing the deterioration in renal function are urgently required.
3. Regenerative Properties of Mesenchymal Stem Cells
Innovative mesenchymal stem cell (MSC) therapies appear to show promise in regenerative medicine [4]. MSCs are multipotent, non-haematopoietic stem cells, which can be collected from various sources (bone marrow (BM), liver, kidney, adipose tissue, urine, umbilical cord blood, umbilical tissue Wharton’s jelly, placenta) [27,28]. MSCs are identified by their expression of surface markers, CD73, CD90, and CD105, and lack of expression of haematopoietic markers (CD11b, CD19, CD34, CD45, CD79, HLA-DR) [29]. Owing to their multipotency, MSCs can replicate and differentiate into specialised cells to repopulate injured tissues [30,31]. However, emerging evidence has shown that they coordinate cellular communication and tissue repair by secreting bioproducts, called “extracellular vesicles” (EVs), which carry a diverse repertoire of trophic factors and genetic material [29,31,32]. The source of MSCs can affect their differentiation capacity and secretome [33]. For example, umbilical-MSCs share similar properties to BM-MSCs and retain primitive characteristics of embryonic stem cells [34]. Moreover, Wharton’s jelly MSCs are considered more immune-privileged and exert greater immunosuppressive properties compared to adipose tissue [35] or BM-MSCs [36]. Nonetheless, it is the ease of harvesting MSCs and their availability that determines their clinical applicability, and so both adult and fetal sources of MSCs will be discussed.
However, there are some disadvantages of using cell-based MSC therapy. These include the difficulty in generating a consistent source of cells with a stable phenotype and the issue of delivering large cells intravenously where there is a risk of entrapment in the pulmonary microvasculature, known as “first-past effect” [37]. Furthermore, there is a risk of MSCs inducing granulocytosis [38], graft rejection, ectopic tissue formation [39], and promotion of tumour growth [40,41]. By comparison, EVs can be prepared from the conditioned media of MSCs and offer greater safety, biological tolerance, easier tissue migration [42], lower propensity to induce an immune response [4], and no tumourigenicity [43].
4. Mesenchymal Stem Cell-Derived Extracellular Vesicles
The regenerative properties of MSCs are mediated by the paracrine action of EVs delivering biomolecules to neighbouring cells [40,43,44] (Figure 1). EVs are membrane-enclosed particles classified by their cellular origin into three categories: apoptotic bodies (1–5 mm), microvesicles (MVs) or microparticles (MPs) (100–1000 nm), and exosomes (30–100 nm) [4,45]. The biogenesis of these EV subtypes is distinct [43,46] (Figure 1). Exosomes are derivatives of the endosomal compartment, secreted into the environment when multivesicular bodies (MVB) fuse with the plasma membrane [44,47,48]. Given the difficulties in differentiating the subtypes of EVs by their size, the International Society for Extracellular Vesicles (ISEV) prefers the collective term of “EVs” to define particles released from cells that are bounded by a lipid bilayer and cannot replicate [49].
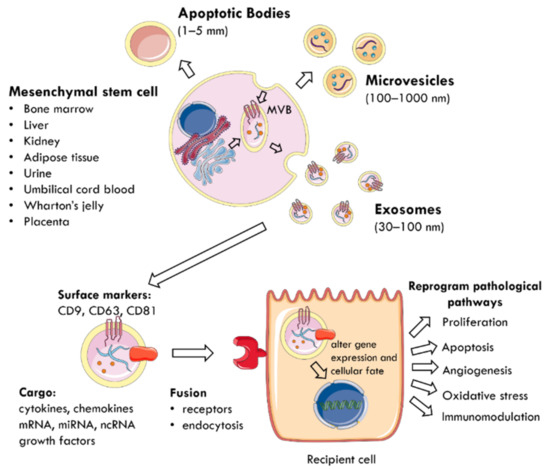
Figure 1.
The mechanism by which EVs are secreted from MSCs and mediate paracrine communication. MSCs can be harvested from multiple tissue sources (bone marrow, liver, kidney, adipose tissue, urine, umbilical cord blood, Wharton’s jelly, placenta). EVs are membrane-enclosed particles classified into three categories: apoptotic bodies, microvesicles, and exosomes. For example, exosomes are secreted when MVB fuse with the plasma membrane and are characterised by surface expression of CD9, CD63, and CD81. They deliver a cargo of mRNA, miRNA, ncRNA, cytokines, chemokines, and growth factors to nearby injured cells. EVs utilise specific receptors or membrane fusion to enter target cells and the delivered material alters gene expression and cellular fate. This reprograms pathophysiological pathways, such as proliferation, apoptosis, angiogenesis, oxidative stress, and immunomodulation. MSC: mesenchymal stem cell; MVB: multivesicular bodies; EVs: extracellular vesicles; CD: cluster of differentiation; ncRNA: non-coding RNA.
EVs carry a cargo reflecting the phenotype of their cells of origin [50], which includes cytokines, chemokines, growth factors, mRNA, miRNA, and other non-coding RNA [28,51,52]. The collection of surface proteins, originating from the endosomal pathway, distinguishes exosomes from MVs and includes tetraspanins (CD9, CD63, CD81), heat shock protein (HSP) 70, ALIX, and tumour suppressor gene 101 [27,40,49]. EVs use specific receptors or membrane fusion to enter target cells and deliver their contents as a form of paracrine communication [53]. Consequently, the delivered material modifies the phenotype of recipient cells by altering gene expression, stimulating transcription, inducing phenotypic switches, or determining cellular fate, self-renewal, and differentiation [29,52]. At the cellular level, this reprograms pathophysiological pathways, such as proliferation, apoptosis, oxidative stress, angiogenesis, and immunomodulation to promote tissue regeneration [4,45,50,54].
Therefore, this review will summarise the methodologies for isolating exosomes, the different clinical applications of MSC-EVs and, more specifically, their regenerative capacity in treating animal models of AKI and CKD.
5. Methodologies of Exosome Isolation
The therapeutic use of exosomes requires the development of methodologies that isolate exosomes of suitable quantity and quality from MSCs [43]. Exosomes can be collected from various biofluids, including plasma, serum, urine, and saliva [55] with conditioned cell culture media being the most widely used material [56]. Current isolation protocols are based on the physical properties of density and size, or chemically through the surface interactions with proteins [57] (Table 1). However, the methods are tedious and non-specific and there is no consensus on a gold standard of isolation [58], with differential ultracentrifugation chosen by 80% of researchers [56]. There is no set characterisation of the three populations of EVs by size, so most methods isolate a heterogeneous population [40,45,59,60]. Further standardisation is required.

Table 1.
Comparison of methods to isolate exosomes from cell culture. RNA: ribonucleic acid; MSpec: mass spectrometry; SEC: size exclusion chromatography; dUC: differential ultracentrifugation; EVs: extracellular vesicles; PEG: polyethylene glycol.
6. Therapeutic Applications of MSC-EVs
In searching the database of https://clinicaltrials.gov (accessed on 18 May 2021), there are currently thirteen clinical trials investigating the therapeutic efficacy of MSC-EVs in various diseases. MSC-EVs have been applied in diabetes mellitus [40,70,71], myocardial infarction [43], conditioning transplants [72,73,74], cancer [42,75], macular degeneration [76,77], repairing bone defects [78], osteoarthritis [79], Alzheimer’s disease (AD) [42,80,81,82], ischaemic stroke [40,83,84], multiple sclerosis [85], and treating COVID-19 [86,87,88,89].
6.1. Macular Degeneration
An ongoing Phase I trial (NCT03437759) is investigating the healing capacity of umbilical-MSC-exosomes to repair areas of large and refractory macular injury [77]. A single dose of 20 μg or 50 μg exosomes will be applied around the injured macular area in 44 patients and the visual outcome will be followed up six months later. Pre-clinical studies propose that MSC-exosomes suppress inflammation and inhibit apoptosis by downregulating MCP-1, a key chemoattractant for monocytes [76].
6.2. Cancer
Tumour cells are known to secrete pathogenic exosomes to facilitate paracrine communication in the tumour microenvironment and promote tumour growth, invasion, metastasis, and drug resistance [90]. Many clinical trials have focused on the role of exosomes as diagnostic (NCT04394572) [91] or prognostic (NCT04288141) [92] indicators in cancer. However, MSC-exosomes may also be therapeutic in targeting cancers with known driver mutations. For example, MSC-exosomes engineered to carry siRNA specific to the oncogenic KrasG12D mutation (iExosomes) have successfully supressed pancreatic ductal adenocarcinoma in mice [93]. In a current Phase I trial (NCT03608631), these iExosomes will be delivered intravenously on days 1, 4, and 10 and repeated every 14 days for three courses of treatment for patients with Stage IV pancreatic ductal adenocarcinoma, harbouring the KrasG12D mutation [94].
6.3. Alzheimer’s Disease
The ability of MSC-EVs to cross the blood brain barrier means they could treat neurodegenerative diseases, such as AD [42]. EVs can directly internalise β-amyloid for lysosomal clearance [81], or transfer an insulin-degrading enzyme [95] or small interfering RNA [96] to reduce β-amyloid production, which is considered pathogenic in AD. A phase I/II trial (NCT04388982) is currently investigating the safety and efficacy of 5 μg, 10 μg, or 20 μg of allogenic adipose-MSC-exosomes administered to patients with AD twice a week for three months [82].
6.4. Ischaemic Stroke
As a result of shared risk factors, patients with Stage 4 CKD are twenty times more likely to die prematurely from cardiovascular events than progressing to end-stage kidney failure [9,20]. Preclinical studies in mice showed that miR-124-enriched exosomes induced neurogenesis, protected against ischaemic injury, and prevented post-ischaemic immunosuppression [84]. In an ongoing Phase II/III trial (NCT03384433), BM-MSC-exosomes, loaded with miR-124, will be administered to the ischaemic area one month following the stroke and the patient’s neurological outcome will be assessed one year later [83].
6.5. ARDS and COVID-19
The anti-inflammatory effect of MSC-exosomes is being investigated in coronavirus (SARS-CoV-2) pneumonia and acute respiratory distress syndrome (ARDS), where only supportive care exists [87]. A few clinical trials are currently investigating the efficacy of aerosol inhalation of allogenic adipose-MSC-exosomes in the treatment of ARDS over seven days (NCT04602104) [88] and intravenous delivery of an escalating dose of MSC-exosomes over five days in COVID-19 pneumonia (NCT04798716) [89]. The mechanism by which EVs elicit their immunomodulatory effects and whether they would be suitable therapeutic candidates is yet to be determined [97].
7. Nephroprotective Role of MSC-EVs in AKI
Multiple animal models have demonstrated that MSC-EVs can ameliorate AKI induced by cisplatin, glycerol, gentamicin, or ischaemic-reperfusion injury (IRI) [43,98,99]. Given the heterogeneous nature of MSC-EVs, it currently remains unknown which subtypes provide renoprotection, so the efficacy of MVs, MPs, and exosomes in rodent AKI will all be discussed (Table 2).
7.1. Tubular Proliferation and Dedifferentiation
Biodistribution analyses illustrate that MSC-EVs specifically accumulate at the site of injury and their cargo of growth factors determines regenerative capacity [100]. In glycerol- [101] or cisplatin-triggered [102] AKI and IRI [53,103,104], MSC-MVs delivered mRNA of the mesenchymal phenotype or IGF-1 receptor [102] to proximal TECs and this induced expression of hepatocyte growth factor (HGF) and macrophage-stimulating protein (MSP). This promoted proliferation and dedifferentiation of proximal TECs. Similarly, BM-MSC-EVs, enriched with pro-regenerative miRNA (miR-10a, miR-486), induced TEC proliferation, reduced BUN, creatinine, and proteinuria, and improved renal function following glycerol-triggered AKI [105]. Additionally, activation of the ERK1/2 pathway and downregulation of p38 MAPK signalling was attributed to increased cell proliferation and the reversal of cisplatin-mediated damage in kidneys treated with umbilical-MSC-exosomes [106] and BM-exosomes [107,108].
7.2. Inhibition of Apoptosis
Numerous studies show EVs protect tubular cells from apoptosis [32,98,101,104,109,110,111,112,113,114,115,116,117,118,119,120,121] and necrosis [98,105,106,109,122,123] following AKI. In severe combined immunodeficient (SCID) mice with cisplatin-induced AKI, a single injection of MSC-MVs increased survival to 40% at three weeks, compared to 100% mortality within five days of receiving the vehicle [109]. Multiple injections of MVs improved survival to 80% and restored renal function, indicating the dose response relationship. The mechanism of renoprotection was attributed to the upregulation of anti-apoptotic genes (Bcl-2, Bcl-xL, BIRC8) and downregulation of executioner genes (Caspase-1,-3,-8, lymphotoxin-α), thereby inhibiting apoptosis of proximal TECs [106]. Furthermore, BM-MSC-exosomes delivered miR-199a-3p into mice with IRI and suppressed apoptosis by downregulating Bax, Caspase-3, and the semaphorin, Sema3A [112]. Blocking Sema3A also led to activation of the Akt and ERK pathways for cell proliferation and offered protection against AKI. Similarly, BM-MSC-exosomes secreted miR-199a-5p, which targeted binding immunoglobulin protein (BIP) to inhibit endoplasmic reticulum stress in IRI within 8–16 h [123]. Another study demonstrated Wharton’s jelly MSC-EVs delivered miR-30b/c/d to injured TECs and mitigated DRP1-induced mitochondrial fragmentation caused by IRI and abrogated apoptosis [120].
7.3. Angiogenesis
The horizontal transfer of proangiogenic factors, such as vasculogenic growth factor (VEGF-A), IGF-1, and basic fibroblast growth factor (bFGF) from MSC-EVs to resident cells mediates nephroprotection by EVs [108,111,113,124]. The downregulation of HIF-1α led to increased density and perfusion of renal capillaries, thereby reducing hypoxia [111].
7.4. Anti-Oxidation
During ischaemia, ATP levels rapidly fall, whilst intracellular calcium, protons, and reactive oxygen species (ROS) levels and lactic acid rise [125,126]. Increased mitochondrial membrane permeability and release of lysosomal enzymes cause breakdown of TECs. BM-MSC-EVs reduced ischaemic damage in isolated rat kidneys by upregulating enzymes involved in cellular metabolism (Calbindin1) and ion membrane transport (Slc16a1, vacuolar H+-ATPase d2 subunit) [74]. Calbindin1 sequesters excess calcium and reduces ROS and apoptosis [127]. Slc16a1 encodes for monocarboxylate transporter 1 and exports accumulated lactic acid [128]. Additionally, H+-ATPase pumps protons across the cell membrane and this reduces intracellular acidosis [129]. Therefore, EV-treated kidneys had lower glucose but higher pyruvate levels compared to ischaemic kidneys [74], indicating the important anti-oxidant activity of EVs [126].
The anti-oxidant activity of EVs may involve upregulation of nuclear factor E2-related factor (Nrf2) [130], which is a transcription factor binding anti-oxidant response elements and improves the expression of ROS scavenging enzymes, such as superoxide dismutase (SOD) and heme oxygenase-1 (HO-1) [131]. Wharton’s jelly MSC-EVs [130] and -MVs [119] alleviated oxidative stress in rats with unilateral kidney ischaemia and IRI, respectively, by downregulating NOX2 expression, which is a NADPH oxidase generating ROS. The authors hypothesised that miRNA delivered by EVs activated Nrf2 [130] or suppressed NOX2 expression [119]. Another IRI study supports this mechanism where human placenta-MSC-EVs delivered a cargo of miR-200a-3p to TECs [132]. The miRNA downregulated Keap1, freeing Nrf2 for nuclear translocation and promoted SOD2 expression. This reinforced antioxidant defence, increased ATP production, and protected TECs from mitochondrial fragmentation.
Melatonin is a strong scavenger of ROS and exosomes derived from MSCs conditioned with melatonin reduced oxidative stress within three days of IRI [133]. Exosomes carried RNA that downregulated expression of ROS such as malondialdehyde, HIF-1α, and NOX2, and upregulated anti-oxidant molecules (HO-1, SOD, catalase, glutathione peroxidase) [113].
7.5. Immunomodulation
The anti-inflammatory effects of EVs can be attributed to their paracrine delivery of immunomodulatory molecules or expression of surface proteins that minimises infiltration of immune cells, such as macrophages, T cells, and NK cells [134]. BM-MSC-exosomes downregulated pro-inflammatory cytokines (IL-6, IL-1β, IFN-γ, TNF-α) and stimulated anti-inflammatory cytokines (IL-10) in rodents with IRI [113], unilateral ureteral obstruction (UUO) [135], or gentamicin- [98] or cisplatin-induced AKI [107,108,136]. Additionally, umbilical-MSC-exosomes upregulated miR-146b, leading to reduced IRAK1 expression and NF-κB transcriptional activity in TECs in mice with sepsis-associated AKI [137]. By dampening the cytokine storm of sepsis, exosomes alleviated AKI and improved survival from 28% to 45% at day three. Recent studies have demonstrated the synergistic role of pulsed focused ultrasound and BM-MSC-EVs in reducing HSP70 expression and inhibiting activation of the NRLP3 inflammasome and its pro-inflammatory cytokines (IL-1β, IL-18) in cisplatin-induced AKI [108,136].
As mentioned earlier, Wharton’s jelly MSCs exert greater immunomodulatory activity than other sources of MSCs [35,36]. A single injection of Wharton’s jelly MSC-MVs delivered miR-15a/-15b/-16 to rats with IRI and this suppressed CX3CL1 expression and CD68+ macrophage infiltration [110,118]. Furthermore, surface expression of CCR2 by exosomes could sequester its extracellular ligand, CCL2, and interfere with macrophage recruitment in IRI [134]. Additional studies demonstrated Wharton’s jelly MSC-EVs decrease NK cell infiltration in ischaemic kidneys through downregulation of CX3CL1 and TLR2 expression [138]. This immunomodulation was preserved in rats with a splenectomy, indicating the spleen was not necessary for EVs to mediate renoprotection, unlike MSCs.
In summary, MSC-EVs ameliorate AKI by inducing tubuloepithelial regeneration and angiogenesis, and dampening apoptosis, oxidative stress, and inflammation [4,139].

Table 2.
Comparison of EVs subtypes from various sources of MSCs in treating rodent models of AKI. Molecules critical for MSC-EVs to mediate nephroprotection are emboldened. Up (↑) indicates increased, and down (↓) indicates decreased levels or activity.
Table 2.
Comparison of EVs subtypes from various sources of MSCs in treating rodent models of AKI. Molecules critical for MSC-EVs to mediate nephroprotection are emboldened. Up (↑) indicates increased, and down (↓) indicates decreased levels or activity.
| MSC Source | In Vivo Model | EV Subtype | Dose | Administration | Pathophysiological Effects | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Bone marrow | Glycerol | EVs | Single: 200 μg | Intravenous | EVs accumulate specifically in injured kidneys | [100] | |
| Bone marrow | Glycerol | MVs | Single: 15 μg | Caudal vein | MVs accumulated within lumen of injured tubules ↑ proliferation ↓ apoptosis ↑ tubuloepithelial regeneration | Delivery of HGF, MSP | [101] |
| Bone marrow | Glycerol | EVs | Single: 16.5 × 107 or 8.25 × 107 | Intravenous | Pro-regenerative miRNA-enriched EVs are superior to naïve EVs at lower doses ↓ BUN, creatinine ↓ necrosis | Pro-regenerative miRNA: miR-10a, miR-486, miR-127 | [105] |
| Bone marrow | Cisplatin | MVs | Multiple: 100 μg, then 50 μg days 2, 6, 10, 14, 18 | Intravenous | ↓ apoptosis, necrosis ↑ proliferation ↓ mortality Did not prevent chronic tubular injury at 3 weeks | ↓ Caspase-1,8, lymphotoxin-α ↑ Bcl-2, Bcl-xL, BIRC8 | [109] |
| Bone marrow | Cisplatin | EVs | Single: 150 μg | Intra-arterial kidney | ↓ BUN, creatinine ↓ tubular cast formation ↑ proliferation ↓ inflammation | ↓ IL-6, TNF-α, NF-κB | [107] |
| Bone marrow | Cisplatin | EVs | Single: 200 μg/100 g body weight on day 3 | Intraperitoneal | Combined pre-treatment with pulsed focused ultrasound on d2 ↓ BUN, creatinine ↓ tissue damage (KIM-1, NGAL) ↓ inflammation | ↓ HSP70, HSP90 activation of NLRP3 inflammasome ↓ IL-1β, IL-18 | [136] |
| Bone marrow | Cisplatin | EVs | Single: 150 μg/100 g body weight on day 3 | Caudal vein | Pulsed focused ultrasound pre-treatment ↓ tissue damage (KIM-1, TIMP-1) ↑ proliferation ↑ angiogenesis ↓ apoptosis ↓ inflammation | ↑ ERK signalling ↑ PI3K/Akt ↑ VEGF, PCNA, survivin ↑ SIRT3, eNOS ↓ Caspase-3, Bax ↓ TNF-α, IL-6, IL-1β | [108] |
| Bone marrow | Gentamicin | Exosomes | Multiple: 100 μg | Caudal vein | ↓ apoptosis, necrosis ↑ proliferation ↓ inflammation | Unknown RNA ↓ IL-6, IFN-γ, TNF-α; ↑ IL-10 | [98] |
| Bone marrow | IRI | Exosomes | Single: 200 μg | Renal capsule | ↓ macrophage infiltration ↓ inflammation | CCR2 expression on exosomes suppress CCL2 activity | [134] |
| Bone marrow | IRI | Exosomes preconditioned with 5 μM melatonin | Single: 250 μg | Perfusion | ↓ BUN, creatinine ↓ apoptosis ↓ oxidative stress ↓ inflammation ↑ regeneration ↑ angiogenesis | Melatonin: ↓ Caspase-3, Bax, PARP1; ↑ Bcl-2 ↓ ROS: MDA, HIF-1α, NOX2 ↑ anti-oxidants (HO-1, SOD, CAT, GPX) ↓ MPO activity, ICAM-1, IL-1β, NF-κB; ↑ IL-10 ↑ bFGF, HGF, Sox9, VEGF | [113] |
| Bone marrow | IRI | Exosomes enriched with miR-199a-3p | Single: 5 × 105 | Caudal vein | ↓ apoptosis | ↓ Sema3A and reactivate Akt and ERK pathways ↓ Caspase-3 | [112] |
| Bone marrow | IRI | Exosomes enriched with miR-199a-5p | Single: 5 × 105 | Caudal vein | ↓ endoplasmic reticulum stress at 8–16 h after reperfusion ↓ apoptosis | Targets BIP | [123] |
| Bone marrow | IRI, nephrectomy | EVs | Single: released from 3 × 106 MSCs | Perfusion | ↓ ischaemic damage | ↑ Expression of proteins in membrane transport and homeostasis (Calb1, Slc16a1, vaculor H+-ATPase d2 subunit) | [74] |
| Bone marrow | UUO | EVs | Single: 0.5 mg/kg | Intravenous | ↓ inflammation ↓ macrophage infiltration (ED-1+) ↓ mitochondrial damage ↓ oxidative stress ↓ apoptosis ↓ fibrosis | Delivered MFG-E8 to inhibit RhoA/ROCK pathway ↓ IL-1β, TNF-α, IL-6 ↓ MDA; ↑ anti-oxidants (SOD, CAT) ↓ Caspase-3, PARP1 ↓ α-SMA, ↓fibronectin, ↑E-cadherin | [135] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↓ apoptosis, necrosis ↓ oxidative stress ↑ proliferation | ↓ Caspase 3 ↓ p38 MAPK pathway | [106] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↑ autophagy: ↑LC3B ↓ BUN, creatinine after 3d ↓ apoptosis ↓ inflammation | ↓ mTOR activity ↓ Bax, ↓ Caspase-3; ↑ Bcl-2, Bcl-XL ↓ IL-1β, IL-6, TNF-α | [114] |
| Umbilical cord | Cisplatin | Exosomes | Single: 200 μg | Renal capsule | ↑ autophagy ↓ BUN, creatinine after 3d ↓ apoptosis | Delivered 14–3-3ζ to ↑ autophagy via promoting the localisation of ATG16L ↓ Caspase 3 | [115] |
| Umbilical cord | IRI | EVs overexpressing Oct4 | Single: 100 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↑ proliferation ↓ fibrosis | Oct4 inhibited fibrosis (↓ SNAIl, α-SMA) | [117] |
| Umbilical cord | Sepsis (caecal ligation and puncture) | Exosomes | Single: 120 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↓ inflammation ↑ survival (45% vs. 28% control) | Upregulation of miR-146b ↓ IRAK1 and ↓ NF-κB expression ↓ IL-1β, TNF-α | [137] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Caudal vein | ↓ BUN, creatinine ↓ apoptosis ↑ tubular cell proliferation ↓ inflammation ↓ CD68+ macrophage infiltration ↓ fibrosis | Delivery of miRN-15a/-15b/-16 reduced CX3CL ↓ α-SMA | [110] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Caudal vein | ↓ oxidative stress ↓ apoptosis ↑ proliferation ↓ fibrosis | ↓ NOX2 expression, ↓ ROS levels ↓ α-SMA | [119] |
| Wharton’s jelly | IRI | MVs | Single: 30 μg | Caudal vein | ↑ tubular cell dedifferentiation and growth | ↑ HGF RNA | [103] |
| Wharton’s jelly | IRI | MVs | Single: 100 μg | Intravenous | ↑ survival ↓ BUN, creatinine ↓ apoptosis ↑ proliferation ↓ inflammation ↓ CD68+ macrophage infiltration ↓ fibrosis | ↓ TNF-α; ↑ IL-10 ↓ α-SMA, TGF-β1 ↑ HGF | [118] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Intravenous | ↓ BUN, creatinine after 24 h ↓ NK cells in kidney without the involvement of the spleen | ↓ CX3CL1, TLR2 | [138] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↑ angiogenesis ↓ fibrosis | Delivery of VEGF and its RNA; ↓ HIF-1α, α-SMA | [124] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↓ mitochondrial fission ↓ apoptosis | Delivery of miR-30b/c/d | [120] |
| Wharton’s jelly | IRI | EVs | Single: 100 μg | Caudal vein | ↓ oxidative stress↓ renal cell injury (↓NGAL) ↓ apoptosis | ↑ Nrf2/ARE activation ↑ ROS scavenging enzymes (HO-1) | [130] |
| Renal | IRI | EVs | Single: 4 × 108 | Intravenous | EVs detected in ischaemic kidneys within 1 h ↓ BUN, creatinine ↑tubular cell proliferation | Identified 62 miRNAs | [53] |
| Renal | IRI | EVs | Single: 2 × 107 | Caudal vein | ↓ apoptosis ↑ peritubular capillary endothelial cell proliferation ↑ angiogenesis | Selective engraftment in ischaemic kidneys Delivery of VEGF-A, bFGF, IGF-1 | [111] |
| Adipose | IRI | Exosomes | Single: 100 μg | Intravenous | Combined ADMSC and exosome therapy is superior to monotherapy: ↓ proteinuria ↓ kidney injury score | [140] | |
| Adipose | Sepsis (caecal ligation and puncture) | Exosomes | Single: 100 μg | Caudal vein | ↓ inflammation ↓ inflammatory cell infiltration ↓ apoptosis ↓ mortality | ↑ SIRT1 inhibited NF-κB and its inflammatory activity ↓ TNF-α, IL-6, MCP-1 ↓ Bax, ↓ Caspase-3; ↑ Bcl-2 | [141] |
| Human induced pluripotent stem cells | IRI | EVs | Single: 1 × 1012 | Intravenous | ↓ necroptosis | Delivery of SP1 to renal cells | [122] |
| Human placenta-derived | IRI | EVs | Single: 80 μg | Intravenous | EVs specifically accumulated in ischaemic kidney and taken up by proximal TECs ↑ mitochondrial antioxidant defence ↓ mitochondrial fragmentation | Keap1-Nrf2 pathway- ↑ SOD2, ↑ATP production | [132] |
| Human placenta-derived | IRI | EVs | Multiple: 100 μg daily for 3 days | EVs travelled to injured kidneys ↑ proliferation and regeneration ↓ BUN, creatinine ↓ apoptosis ↓ fibrosis d28 | ↑ Sox9+ expression in tubular epithelial cells ↓ α-SMA, fibronectin, collagen I, TGF-β1 | [142] |
Abbreviations: MSC: mesenchymal stem cell; AKI: acute kidney injury; EVs: extracellular vesicles; MVs: microvesicles; miR: miRNA; HGF: hepatocyte growth factor; MSP: macrophage-stimulating protein; BUN: blood urea nitrogen; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra-large; BIRC8: baculoviral IAP repeat containing 8; IL-6: interleukin-6; TNF-α: tumour necrosis factor alpha; NF-κB: nuclear factor kappa-light-chain enhancer of activated B cells; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin; HSP: heat shock protein; NRLP3: NLR family pyrin domain containing 3; ERK: extracellular signal-regulated kinase; PI3K: phosphoinositide 3-kinase; Akt: protein kinase B; TIMP-1: tissue inhibitor matrix metalloproteinase 1; VEGF: vascular endothelial growth factor; PCNA: proliferating cell nuclear antigen; SIRT3: sirtuin 3; p-eNOS: phosphorylated endothelial nitric oxide synthase; IRI: ischaemia-reperfusion injury; IFN-γ: interferon-γ; CCR2: C-C motif chemokine receptor type 2; CCL2: C-C motif chemokine ligand 2; PARP1: poly [ADP-ribose] polymerase 1; ROS: reactive oxygen species; MDA: malondialdehyde; HIF-1α: hypoxia-inducible factor 1 alpha; NAPDH: nicotinamide-adenine dinucleotide phosphate; NOX2: NADPH oxidase 2; HO-1: haeme oxygenase 1; SOD: superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; MPO: myeloperoxidase; ICAM-1: intercellular adhesion molecule 1; bFGF: basic fibroblast growth factor; Sox9: SRY-box transcription factor 9; Sema3A: semaphorin-3A; BIP: binding immunoglobulin protein; Calb1: calbindin 1; Slc16a1: solute carrier family 16 member 1; UUO: unilateral ureteral obstruction; MFG-E8: milk fat globule-EGF factor 8 protein; RhoA: Ras homolog family member A; ROCK: Rho-associated protein kinase; α-SMA: alpha smooth muscle actin; MAPK: mitogen-activated protein kinase; LC3: microtubule-associated protein light chain 3; mTOR: mammalian target of rapamycin; ATG16L: autophagy related 16 like 1; CX3CL: C-X3-C motif chemokine ligand 1; TLR: toll-like receptor; Oct4: octamer-binding transcription factor 4; SNAI1: snail family transcriptional repressor 1; IRAK1: interleukin-1 receptor associated kinase 1; CD68: cluster of differentiation; TGF-β: transforming growth factor beta; ROS: reactive oxygen species; Nrf2: nuclear factor erythroid 2-related factor 2; ARE: antioxidant response element; IGF-1: insulin growth factor-1; ADMSC: adipose-derived mesenchymal stem cell; MCP-1: monocyte chemoattractant protein-1; SP1: proximal specificity protein 1; TEC: tubular epithelial cells; ATP: adenosine triphosphate; Keap1: Kelch-like ECH-associated protein 1.
8. Anti-Fibrotic Effect of MSC-EVs in CKD
Renal glomerulosclerosis and tubulointerstitial fibrosis are hallmarks of diabetic nephropathy and indeed all types of CKD [4]. MSC-EVs promote tissue regeneration by targeting kidney fibrosis, reducing tubular atrophy and inflammation, and facilitating angiogenesis to abrogate pathogenic insults in CKD (Table 3).
8.1. Downregulate Pro-Fibrotic Gene Expression and the EMT
TGF-β is a key inducer of the EMT in CKD [12]. In β-integrin signalling, TGF-β forms a complex with Smad and binds transcription factors, such as Snail, to downregulate expression of epithelial cell markers (E-cadherin), and activate expression of fibrosis-associated stromal cell markers (α-SMA, fibronectin, collagen I) [143]. Erythropoietin (EPO)-transfected microparticles in mice with UUO [144,145], Wharton jelly’s MSC-EVs in cyclosporin A injury [146], and adipose-MSC-EVs in renal artery stenosis [147] all inhibited the EMT and tubulointerstitial fibrosis by downregulating phosphorylated Smad2, Smad3, and p38 MAPK signalling, and increasing E-cadherin.
The anti-fibrotic mechanism of MSC-EVs is mediated through the transfer of miRNA targeting fibrosis-associated genes [4,54,116,117,148]. For example, MSC-exosomes delivered miRNA-let7c to mouse TECs with UUO and downregulated the expression of collagen IVα1, metalloproteinase-9 (MMP9), α-SMA, and TGF-β1 and its receptor [149]. Similarly, NOD SCID gamma mice with streptozotocin (STZ)-induced T1DM were treated with BM-MSC-EVs and their cargo of miRNA suppressed fibrotic (collagen I, MMP3, TIMP1) and apoptotic (FasL, Serpina1a) gene expression [54]. Umbilical-MSCs-MVs, enriched with miR-451a, reversed the EMT in STZ-induced diabetic nephropathy by increasing E-cadherin expression and reducing fibrosis [150]. The shuttled miR-451a targeted the 3′UTR sites of cell cycle inhibitors, P15INK4b and P19INK4d, enabling a resumption of the blocked cell cycle and amelioration of the EMT. In aristolochic acid-induced herbal nephropathy, BM-MSC-EVs reduced tubular necrosis and interstitial fibrosis by suppressing expression of fibrotic genes (α-SMA, TGF-β1, collagen Iα1) [151]. This was postulated by MSC-EVs downregulating various miRNAs (miR21-5p, 34a-5p, 34c-5p, 132-3p, 212-3p, 214-3p, 342-3p) that mediate fibrosis, inflammation, and apoptosis. Hence, EVs can revert the progression of tubulointerstitial fibrosis and the EMT and restore function in CKD [16,54,152].
8.2. Reduce Tubular Atrophy
EVs exhibit anti-apoptotic activity to prevent the transition of AKI into CKD [109,145,153]. Six months following treatment with umbilical-MSC-MVs, rats with IRI showed dwindling tubular atrophy, improved functioning, and decreased glomerular ECM accumulation and fibrosis [104]. Reduced-to-absent tubular atrophy and repaired renal morphology were also observed in mice with 5/6 subtotal nephrectomy treated with MSC-MVs [153]. Furthermore, BM-MSC-EVs reduced degeneration, vacuolisation, tubular cyst formation, and atrophic changes of proximal TECs in mice with T1DM, T2DM [16], or cyclosporin nephrotoxicity [154].
8.3. Vascular Regeneration
Urinary MSC-exosomes delivered VEGF, TGF-β1, angiogenin, and BMP7 for vascular and tubular regeneration in STZ-induced T1DM [121]. Renal MSC-microparticles offered similar protection by attenuating TGF-β1-induced endothelial-to-mesenchymal transition of PTC, thereby improving capillary density and reducing fibrosis in kidneys with UUO [155].
A high cholesterol/fructose diet induces Metabolic Syndrome that not only increases the risk of CKD progressing to end-stage kidney failure [156], but also hinders the proliferative and differentiation potential of MSCs [157]. In a swine model of unilateral renovascular disease and Metabolic Syndrome, autologous adipose-MSC-EVs, enriched with pro-angiogenic factors (VEGF-A,C, VEGF receptor, angiopoietin-like 4, HGF), were internalised by tubular and endothelial cells within four weeks and improved cortical microvascular density, renal blood flow, and GFR [158].
8.4. Anti-Inflammatory
Intercellular adhesion molecule 1 (ICAM-1) is a glycoprotein expressed by TECs and PTCs to support the recruitment of inflammatory cells into injured kidneys [159]. In T1DM and T2DM mice, BM-MSC-exosomes reduced expression of ICAM-1 in PTCs and reversed infiltration of dendritic cells, thereby preventing the development of diabetic nephropathy [16]. Additionally, BM-MSC-EVs downregulated CCL3 and hindered recruitment of macrophages and T cells [54,160]. Another study demonstrated that adipose-MSC-EVs delivered miR-26a-5p to inhibit TLR4 and the NF-κB/VEGFA inflammatory pathway, thereby alleviating diabetic nephropathy [161]. BM-MSC-EVs reduced TNF-α expression and inflammation, leading to an improvement in CKD outcomes [16].
TGF-β induces gene expression of forkhead box-P3 (FoxP3) to create a population of regulatory CD4+ T cells (Tregs) that police excessive inflammation and this can be utilised to ameliorate CKD [162]. For example, MSC-EVs, harvested from lean pigs, upregulated TGF-β expression and induced Treg differentiation in pigs with Metabolic Syndrome and unilateral renal artery stenosis, thereby decreasing inflammation and tubulointerstitial fibrosis [163]. Lean-EVs shifted the balance of macrophages from a pro-inflammatory M1 to anti-inflammatory M2 phenotype, and reduced the numbers of cytotoxic CD8+ T cells and IL-1β expression. By contrast, MSC-EVs, derived from pigs with Metabolic Syndrome, failed to alleviate CKD. This indicates the importance of the source and phenotype of MSC-EVs, where Metabolic Syndrome altered the cargo of 19 mitochondria-related miRNAs and therefore impaired the therapeutic efficacy of EVs [164].
Ultimately, MSC-EVs offer nephroprotection against CKD through reversing fibrosis, reducing the EMT, inhibiting apoptosis, promoting angiogenesis, and suppressing inflammation.

Table 3.
Comparison of EVs subtypes from various sources of MSCs in treating rodent models of CKD. Molecules critical for MSC-EVs to mediate nephroprotection are emboldened. Up arrow (↑) indicates increased and down arrow (↓) indicates decreased levels or activity.
Table 3.
Comparison of EVs subtypes from various sources of MSCs in treating rodent models of CKD. Molecules critical for MSC-EVs to mediate nephroprotection are emboldened. Up arrow (↑) indicates increased and down arrow (↓) indicates decreased levels or activity.
| MSC Source | In Vivo Model | EV Subtypes | Dose | Administration | Pathophysiological Effects | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Bone marrow | IRI | MVs | Single: 30 μg | Intravenous | ↓ BUN, creatinine, proteinuria ↓ fibrosis, ↓glomerular matrix accumulation ↓ interstitial lymphocyte infiltrate ↓ tubular atrophy | Dependent on RNA cargo | [104] |
| Bone marrow | Chronic CsA | EVs | Multiple: 100 μg Preventive: 24 h after CsA, weekly for 4 weeks Curative: 2 weeks after CsA, weekly for 4 weeks | Intraperitoneal | Greater improvement when administered after damage (curative regime), rather than prophylactically ↓ tubular casts | ↓ PAI-1, TIMP-1, IFN-γ | [154] |
| Bone marrow | Aristolochic acid | EVs | Single: 1 × 1010 on day 3 | Intravenous | ↓ BUN, creatinine ↓ necrosis ↓ CD45+ immune cells, fibroblast, pericyte infiltration ↓ interstitial fibrosis | Downregulation of hsa-miR-21-5p, 34a-5p, 34c-5p, 132-3p, 214-3p, 342-3p; and mmu-miR-212-3p Upregulation of hsa-miR-194-5p, 192-5p; and mmu-miR-378-3p ↓ α-SMA, TGF-β1, collagen Iα1 | [151] |
| Bone marrow | 5/6 subtotal nephrectomy | MVs | Multiple: 30 μg, days 2, 3, 5 | Caudal vein | ↓ BUN, creatinine, uric acid, proteinuria prevent fibrosis ↓ tubular atrophy ↓ interstitial lymphocyte infiltrate | [153] | |
| Bone marrow | UUO | MVs | Single: 30 μg | Caudal vein | ↓ BUN, creatinine ↓ fibrosis | ↓ TGF-β1, α-SMA ↑ E-cadherin | [152] |
| Bone marrow | UUO | Exosomes enriched with miR-let7c | Single: released from 1 × 106 MSCs | Intravenous | Exosomes home to injured kidneys ↓ fibrosis | Delivery of miRNA-let7c ↓ collagen, MMP-9, α-SMA, TGF-βR1 | [149] |
| Bone marrow | Type 2 diabetes, STZ Type 1 diabetes | Exosomes | Single: 5.3 × 107 | Renal subcapsular | ↓ degeneration, vacuolation and tubular atrophy ↓ EMT ↓ ICAM-1-mediated interstitial inflammatory infiltration | ↓ TGF-β ↓ TNF-α | [16] |
| Bone marrow | STZ Type 1 diabetes | Exosomes | Single: 100 μg | Intravenous | ↑ Autophagy: ↑ LC3-II, Beclin-1 ↓ BUN, creatinine, blood glucose, proteinuria at 10 and 12 weeks ↓ fibrosis | ↓ mTOR activity ↓ collagen, TGF-β | [165] |
| Bone marrow, Liver | STZ Type 1 diabetes | EVs | Multiple: 1 × 1010 | Intravenous | ↓ BUN, creatinine ↓ fibrosis, ↓ EMT ↓ inflammatory cell recruitment | ↓ collagen I, MMP3, TIMP1, FasL, Serpina1a, SNAI1 ↓ CCL3 | [54] |
| Umbilical cord | STZ-induced DN with hyperuricaemia | MVs enriched with miR-451a | Single: 1.5 mg/kg | Caudal vein | ↓ BUN, creatinine ↓ fibrosis, ↓ EMT ↑ proliferation and removed arrest on cell cycle | ↓ α-SMA, ↑ E-cadherin miR-451a targeted 3′UTR sites of cell cycle inhibitors (P15INK4b, P19INK4d) | [150] |
| Umbilical cord | UUO | Exosomes | Single: 200 μg | Intravenous | ↓ tubulointerstitial fibrosis | Exosomes delivered casein kinase 1δ and E3 ubiquitin ligase β-TRCP to degrade YAP | [166] |
| Umbilical cord | UUO | Exosomes | Single: 200 μg | Intra-arterial kidney | ↓ BUN, creatinine ↓ apoptosis ↓ oxidative stress ↓ tubulointerstitial fibrosis | ↓ ROS-mediated p38 MAPK/ERK signalling pathway ↓ ROS: MDA ↑ anti-oxidants: GSH | [145] |
| Wharton’s jelly | CsA | EVs | Multiple: 100 μg at day 7, 21 | Intravenous | ↓ creatinine ↓ fibrosis, ↓ EMT ↓ oxidative stress | ↓ α-SMA ↓ ROS: MDA ↑ anti-oxidants: SOD | [146] |
| Renal | UUO | MPs | Single: 2 × 107 | Caudal vein | ↓ EndoMT of PTC endothelial cells ↓ PTC rarefaction ↓ F4/80+ inflammatory cell infiltration ↓ tubulointerstitial fibrosis | ↓ α-SMA | [155] |
| Renal | UUO | EPO-enriched MPs | Single: 80 μg | Caudal vein | ↓ tubulointerstitial fibrosis, ↓ EMT ↓ myofibroblast and F4/80+ macrophage infiltration | ↓ phosphorylated Smad2, Smad3, MAPK 38 expression to inhibit EMT ↓ α-SMA, fibronectin, collagen | [144] |
| Adipose (transfected with GDNF) | UUO | Exosomes | Single: 200 μg | Caudal vein | ↓ PTC rarefaction ↓ tubulointerstitial fibrosis ↑ endothelial function and angiogenesis | GDNF: ↑ SIRT1/p-eNOS pathway ↓ α-SMA ↑ VEGF, ↓ HIF-1α | [167] |
| Adipose | IRI | Exosomes | Single: 100 μg | Caudal vein | ↑ tubular proliferation, regeneration ↓ TGF-β1-induced transformation of TECs to pro-fibrotic phenotype ↓ AKI to CKD transition | ↑ Sox9 ↓ α-SMA, PDGFR-β | [168] |
| Adipose | Type 1 diabetes | Exosomes | Single: not stated, 12-week therapy | Caudal vein | ↓ BUN, creatinine, proteinuria ↑ autophagy, ↓ apoptosis podocytes | miR-486 reduced Smad1 expression, leading to ↓ mTOR activation | [169] |
| Adipose | Hindlimb Ischaemia | Melatonin-stimulated exosomes | CKD-MSCs treated with 30 μg exosomes, and 1 × 106 cells injected | Injection into ischaemic site | CKD-MSCs were treated with melatonin-stimulated exosomes and injected into mice ↑ neovascularisation ↑ functional recovery | Upregulation of miR-4516 ↑ PrPc in exosomes | [170] |
| Adipose | DN (C57BL/KsJ db/db) | EVs | Single | Caudal vein | ↓ histopathology of DN, ↓ BUN, creatinine ↓ VEGFA leads to ↓ podocyte apoptosis | miR-26a-5p inhibited TLR4 and inactivated NF-κB/VEGFA pathway (↓ IKKβ, IκBα, p65) ↓ Caspase-3, Bax, ↑ Bcl-2 | [161] |
| Adipose | Unilateral renovascular disease on background of Metabolic Syndrome | EVs | Single: 1 × 107 | Intra-renal vein | ↑ cortical microvascular, PTC density ↑ RBF, GFR ↓ glomerular, tubulointerstitial fibrosis ↓ apoptosis ↓ oxidative stress | Delivered proangiogenic factors: VEGF-A,C, VEGF receptor, angiopoietin like 4, HGF ↓ Caspase-3 ↓ ROS: superoxides, CD31, nitro tyrosine | [158] |
| Adipose | Unilateral renal artery stenosis on background of Metabolic Syndrome | EVs | Single: 1 × 1010 | Intrarenal artery | EVs derived from lean pigs were injected into pigs with Metabolic Syndrome | ↑ TGF-β induction of Tregs ↓ IL-1β | [163] |
| ↑ anti-inflammatory M2 macrophages | |||||||
| ↓ pro-inflammatory M1 macrophages | |||||||
| ↓ CD8+ T cells | |||||||
| Adipose | Unilateral renal artery stenosis on background of Metabolic Syndrome | EVs | Single: 1 × 1010 | Intrarenal artery | Metabolic Syndrome alters the cargo of 19 mitochondria-related miRNA, impairing regenerative capacity | ↑ miR-196a, 132 ↓ miR-192, 320 | [164] |
| Adipose | Unilateral renal artery stenosis | MVs, exosomes | Single: 100 μg | Caudal vein | ↓ HIF-1α Stabilised systolic blood pressure ↓ proteinuria (MVs only) ↑ natriuresis (exosomes only) ↓ fibrosis ↓ inflammation | ↓ collagen I, TGF-β ↑ IL-10 | [147] |
| Urine | STZ Type 1 diabetes | Exosomes | Multiple: 100 μg weekly x × 12 | Intravenous | ↓ apoptosis of podocyte and tubular cells ↑ glomerular endothelial cell proliferation ↑ angiogenesis | ↓ Caspase-3 Delivery of VEGF, TGF-β1, angiogenin, BMP7 | [121] |
Abbreviations: MSC: mesenchymal stem cell; CKD: chronic kidney disease; EVs: extracellular vesicles; IRI: ischaemia-reperfusion injury; MVs: microvesicles; BUN: blood urea nitrogen; CsA: cyclosporin A; PAI-1: plasminogen activator inhibitor-1; TIMP-1: tissue inhibitor matrix metalloproteinase 1; IFN-γ: interferon-γ; α-SMA: alpha smooth muscle actin; TGF-β: transforming growth factor beta; miR: miRNA; CD45: cluster of differentiation 45; UUO: unilateral ureteral obstruction; MMP: matrix metalloproteinase; STZ: streptozotocin; ICAM-1: intercellular adhesion molecule 1; TNF-α: tumour necrosis factor alpha; EMT: epithelial-to-mesenchymal transition; LC3-II: microtubule-associated protein light chain 3; mTOR: mammalian target of rapamycin; Sepina1a: serpin family A member 1; SNAI1: snail family transcriptional repressor 1; CCL3: C-C motif chemokine ligand 3; DN: diabetic nephropathy; β-TRCP: β-transducin repeats-containing protein; YAP: yes-associated protein; MAPK: mitogen-activated protein kinase; ROS: reactive oxygen species; MDA: malondialdehyde; GSH: glutathione; SOD: superoxide dismutase; MPs: microparticles; EndoMT: endothelial-to-mesenchymal transition; PTC: peritubular capillaries; EPO: erythropoietin; GDNF: glial cell line-derived neurotrophic factor; VEGF: vascular endothelial growth factor; Bcl-2: B-cell lymphoma 2; Bax: Bcl-2-associated X protein; HIF-1α: hypoxia-inducible factor 1 alpha; Sox9: SRY-box transcription factor 9; TEC: tubular epithelial cells; PDGFR: platelet-derived growth factor receptor; AKI: acute kidney injury; PrPc: cellular prion protein; TLR: toll-like receptor; NF-κB: nuclear factor kappa-light-chain enhancer of activated B cells; HGF: hepatocyte growth factor; RBF: renal blood flow; GFR: glomerular filtration rate; Tregs: regulatory T cells; IL-1β: interleukin-1β; BMP7: bone morphogenetic protein 7.
9. Biological Cargo Carried by MSC-EVs to Alleviate AKI and CKD
MSC-EVs can be engineered to carry different biomolecules and target the interplay of signalling pathways responsible for AKI and CKD (Figure 2).
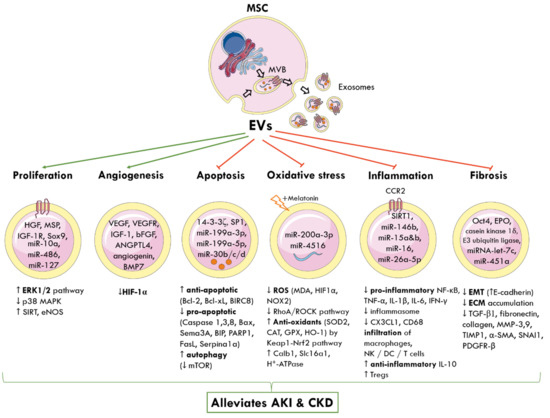
Figure 2.
Molecular and signalling mechanisms by which MSC-derived EVs elicit nephroprotection following AKI and CKD. The cellular origin of one subtype of EVs, known as exosomes, from MVBs is shown. MSC-EVs deliver a cargo of miRNA, proteins, cytokines, and growth factors to nearby injured renal cells and alter gene expression. This reprograms pathophysiological pathways and promotes tubular proliferation and angiogenesis, and inhibits apoptosis, oxidative stress, inflammation, and fibrosis to alleviate AKI and CKD. Green arrows represent promotion; red arrows represent inhibition; up arrow (↑) indicates increased and down arrow (↓) indicates decreased levels or activity. MSC: mesenchymal stem cell; MVB: multivesicular bodies; EVs: extracellular vesicles; HGF: hepatocyte growth factor; MSP: macrophage-stimulating protein; IGF-1R: insulin growth factor-1 receptor; Sox9: SRY-box transcription factor; miR: miRNA; ERK: extracellular signal-regulated kinase; MAPK: mitogen-activated protein kinase; SIRT: sirtuin 1; eNOS: endothelial nitric oxide synthase; VEGF: vascular endothelial growth factor; VEGFR: VEGF-receptor; bFGF: basic fibroblast growth factor; ANGPTL4: angiopoietin-like 4; BMP7: basic morphogenetic protein 7; HIF-1α: hypoxia-inducible factor 1 alpha; SP1: proximal specificity protein 1; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra-large; BIRC8: baculoviral IAP repeat containing 8; Bax: Bcl-2-associated X protein; Sema3A: semaphorin-3A; BIP: binding immunoglobulin protein; PARP1: poly [ADP-ribose] polymerase 1; Sepina1a: serpin family A member 1; mTOR: mammalian target of rapamycin; ROS: reactive oxygen species; MDA: malondialdehyde; NOX2: NADPH oxidase 2; RhoA: Ras homolog family member A, ROCK: Rho-associated protein kinase; SOD: superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; HO-1: haeme oxygenase 1; Keap1: Kelch-like ECH-associated protein 1; Nrf2: nuclear factor erythroid 2-related factor 2; Calb1: calbindin 1; Slc16a1: solute carrier family 16 member 1; ATP: adenosine triphosphate; CCR2: chemokine receptor type 2; NF-κB: nuclear factor kappa-light-chain enhancer of activated B cells; TNF-α: tumour necrosis factor alpha; IL-1β: interleukin-1β; CX3CL1: C-X3-C motif chemokine ligand 1; CD68: cluster of differentiation 68; NK: natural killer cells; DC: dendritic cells; Tregs: regulatory T cells; Oct4: octamer-binding transcription factor 4; EPO: erythropoietin; EMT: epithelial-to-mesenchymal transition; ECM: extracellular matrix; TGF-β1: transforming growth factor β1; MMP: matrix metalloproteinase; TIMP-1: tissue inhibitor matrix metalloproteinase 1; α-SMA: alpha smooth muscle actin; SNAI1: snail family transcriptional repressor 1; PDGFR-β: platelet-derived growth factor receptor β.
9.1. mTOR
The mechanistic target of rapamycin (mTOR) is a nutrient-sensing protein kinase that suppresses autophagy and exacerbates podocyte damage in CKD, and its deactivation represents a target for MSC-exosomes [171,172]. Umbilical-MSC-exosomes increased expression of the autophagic marker protein, LC3B, and this inhibited phosphorylation of mTOR, thereby activating autophagy in cisplatin-triggered AKI [114] and STZ-induced T1DM [165]. Similarly, miR-486 from adipose-MSC-exosomes targeted Smad1 and this suppressed mTOR activity [169].
9.2. 14-3-3ζ
14-3-3 is a family of adaptor proteins involved in regulating protein trafficking, cell cycling, signal transduction, and apoptosis, and it operates synergistically with mTOR to coordinate autophagy [173,174]. One isoform, 14-3-3ζ, offers protection from cell death due to hypoxia, chemotherapy, and growth factor deprivation [175]. Umbilical-MSC-exosomes, enriched with 14-3-3ζ, enhanced localisation of autophagy-related protein, 16L, to the outer surface of autophagosome precursors and this increased formation of autophagosomes [115]. By 14-3-3ζ inducing autophagy, renal cells were protected from apoptosis, cell proliferation increased, and this alleviated nephrotoxicity.
9.3. YAP
YAP is a transcription factor in the Hippo signalling pathway and co-localises with α-SMA in the nucleus of TECs to promote fibrosis through an unclear mechanism [176]. Umbilical-MSC-exosomes delivered casein kinase 1δ and E3 ubiquitin ligase β-transducin repeats-containing protein to trigger ubiquitination and degradation of YAP in TECs [166]. This reduced collagen and ECM deposition and attenuated fibrosis associated with UUO.
9.4. Oct-4
Oct-4 is known as one of the four transcription factors capable of reprogramming fibroblasts into induced pluripotent stem cells (iPSCs) [177] and it can downregulate Snail and the EMT [178]. Umbilical-MSC-EVs overexpressing Oct4 reduced apoptosis, promoted TEC proliferation, and rescued mice with IRI from fibrosis within two weeks [117].
9.5. SP1
MSC-EVs from human iPSCs can deliver sphinganine-1-phosphate 1 (SP1) to PTCs to directly bind the promoter region of sphingosine kinase 1 [122]. This increased SP1 expression and inhibited necroptosis in rats with IRI, elucidating a novel mechanism of EVs in nephroprotection.
9.6. Sox-9
Sox-9 is a transcription factor of the sex-determining region Y box family and may repair injured TECs [179]. Adipose-MSCs-exosomes upregulated Sox9 and prevented TGF-β1-induced transformation of TECs into a pro-fibrotic phenotype in mice with IRI [168]. Increased Sox9 stimulated TEC proliferation, attenuated AKI, and protected the development of tubulointerstitial fibrosis. Another study used two-photon microscopy to track human placenta-MSC-EVs migrating to kidneys injured by IRI. MSC-EVs promoted Sox9 activation in TECs to stimulate regeneration and reduce fibrosis within four weeks [142].
9.7. SIRT1
Sirtuin 1 (SIRT1) is an NAD+-dependent deacetylase of the sirtuin family that is expressed by various kidney cells during stress and inhibits inflammation, apoptosis, and fibrosis [180]. In sepsis-induced AKI, adipose-MSC-exosomes inhibited NF-κB-mediated transcription of pro-inflammatory cytokines in the SIRT1 pathway and reduced immune cell infiltration and apoptosis [141]. Furthermore, glial cell line-derived neurotrophic factor (GDNF) was transfected into adipose-MSCs, and their exosomes ameliorated fibrosis in mice with UUO [167]. This was mediated by SIRT1 signalling and its downstream target, phosphorylated endothelial nitric oxide synthase (p-eNOS), which activated endothelial function and angiogenesis and reduced PTC loss. Upregulation of SIRT3/eNOS by BM-MSC-EVs also improved angiogenesis and regeneration in cisplatin-triggered AKI [108].
9.8. MFG-E8
Milk fat globule-epidermal growth factor-factor 8 (MFG-E8) is a glycoprotein that inhibits the RhoA/ROCK signalling pathway. BM-MSC-EVs delivered MFG-E8 to rats with UUO and reduced inflammation, macrophage infiltration, mitochondrial damage, apoptosis, oxidative stress, and the EMT within two weeks [135].
9.9. Melatonin and PrPc
A recent study focused on the efficacy of melatonin in autologous MSC-based therapeutics for CKD [170]. Exposure of adipose-MSCs to melatonin upregulated expression of miR-4516 and cellular prion protein (PrPC), and “MT exosomes” were harvested. Adipose-MSCs were also collected from patients with CKD (CKD-MSCs) and incubated with MT exosomes, which promoted proliferation, mitochondrial activity, and angiogenic proteins, and protected cells from senescence. These MT exosome-treated CKD-MSCs improved neovascularisation and functional recovery when administered to mice with hindlimb ischaemia, which was mediated through miR-4516-PrPc signalling.
10. Conclusions
MSCs have shown increasing potential in immunomodulation and regenerative medicine and their paracrine effects are mediated by the secretion of EVs [42,181,182,183,184,185]. MSC-EVs are advantageous over their counterpart whole cells due to a higher safety profile, lower immunogenicity, and the inability to directly form tumours [42,181,182,183,184,185]. The regenerative capacity of MSC-EVs is based on the cargo of biomolecules they deliver to injured renal cells, particularly the types of miRNA and ncRNA [60]. To minimise the level of reporting and publication bias in this review, multiple databases were searched, and two extensive tables were created to methodologically analyse 34 preclinical animal models of AKI and 26 of CKD. However, the heterogeneous nature of EVs means the extrapolated results are difficult to generalise. It can be concluded that MSC-EVs induce tubular proliferation, regeneration, and angiogenesis, and suppress apoptosis, oxidative stress, inflammation, the EMT, and tubulointerstitial fibrosis. By altering the pathogenesis of disease, MSC-EVs show promise in mitigating AKI and CKD and offering a novel therapeutic for patients.
There are some limitations of this review. There is no consensus regarding the reporting of studies using EVs as it is an emerging therapeutic and there is a lack of global standardisation in isolation, characterisation, and validation protocols [4,40,49]. Moreover, there are no established methods to differentiate the subtypes of EVs and therefore studies claiming to use a certain subtype cannot be verified and this makes comparison difficult. Additionally, there are functional differences in efficacy between EV subtypes. A recent study found adipose-MSC-MVs reduced proteinuria while only exosomes promoted natriuresis following chronic renal artery stenosis [147]. To minimise these effects, studies were compared based on the ISEV recommendations, according to the source of MSCs, EV subtype, protein content of administered EVs, and route of injection [49].
Furthermore, clinical translation is in its infancy and the conclusions are limited to preclinical animal models. They are monocausal and simplistic when compared to the multifactorial aetiologies of CKD and comorbidities, such as increasing age and cardiovascular disease, from which patients suffer [5]. Moreover, the selected animals are young and only a short duration of disease (weeks—months) is observed. Therefore, there is an increasing need for human clinical trials. In a phase II/III trial, twenty patients with Stage III and IV CKD (eGFR 15–60 mg/mL) received two doses of umbilical-MSC-EVs (100 μg/kg/dose) one week apart, and this increased eGFR and reduced serum creatinine, BUN, and urinary albumin creatinine ratio within one year [186]. The clinical improvement was attributed to increased anti-inflammatory cytokines and decreased TNF-α.
For translation of EV therapy to clinical practice, the following manufacturing issues surrounding optimal dosing, mode of injection, schedule of administration, potency assays, minimising dose toxicity, uniformity between batches, identification of EVs, and safety must be standardised [4,40,187]. This is inherently difficult when considering the heterogeneity of EVs, so each batch will display both donor and clone-specific differences [97,187]. Most studies used a single dose of EVs, but this may be insufficient to achieve a sustained effect in humans [113]. Multiple doses of EVs showed greater efficacy than single dosing but repeated injections decrease feasibility [98,109]. Most studies focused on intravenous injection but there is a shifting focus to delivering therapeutics to organs via their arterial blood supply. This maximises the efficacy at the target site while reducing its metabolism and systemic side effects [107]. The timing of EV injection is also significant whereby a recent study confirmed administration of BM-MSC-EVs after renal damage is more effective than delivering them prophylactically [154]. Most studies use in-house manufacturing and characterisation protocols to isolate EVs [97] and only a handful have published their adherence to good manufacturing practice criteria [187,188,189]. Further pharmaceutical regulation of the manufacture and delivery of EV-based therapeutics is required before they can be safely translated from the laboratory bench to the bedside [187].
In conclusion, MSC-EV therapy shows increasing potential for alleviating AKI and slowing the progression of CKD. Future studies should engineer the surface and cargo of EVs for superior specificity and develop optimal protocols for delivery and safe transition into clinical practice.
Author Contributions
Funding
This research was supported by research fund donations to Renal Research Laboratory, Kolling Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, Y.; Bowe, B.; Mokdad, A.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 1–138. [Google Scholar] [CrossRef]
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, pathophysiology and clinical phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem cell-derived extracellular vesicles and kidney regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Westenfelder, C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014, 6, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, K.; Luo, J.; Dong, Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 2010, 176, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Lalli, M.; Kramann, R.; Kobayashi, A.; Humphreys, B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA 2014, 111, 1527–1532. [Google Scholar] [CrossRef]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E., II; Perkins, R.M. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Wu, V.-C.; Wu, C.-H.; Huang, T.-M.; Wang, C.-Y.; Lai, C.-F.; Shiao, C.-C.; Chang, C.-H.; Lin, S.-L.; Chen, Y.-Y.; Chen, Y.-M.; et al. Long-term risk of coronary events after AKI. Clin. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef]
- Tuttle, K. A turning point for chronic kidney disease in diabetes. Lancet 2019, 393, 1913–1914. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. The role of TGF-beta and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar]
- Kida, Y.; Tchao, B.N.; Yamaguchi, I. Peritubular capillary rarefaction: A new therapeutic target in chronic kidney disease. Pediatr. Nephrol. 2014, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D. Renal tubulointerstitial fibrosis: Common but never simple. Am. J. Physiol. Ren. Physiol. 2009, 296, F1239–F1244. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of tubulointerstitial fibrosis and the chronic kidney disease phenotype—Role of risk factors and epigenetics. Front. Pharm. 2017, 8, 520–528. [Google Scholar] [CrossRef]
- Nagaishi, K.; Mizue, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Konari, N.; Fujimiya, M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016, 6, 34842. [Google Scholar] [CrossRef]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2015, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of global kidney health care status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Admitted Patient Care 2014–15: Australian Hospital Statistics; Australian Institute of Health and Welfare: Canberra, ACT, Australia, 2018.
- Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care, 4th ed.; Kidney Health Australia: Melbourne, VIC, Australia, 2020. [Google Scholar]
- Chadban, S.; Cass, A.; Gallagher, M.; Howard, K.; Jones, A.; McDonald, S.; Snelling, P.; White, S. The Economic Impact of End-Stage Kidney Disease in Australia: Projections to 2020; Kidney Health Australia: Melbourne, VIC, Australia, 2010. [Google Scholar]
- Wyld, M.; Morton, R.L.; Hayen, A.; Howard, K.; Webster, A.C. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012, 9, e1001307. [Google Scholar] [CrossRef]
- Wright, J.; Narayan, S. Analysis of Kidney Allocation during 2015; National Organ Matching Service: Adelaide, SA, Australia, 2016.
- ANZDATA Registry. The 40th Annual ANZDATA Report; Australia and New Zealand Dialysis and Transplant Registry: Adelaide, SA, Australia, 2017. [Google Scholar]
- Wong, G.; Turner, R.M.; Chapman, J.R.; Howell, M.; Lim, W.H.; Webster, A.C.; Craig, J.C. Time on dialysis and cancer risk after kidney transplantation. Transplantation 2013, 95, 114–121. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Chronic Kidney Disease; Australian Institute of Health Welfare: Canberra, ACT, Australia, 2019.
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on mesenchymal stem cell-derived exosomes: Opportunities and challenges in cell-free therapy. Stem. Cells Int. 2017, 2017, 6305295–6305305. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular vesicles: Evolving factors in stem cell biology. Stem. Cells Int. 2016, 2016, 1073140. [Google Scholar] [CrossRef]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef]
- Bochon, B.; Kozubska, M.; Surygala, G.; Witkowska, A.; Kuzniewicz, R.; Grzeszczak, W.; Wystrychowski, G. Mesenchymal stem cells—Potential applications in kidney diseases. Int. J. Mol. Sci. 2019, 20, 2462. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef]
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.C.; Borlongan, C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015, 17, 18–24. [Google Scholar] [CrossRef]
- Kamal, M.M.; Kassem, D.H. Therapeutic potential of Wharton’s jelly mesenchymal stem cells for diabetes: Achievements and challenges. Front. Cell Dev. Biol. 2020, 8, 16. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem. Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.; Wu, Y. Excess integrins cause lung entrapment of mesenchymal stem cells. Stem. Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef]
- Tögel, F.; Isaac, J.; Westenfelder, C. Hematopoietic stem cell mobilization–associated granulocytosis severely worsens acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1261–1267. [Google Scholar] [CrossRef]
- Fennema, E.M.; Tchang, L.A.H.; Yuan, H.; van Blitterswijk, C.A.; Martin, I.; Scherberich, A.; de Boer, J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J. Tissue Eng. Regen. 2018, 12, e150–e158. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Katsuda, T.; Gailhouste, L.; Nakagama, H.; Ochiya, T. Mesenchymal stem cell-derived extracellular vesicles: A glimmer of hope in treating Alzheimer’s disease. Int. Immunol. 2017, 29, 11–19. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Deng, Y.; Yang, C. An update on isolation methods for proteomic studies of extracellular vesicles in biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017, 7, 17095–17104. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087–30118. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wang, K.J.Z.; Webster, K.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; Ni, C.; et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem. Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark Res. 2019, 7, 8–16. [Google Scholar] [CrossRef]
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: Deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front. Genet. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Bruno, S.; Bussolati, B.; Moggio, A.; Dimuccio, V.; Tapparo, M.; Biancone, L.; Gontero, P.; Frea, B.; Camussi, G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem. Cell Res. 2017, 8, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468–4481. [Google Scholar] [CrossRef]
- Nilsson, J.; Skog, J.; Nordstrand, A.; Baranov, V.; Mincheva-Nilsson, L.; Breakefield, X.O.; Widmark, A. Prostate cancer-derived urine exosomes: A novel approach to biomarkers for prostate cancer. Br. J. Cancer 2009, 100, 1603–1607. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell Vesicles 2016, 5, 32945–32950. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles 2014, 3, 26913–26918. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.d.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335–5345. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorum, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319–17335. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-L.; Zhu, J.; Liu, J.-X.; Jiang, F.; Ni, W.-K.; Qu, L.-S.; Ni, R.-Z.; Lu, C.-H.; Xiao, M.-B. A comparison of traditional and novel methods for the separation of exosomes from human samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Morón-Font, M.; Gámez-Valero, A.; Carreras-Planella, L.; Borràs, F.E.; Franquesa, M. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr. Protoc. Cell Biol. 2019, 49, e82. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of extracellular vesicles from human synovial fluid using size exclusion chromatography. J. Extracell Vesicles 2018, 7, 1490145–1490158. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.M.; Kuhnell, D.; Orr-Asman, M.A.; Biesiada, J.; Zhang, X.; Medvedovic, M.; Thomas, H.E. Balancing yield, purity and practicality: A modified differential ultracentrifugation protocol for efficient isolation of small extracellular vesicles from human serum. RNA Biol. 2019, 16, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V.; Nagaeva, O.; Dehlin, E. Isolation and characterization of exosomes from cultures of tissue explants and cell lines. Curr. Protoc. Immunol. 2016, 115, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W. Effect of Microvesicles and Exosomes Therapy on β-cell Mass in Type I Diabetes Mellitus (T1DM). Available online: https://clinicaltrials.gov/ct2/show/NCT02138331 (accessed on 18 May 2021).
- Ezquer, F.E.M.; Contador, D.; Ricca, M.; Simon, V.; Conget, P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem. Cells 2012, 30, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Sherman, L.; Munoz, J.; Rameshwar, P. Immunological properties of mesenchymal stem cells and clinical implications. Arch. Immunol. Exp. 2008, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bank, J.R.; Rabelink, T.J.; de Fijter, J.W.; Reinders, M.E.J. Safety and efficacy endpoints for mesenchymal stromal cell therapy in renal transplant recipients. J. Immunol. Res. 2015, 2015, 391797–391811. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with mesenchymal stromal Cells/extracellular vesicles prevents ischaemic injury. J. Cell Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.-u.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, 63–73. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562–34574. [Google Scholar] [CrossRef]
- Zhang, X.; Tianjin Medical University. MSC-Exos Promote Healing of MHs (MSCs). Available online: https://clinicaltrials.gov/ct2/show/NCT03437759 (accessed on 18 May 2021).
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Jt. Res. 2018, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Istituto Ortopedico Galeazzi. Effects of ASC Secretome on Human Osteochondral Explants (ASC-OA). Available online: https://clinicaltrials.gov/ct2/show/NCT04223622 (accessed on 18 May 2021).
- Takahashi, R.H.; Milner, T.A.; Li, F.; Nam, E.E.; Edgar, M.A.; Yamaguchi, H.; Beal, M.F.; Xu, H.; Greengard, P.; Gouras, G.K. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 2002, 161, 1869–1879. [Google Scholar] [CrossRef]
- Yuyama, K.; Sun, H.; Mitsutake, S.; Igarashi, Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 2012, 287, 10977–10989. [Google Scholar] [CrossRef]
- Wang, G.; Gao, X.; Ruijin Hospital. Safety and Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients with Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04388982 (accessed on 18 May 2021).
- Dehghani, L.; Soleimani, M.; Isfahan University of Medical Sciences. Allogenic Mesenchymal Stem Cell Derived Exosome in Patients with Acute Ischemic Stroke. Available online: https://clinicaltrials.gov/ct2/show/NCT03384433 (accessed on 18 May 2021).
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Nucleic Acids 2017, 7, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front. Behav. Neurosci. 2019, 13, 163–172. [Google Scholar] [CrossRef]
- State-Financed Health Facility “Samara Regional Medical Center Dinasty”. Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia (COVID-19EXO). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04491240 (accessed on 18 May 2021).
- Qu, J.-M.; Ruijin Hospital. A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/NCT04276987 (accessed on 18 May 2021).
- Qu, J.-M.; Ruijin Hospital. A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS. Available online: https://clinicaltrials.gov/ct2/show/NCT04602104 (accessed on 18 May 2021).
- Luttrell, T.C.; Chawla, S.P.; Mission Community Hospital. The Use of Exosomes for the Treatment of Acute Respiratory Distress Syndrome or Novel Coronavirus Pneumonia Cuased by COVID-19 (ARDOXSO). Available online: https://clinicaltrials.gov/ct2/show/NCT04798716 (accessed on 18 May 2021).
- Li, I.; Nabet, B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol. Cancer 2019, 18, 32. [Google Scholar] [CrossRef]
- Bouché, O.; de Reims, C.H.U. Identification of New Diagnostic Protein Markers for Colorectal Cancer in Circulating Tumor Exosomes. Available online: https://clinicaltrials.gov/ct2/show/NCT04394572 (accessed on 18 May 2021).
- McDonald, L.; King’s College London. A Study to Measure the Expression of HER2-HER3 Dimer in Tumour and Blood (Exosomes) Samples from Patients with HER2 Positive Breast Cancer Receiving HER2 Targeted Therapies (HERdi PREDICT). Available online: https://clinicaltrials.gov/ct2/show/NCT04288141 (accessed on 18 May 2021).
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.M.D.; Anderson Cancer Center. iExosomes in Treating Participants with Metastatic Pancreas Cancer with KrasG12D Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT03608631 (accessed on 18 May 2021).
- Leissring, M.A.; Farris, W.; Chang, A.Y.; Walsh, D.M.; Wu, X.; Sun, X.; Frosch, M.P.; Selkoe, D.J. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003, 40, 1087–1093. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Börger, V.; Weiss, D.J.; Anderson, J.D.; Borràs, F.E.; Bussolati, B.; Carter, D.R.F.; Dominici, M.; Falcón-Pérez, J.M.; Gimona, M.; Hill, A.F.; et al. International society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: Considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy 2020, 22, 482–485. [Google Scholar] [PubMed]
- Reis, L.A.; Borges, F.T.; Simões, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Bruno, S.; Chatterjee, D.; Quesenberry, P.J.; Tetta, C.; Camussi, G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 2014, 33, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Deregibus, M.C.; Calogero, R.A.; Saviozzi, S.; Collino, F.; Morando, L.; Busca, A.; Falda, M.; Bussolati, B.; et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Clin. J. Am. Soc. Nephrol. 2009, 20, 1053–1067. [Google Scholar] [CrossRef]
- Tomasoni, S.L.L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem. Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.-Q.; Cheng, J.; Zhong, L.; Wu, S.; Zou, X.-Y.; Zhang, G.-Y.; Gu, D.; Miao, S.; Zhu, Y.-J.; Sun, J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS ONE 2015, 10, e0121534. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion–induced acute and chronic kidney injury. Nephrol. Dial. Transpl. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Tapparo, M.; Bruno, S.; Collino, F.; Togliatto, G.; Deregibus, M.C.; Provero, P.; Wen, S.; Quesenberry, P.J.; Camussi, G. Renal regenerative potential of extracellular vesicles derived from miRNA-engineered mesenchymal stromal cells. Int. J. Mol. Sci. 2019, 20, 2381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem. Cell Res. 2013, 4, 34–47. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Choi, J.; Wang, J.; Concepcion, W.; Thakor, A.S. A novel approach to deliver therapeutic extracellular vesicles directly into the mouse kidney via its arterial blood supply. Cells 2020, 9, 937. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Razavi, M.; Concepcion, W.; Thakor, A.S. Pulsed focused ultrasound enhances the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles in acute kidney injury. Stem. Cell Res. 2020, 11, 398. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem. Cell Res. 2014, 5, 40–53. [Google Scholar] [CrossRef]
- Choi, H.Y.; Moon, S.J.; Ratliff, B.B.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, S.; Lim, B.J.; Kim, B.S.; Plotkin, M.D.; et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS ONE 2014, 9, e87853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Lin, F.; Yin, H.; Li, X.; He, W.; Liu, N.; Gou, X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell Physiol. 2019, 234, 23736–23749. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A. Melatonin improves therapeutic potential of mesenchymal stem cells-derived exosomes against renal ischemia-reperfusion injury in rats. Am. J. Transl. Res. 2019, 11, 2887–2907. [Google Scholar] [PubMed]
- Wang, B.Y.; Jia, H.Y.; Zhang, B.; Wang, J.J.; Ji, C.; Zhu, X.M.; Yan, Y.M.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem. Cell Res. 2017, 8, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Liu, W.Z.; Zhang, B.; Wang, J.J.; Wu, P.P.; Tandra, N.; Liang, Z.F.; Ji, C.; Yin, L.; Hu, X.Y.; et al. HucMSC exosomes-delivered 14-3-3 zeta enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am. J. Transl. Res. 2018, 10, 101–113. [Google Scholar]
- Zhang, J.; Zhang, H.; Liu, J.; Tu, X.; Zang, Y.; Zhu, J.; Chen, J.; Dong, L.; Zhang, J. miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem. Biophys. Res. Commun. 2012, 417, 1100–1105. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Hou, Y.P.; Zou, X.Y.; Xing, X.Y.; Ju, G.Q.; Zhong, L.; Sun, J. Oct-4 enhanced the therapeutic effects of mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Kidney Blood Press. Res. 2020, 45, 95–108. [Google Scholar] [CrossRef]
- Wu, X.; Yan, T.; Wang, Z.; Wu, X.; Cao, G.; Zhang, C.; Tian, X.; Wang, J. Micro-vesicles derived from human Wharton’s Jelly mesenchymal stromal cells mitigate renal ischemia-reperfusion injury in rats after cardiac death renal transplantation. J. Cell Biochem. 2018, 119, 1879–1888. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem. Cells Int. 2016, 2016, 2093940–2093952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N.-S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem. Cell Res. 2016, 7, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200–3218. [Google Scholar] [PubMed]
- Wang, C.Y.; Zhu, G.M.; He, W.Y.; Yin, H.B.; Lin, F.; Gou, X.; Li, X.Y. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019, 33, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Gu, D.; Xing, X.; Cheng, Z.; Gong, D.; Zhang, G.; Zhu, Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am. J. Transl. Res. 2016, 8, 4289–4299. [Google Scholar]
- Devarajan, P. Update on mechanisms of ischemic acute kidney injury. Clin. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Johnson, A.C.M.; Becker, K. Renal cortical pyruvate depletion during AKI. J. Am. Soc. Nephrol. 2014, 25, 998–1012. [Google Scholar] [CrossRef]
- Baniene, R.; Trumbeckas, D.; Kincius, M.; Pauziene, N.; Raudone, L.; Jievaltas, M.; Trumbeckaite, S. Short ischemia induces rat kidney mitochondria dysfunction. J. Bioenerg. Biomembr. 2016, 48, 77–85. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez, S.J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Borthwick, K.J.; Karet, F.E. Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H(+)—ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene 2002, 297, 169–177. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res. 2016, 41, 119–128. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.C.; Peng, H.; Ye, Z.; Zhang, J.; Liu, X.; Lou, T. Activation of the Nrf2-ARE pathway attenuates hyperglycemia-mediated injuries in mouse podocytes. Cell Physiol. Biochem. 2014, 34, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.M.; Cheng, Y.Q.; Gao, H.Q.; Zhuang, J.; Zhang, W.G.; Bian, Q.; Wang, F.; Du, Y.; Li, Z.J.; Kong, D.L.; et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano 2020, 14, 4014–4026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Liu, J.; Zhang, F.; Wang, Y.; Qin, Y.; Zhou, Z.; Qiu, J.; Fan, Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem. Cells Int. 2016, 2016, 1240301–1240311. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Zhang, Y.; Jiang, D. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem. Cell Res. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Rai, S.; Concepcion, W.; Thakor, A.S. HSP70-mediated NLRP3 inflammasome suppression underlies reversal of acute kidney injury following extracellular vesicle and focused ultrasound combination therapy. Int. J. Mol. Sci. 2020, 21, 4085. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhu, Y.; Li, Y.; Liu, W.Z.; Yin, L.; Yin, S.Q.; Ji, C.; Hu, Y.Y.; Wang, Q.N.; Zhou, X.R.; et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol. Lett. 2020, 42, 669–679. [Google Scholar] [CrossRef]
- Zou, X.; Gu, D.; Zhang, G.; Zhong, L.; Cheng, Z.; Liu, G.; Zhu, T. NK Cell regulatory property is involved in the protective role of MSC-derived extracellular vesicles in renal ischemic reperfusion injury. Hum. Gene 2016, 27, 926–935. [Google Scholar] [CrossRef]
- Bruno, S.; Chiabotto, G.; Favaro, E.; Deregibus, M.C.; Camussi, G. Role of extracellular vesicles in stem cell biology. Am. J. Physiol. Cell Physiol. 2019, 317, C303–C313. [Google Scholar] [CrossRef]
- Lin, K.C.; Yip, H.K.; Shao, P.L.; Wu, S.C.; Chen, K.H.; Chen, Y.T.; Yang, C.C.; Sun, C.K.; Kao, G.S.; Chen, S.Y.; et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int. J. Cardiol. 2016, 216, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020, 255, 117719. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, S.; Huimin, S.; Wang, L.; Li, H.; Zhao, J.; Zhang, C.; Li, N.; Guo, Z.; Han, Z.; et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J Biol. Chem. 2020, 295, 12203–12213. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, K.; Umezono, T.; Abe, M.; Kobayashi, T.; Kato, M.; Miyauchi, M.; Yamamoto, N.; Kimura, M.; Toyoda, M.; Suzuki, D. Expression of transcription factor Snai1 and tubulointerstitial fibrosis in progressive nephropathy. J. Nephrol. 2011, 25, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.-h.; Jhee, J.H.; Kim, T.Y.; Choi, H.Y.; Kim, H.J.; Park, H.C. Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis. Stem. Cell Res. 2020, 11, 422. [Google Scholar] [CrossRef]
- Liu, B.; Hu, D.; Zhou, Y.; Yu, Y.; Shen, L.; Long, C.; Butnaru, D.; Timashev, P.; He, D.; Lin, T.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am. J. Transl. Res. 2020, 12, 4998–5014. [Google Scholar] [PubMed]
- Zhang, G.Y.; Yu, S.Y.; Sun, S.; Zhang, L.; Zhang, G.L.; Xu, K.; Zheng, Y.X.; Xue, Q.; Chen, M. Extracellular-vesicles derived from human Wharton-Jelly mesenchymal stromal cells ameliorated cyclosporin A-induced renal fibrosis in rats. Int. J. Clin. Exp. Med. 2019, 12, 8943–8949. [Google Scholar]
- Ishiy, C.S.R.A.; Ormanji, M.S.; Maquigussa, E.; Ribeiro, R.S.; da Silva Novaes, A.; Boim, M.A. Comparison of the effects of mesenchymal stem cells with their extracellular vesicles on the treatment of kidney damage induced by chronic renal artery stenosis. Stem. Cells Int. 2020, 2020, 8814574. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.-H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, G.; Wang, X.; Li, L.; Zhang, J.; Chen, Y.; Liu, J.; Liu, S.; Wei, L.; Zhang, W.; et al. Mesenchymal stem cells-microvesicle-miR-451a ameliorate early diabetic kidney injury by negative regulation of P15 and P19. Exp. Biol. Med. 2019, 243, 1233–1242. [Google Scholar] [CrossRef]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Bruno, S.; Antico, F.; Brizzi, M.F.; Quesenberry, P.J.; et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front. Cell Dev. Biol. 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- Ramirez-Bajo, M.J.; Martin-Ramirez, J.; Bruno, S.; Pasquino, C.; Banon-Maneus, E.; Rovira, J.; Moya-Rull, D.; Lazo-Rodriguez, M.; Campistol, J.M.; Camussi, G.; et al. Nephroprotective potential of mesenchymal stromal cells and their extracellular vesicles in a murine model of chronic cyclosporine nephrotoxicity. Front. Cell Dev. Biol. 2020, 8, 296–310. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, H.G.; Kim, B.S.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, J.E.; Kim, H.J.; Ha, S.K.; Park, H.C. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem. Cell Res. 2015, 6, 18. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Lupo, A.; Yabarek, T.; Graziani, M.S.; Bonfante, L.; Abaterusso, C.; Gambaro, G. Metabolic syndrome, cardiovascular disease, and risk for chronic kidney disease in an Italian cohort: Analysis of the INCIPE study. Metab. Syndr. Relat. Disord. 2011, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and Type 2 Diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem. Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transpl. 2018, 27, 1080–1095. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Leung, J.C.K.; Chan, L.Y.Y.; Tsang, A.W.L.; Lai, K.N. Activation of tubular epithelial cells in diabetic nephropathy and the role of the peroxisome proliferator–activated receptor-γ agonist. J. Am. Soc. Nephrol. 2006, 17, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.C.K.; Lan, H.Y. Chemokines in renal injury. Clin. J. Am. Soc. Nephrol. 2011, 22, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25 highforkhead box P3+ regulatory T cells. J. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef]
- Song, T.R.; Eirin, A.; Zhu, X.Y.; Zhao, Y.; Krier, J.D.; Tang, H.; Jordan, K.L.; Woollard, J.R.; Taner, T.; Lerman, A.; et al. Mesenchymal stem cell-derived extracellular vesicles induce regulatory T cells to ameliorate chronic kidney injury. Hypertension 2020, 75, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Farahani, R.A.; Zhu, X.-Y.; Tang, H.; Jordan, K.L.; Lerman, A.; Lerman, L.O.; Eirin, A. Metabolic syndrome alters the cargo of mitochondria-related microRNAs in swine mesenchymal stem cell-derived extracellular vesicles, impairing their capacity to repair the stenotic kidney. Stem. Cells Int. 2020, 2020, 8845635. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; Gazzar, W.B.E.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhang, J.H.; Zhu, Y.; Shi, H.; Yin, S.Q.; Sun, F.T.; Wang, Q.N.; Zhang, L.L.; Yan, Y.M.; Zhang, X.; et al. Exosomes derived from hucMSC attenuate renal fibrosis through CK1 delta/beta-TRCP-mediated YAP degradation. Cell Death Dis. 2020, 11, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Li, S.; Zuo, B.; Zhang, X.; Wang, F.; Sun, D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 2020, 10, 9425–9442. [Google Scholar] [CrossRef]
- Zhu, F.; Chong Lee Shin, O.L.S.; Pei, G.; Hu, Z.; Yang, J.; Zhu, H.; Wang, M.; Mou, J.; Sun, J.; Wang, Y.; et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget 2017, 8, 70707–70726. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem. Cell Res. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Lee, J.H.; Song, K.H.; Noh, H.; Lee, S.H. Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J. Pineal Res. 2020, 68, e12632. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, Y.; Cao, H.; Wen, P.; Jiang, L.; He, W.; Dai, C.; Yang, J. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS ONE 2013, 8, e60546. [Google Scholar] [CrossRef] [PubMed]
- Gödel, M.; Hartleben, B.; Herbach, N.; Liu, S.; Zschiedrich, S.; Lu, S.; Debreczeni-Mór, A.; Lindenmeyer, M.T.; Rastaldi, M.-P.; Hartleben, G.; et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Investig. 2011, 121, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Portt, L.; Khoury, C.; Sheibani, S.; Norman, G.; Ebner, P.; Eid, R.; Vali, H.; Mandato, C.A.; Madeo, F.; et al. 14-3-3 protects against stress-induced apoptosis. Cell Death Dis. 2012, 3, e348. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2–mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem. Cell 2010, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Liu, J.; Pang, P.; Krautzberger, A.M.; Reginensi, A.; Akiyama, H.; Schedl, A.; Humphreys, B.D.; McMahon, A.P. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015, 12, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Huang, W.; Li, X.; Gao, L.; Su, T.; Li, X.; Ma, S.; Liu, T.; Li, C.; Chen, J.; et al. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J. Pineal Res. 2016, 60, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem. Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Wang, Z.; Gutkind, J.S.; Wang, Z.; Wang, F.; Lu, J.; Niu, G.; Teng, G.; Chen, X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem. Cells 2015, 33, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Huang, J.; Geng, Y.; Qian, H.; Wang, F.; Liu, X.; Shang, M.; Nie, S.; Liu, N.; Du, X.; et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS ONE 2015, 10, e0129164. [Google Scholar] [CrossRef]
- Heldring, N.; Mäger, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum. Gene 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21–32. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).