Potential Biomarkers in Diagnosis of Renal Acanthamoebiasis
Abstract
1. Introduction
2. Results
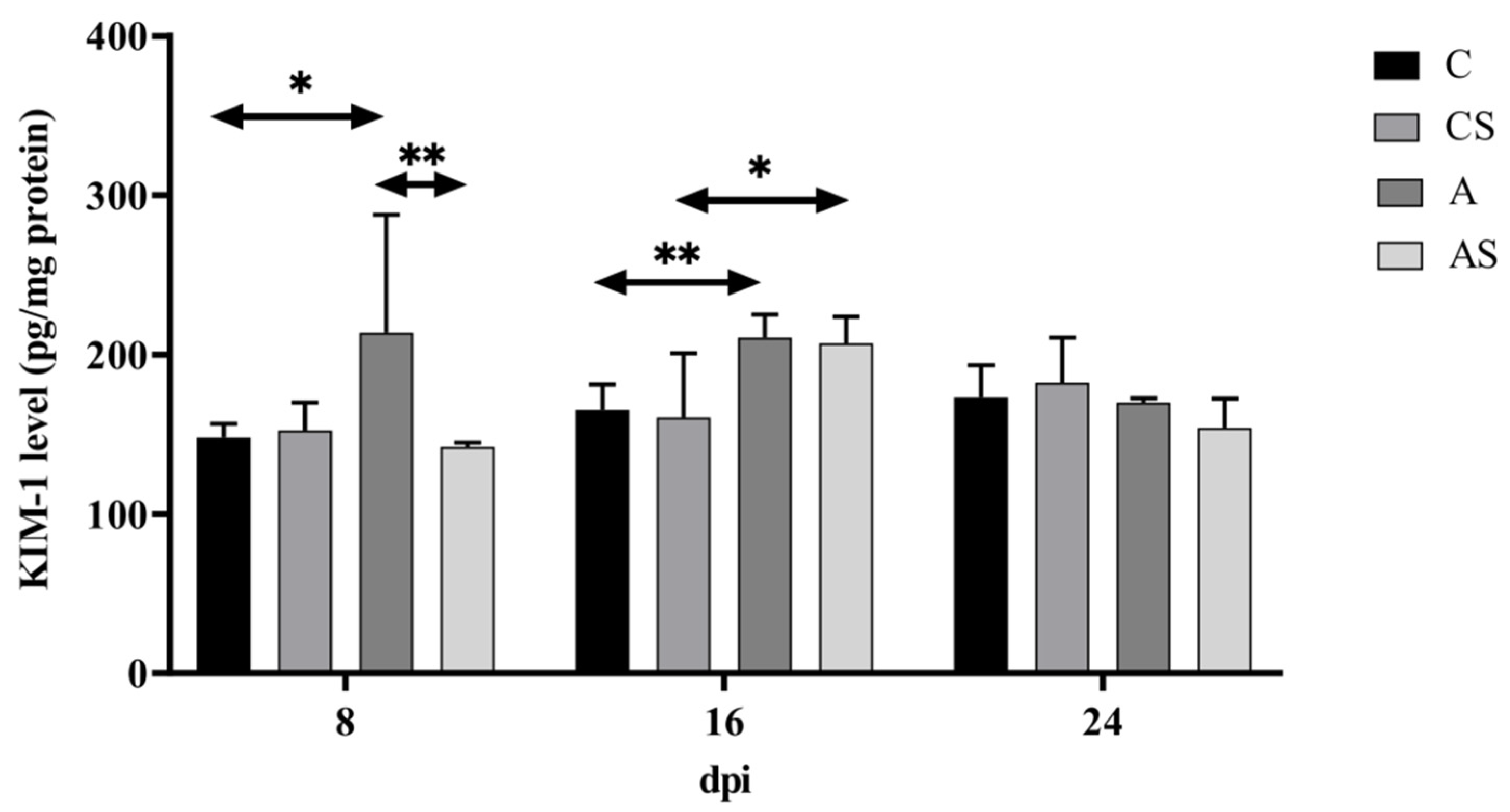
2.1. KIM-1
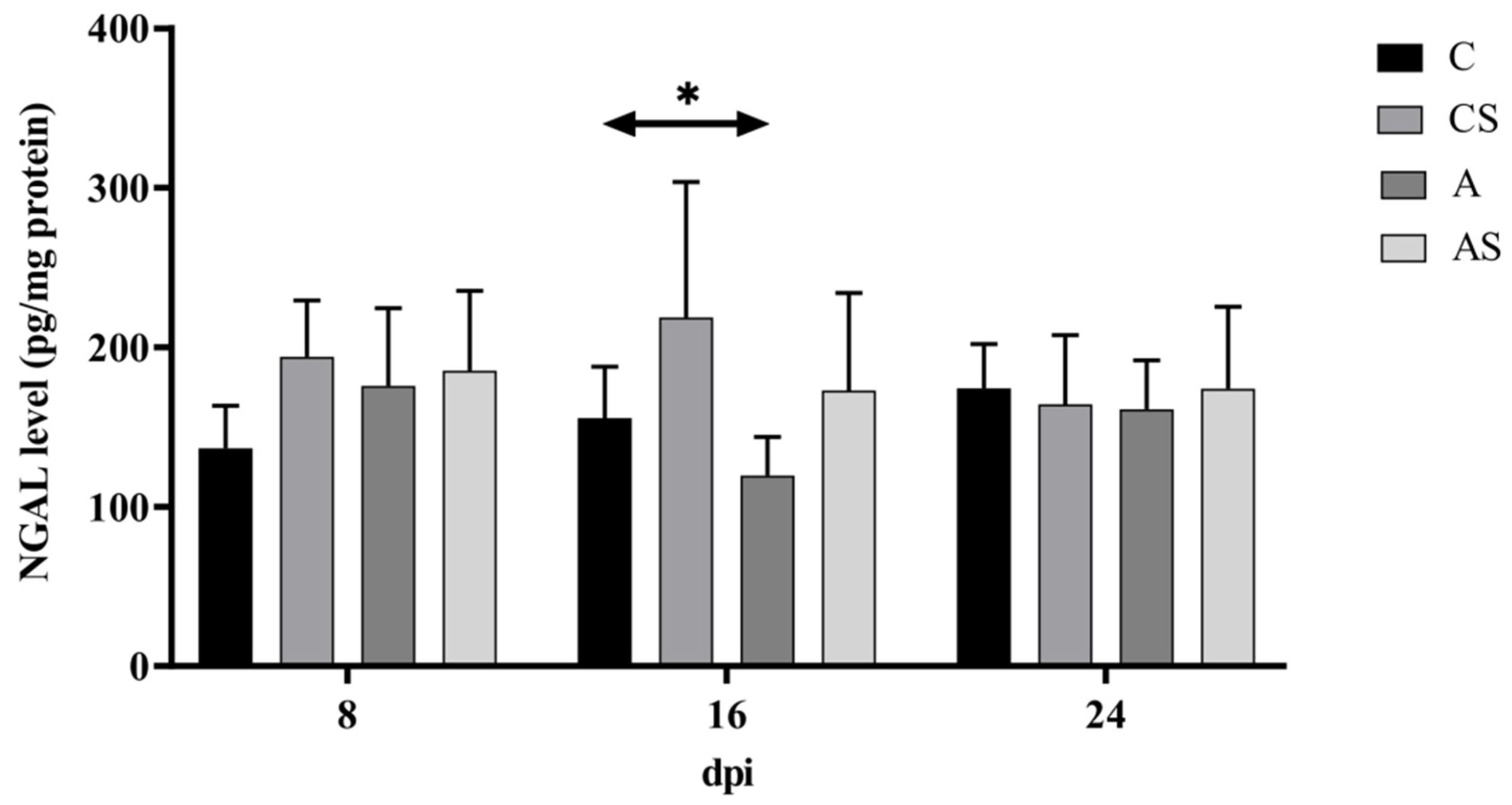
2.2. NGAL
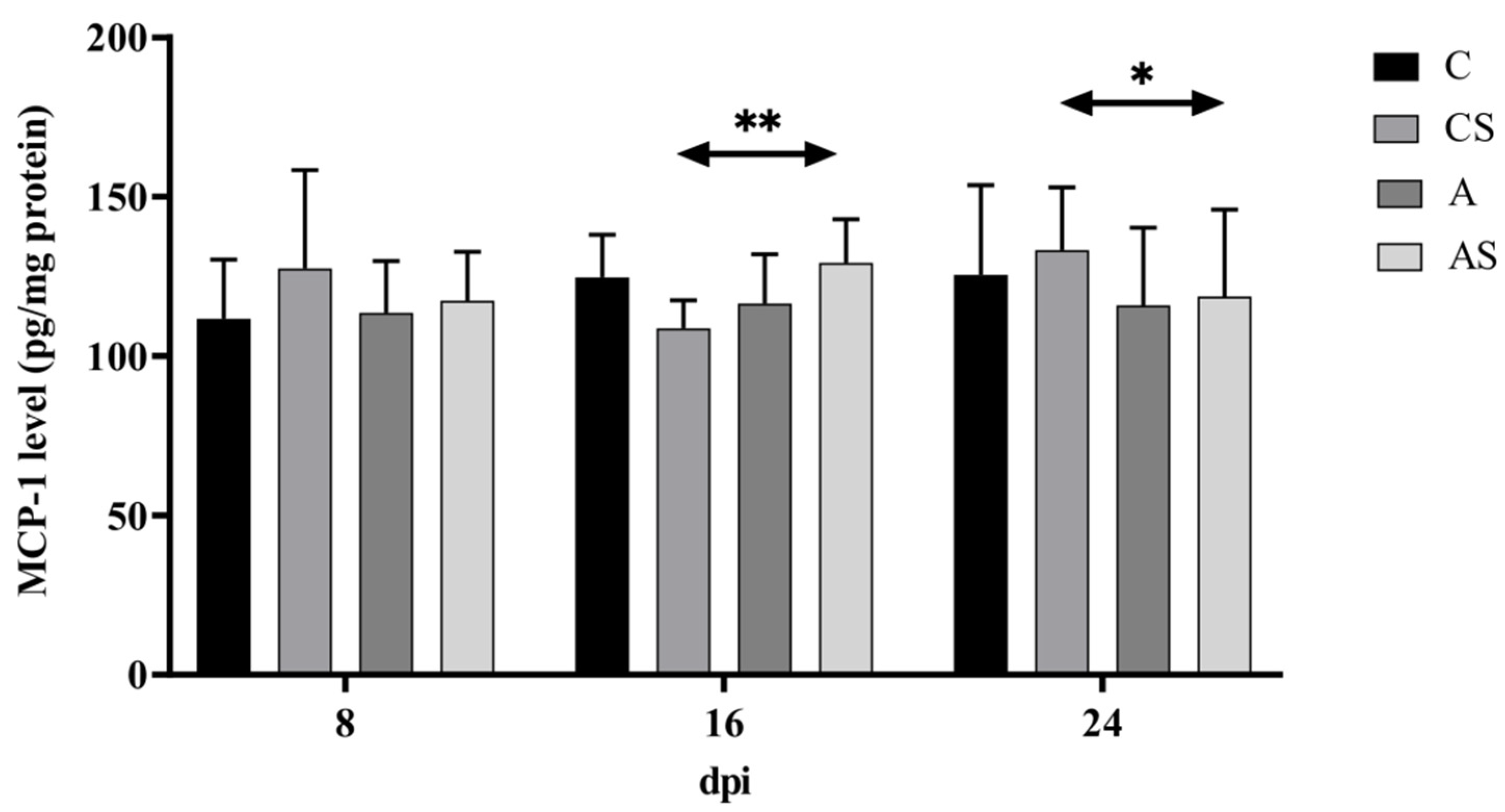
2.3. MCP-1
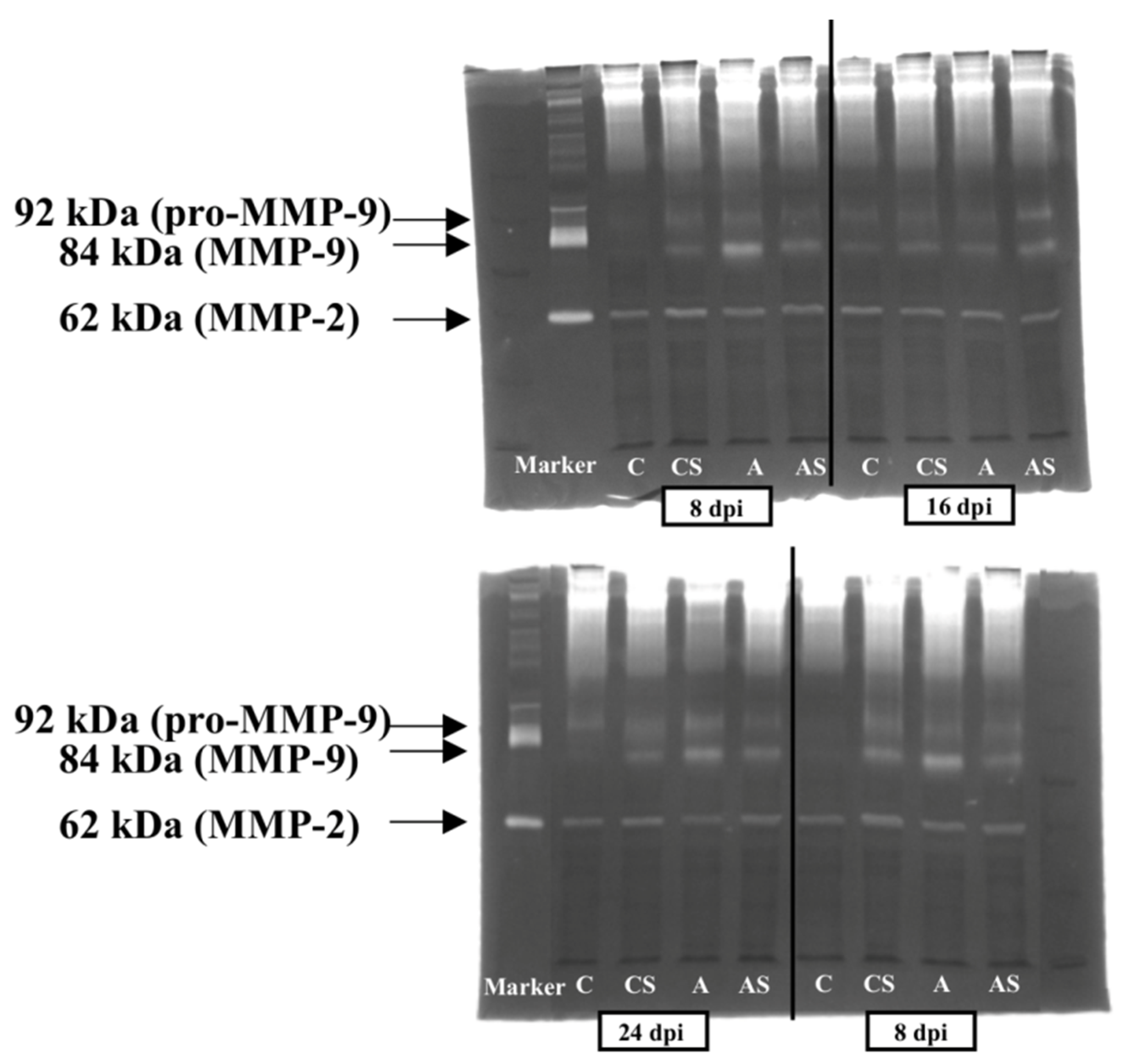
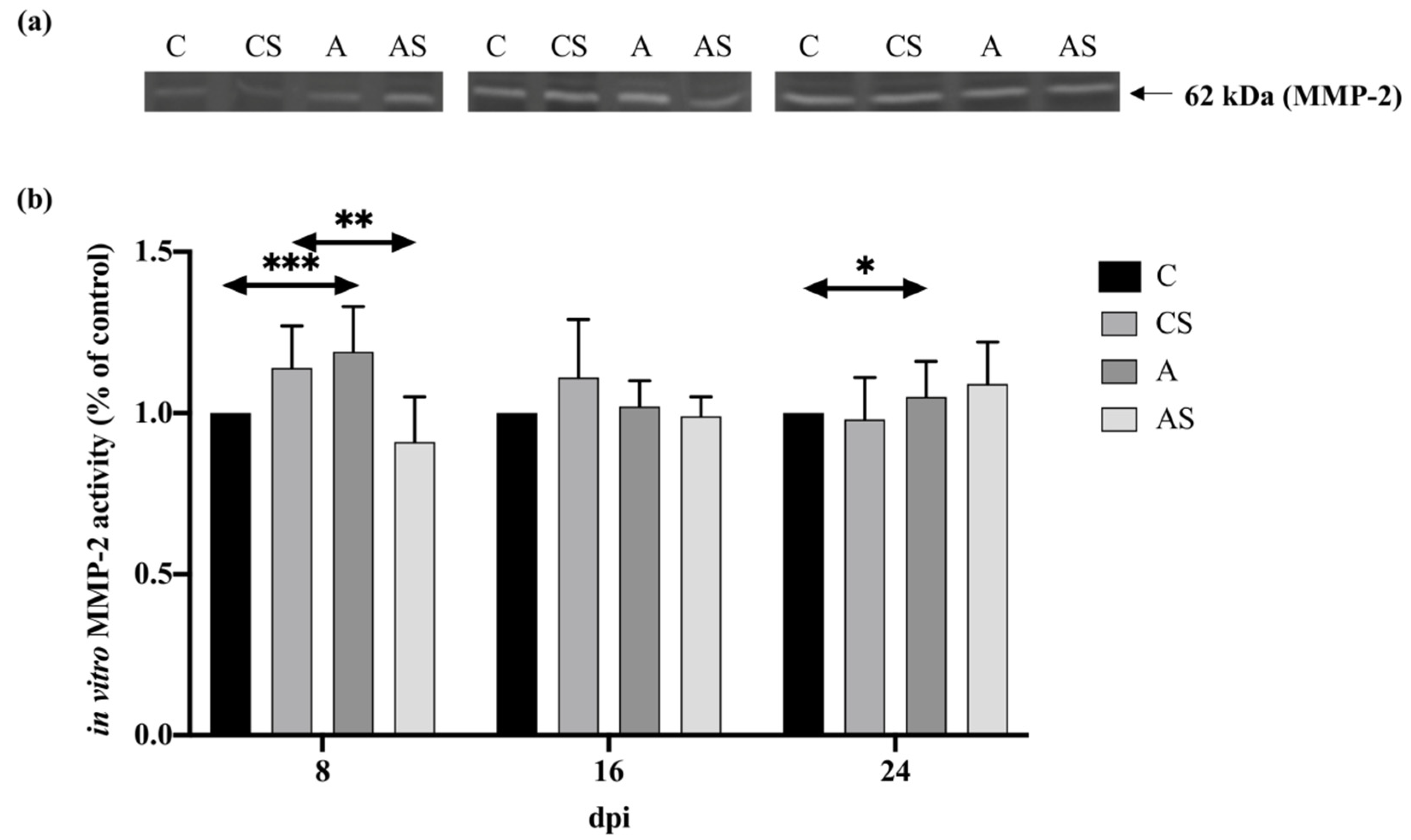
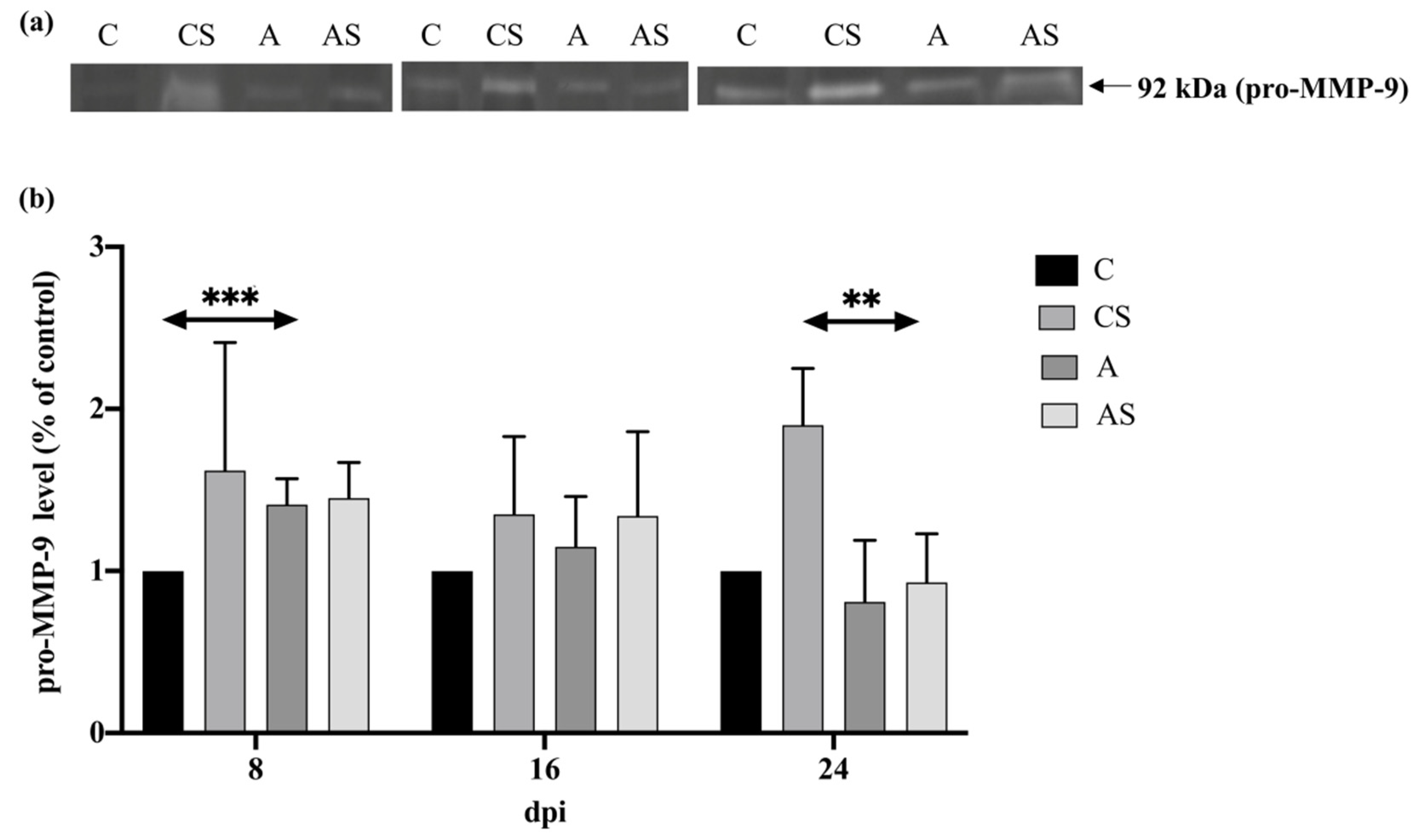
2.4. MMPs
2.4.1. MMP-2
2.4.2. Pro-MMP-9
2.4.3. MMP-9
2.5. MMP-9/NGAL
3. Discussion
3.1. KIM-1
3.2. NGAL
3.3. MCP-1
3.4. MMPs
3.5. MMP-9/NGAL
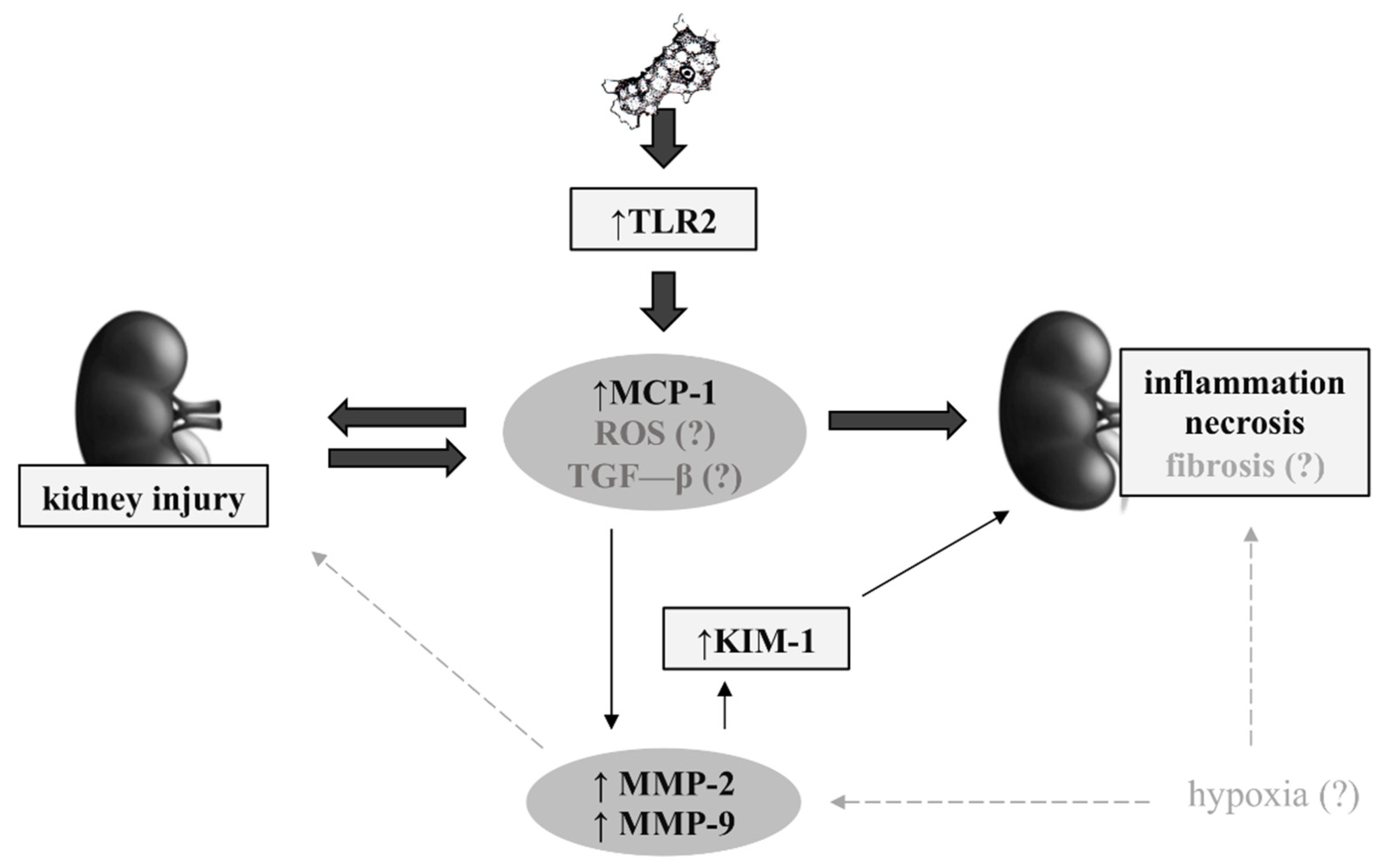
3.6. Possible Mechanisms in Renal Acanthamoebiasis
4. Materials and Methods
4.1. Ethics Statement
4.2. Acanthamoeba Spp.
4.3. Animal Model
4.4. Homogenization of Samples
4.5. Determination of Protein Content
4.6. KIM-1
4.7. NGAL
4.8. MCP-1
4.9. MMPs
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Ringsted, J.; Jager, B.V.; Suk, D.S.; Visvesvara, G.S. Probable Acanthamoeba meningoencephalitis in a Korean child. Am. J. Clin. Pathol. 1976, 66, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.A.; Kosik-Bogacka, D.I. Amoebas from the genus Acanthamoeba and their pathogenic properties. Ann. Parasitol. 2018, 64, 299–308. [Google Scholar] [CrossRef]
- Kot, K.; Kosik-Bogacka, D.; Wojtkowiak-Giera, A.; Kolasa-Wołosiuk, A.; Łanocha-Arendarczyk, N. The expression of TLR2 and TLR4 in the kidneys and heart of mice infected with Acanthamoeba spp. Parasit. Vectors 2020, 13, 480. [Google Scholar] [CrossRef]
- Górnik, K.; Kuźna-Grygiel, W. Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol. 2005, 64, 161–167. [Google Scholar]
- Simsek, A.; Tugcu, V.; Tasci, A.I. New biomarkers for the quick detection of acute kidney injury. ISRN Nephrol. 2012, 2013, 394582. [Google Scholar] [CrossRef]
- Mansour, S.G.; Puthumana, J.; Coca, S.G.; Gentry, M.; Parikh, C.R. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: A systematic review. BMC Nephrol. 2017, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Wołyniec, W.; Ratkowski, W.; Renke, J.; Renke, M. Changes in Novel AKI Biomarkers after Exercise. A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5673. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Lim, A.I.; Tang, S.C.; Lai, K.N.; Leung, J.C. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell Physiol. 2013, 228, 917–924. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Farmer, T.; Kapke, G.F. Assay validation for KIM-1: Human urinary renal dysfunction biomarker. Int. J. Biol. Sci. 2009, 5, 128–134. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, K.; Nie, Z.; Li, Q.; Jin, F. Kidney injury molecule-1 (KIM-1): A novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant. Rev. 2010, 24, 143–146. [Google Scholar] [CrossRef]
- Khreba, N.A.; Abdelsalam, M.; Wahab, A.M.; Sanad, M.; Elhelaly, R.; Adel, M.; El-Kannishy, G. Kidney injury molecule 1 (KIM-1) as an early predictor for acute kidney injury in post-cardiopulmonary bypass (CPB) in open heart surgery patients. Int. J. Nephrol. 2019, 2019, 6265307. [Google Scholar] [CrossRef]
- Krawczeski, C.D.; Goldstein, S.L.; Woo, J.G.; Wang, Y.; Piyaphanee, N.; Ma, Q.; Bennett, M.; Devarajan, P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J. Am. Coll. Cardiol. 2011, 58, 2301–2309. [Google Scholar] [CrossRef]
- van Timmeren, M.M.; Vaidya, V.S.; van Ree, R.M.; Oterdoom, L.H.; de Vries, A.P.; Gans, R.O.; van Goor, H.; Stegeman, C.A.; Bonventre, J.V.; Bakker, S.J. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 2007, 84, 1625–1630. [Google Scholar] [CrossRef]
- Di Carlo, A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol. Lett. 2013, 5, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Marchewka, Z.; Tacik, A.; Piwowar, A. KIM-1 and NGAL as potential biomarkers for the diagnosis and cancer progression. Postepy Hig. Med. Dosw. 2016, 70, 329–336. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef] [PubMed]
- Doborek, Ł.; Thor, P. Selected proteins as biomarkers of kidney injury used in the nephrological diagnosis. Post. Biochem. 2016, 62, 482–494. [Google Scholar]
- Nabity, M.B.; Lees, G.E.; Cianciolo, R.; Boggess, M.M.; Steiner, J.M.; Suchodolski, J.S. Urinary biomarkers of renal disease in dogs with X-linked hereditary nephropathy. J. Vet. Intern. Med. 2012, 26, 282–293. [Google Scholar] [CrossRef]
- Flower, D.R.; North, A.C.; Sansom, C.E. The lipocalin protein family: Structural and sequence overview. Biochim. Biophys. Acta 2000, 1482, 9–24. [Google Scholar] [CrossRef]
- Kuribayashi, R.; Suzumura, H.; Sairenchi, T.; Watabe, Y.; Tsuboi, Y.; Imataka, G.; Kurosawa, H.; Arisaka, O. Urinary neutrophil gelatinase-associated lipocalin is an early predictor of acute kidney injury in premature infants. Exp. Ther. Med. 2016, 12, 3706–3710. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Bertram, A.; Nadrowitz, F.; Menne, J. Monocyte chemoattractant protein-1 and the kidney. Curr. Opin. Nephrol. Hypertens. 2016, 25, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Tam, F.W. Urinary monocyte chemoattractant protein-1 in renal disease. Clin. Chim. Acta 2011, 412, 2022–2030. [Google Scholar] [CrossRef]
- Yadav, A.; Saini, V.; Arora, S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin. Chim. Acta. 2010, 411, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Sung, F.L.; Zhu, T.Y.; Au-Yeung, K.K.; Siow, Y.L.; Karmin, O. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int. 2002, 62, 1160–1170. [Google Scholar] [CrossRef]
- Moledina, D.G.; Isguven, S.; McArthur, E.; Thiessen-Philbrook, H.; Garg, A.X.; Shlipak, M.; Whitlock, R.; Kavsak, P.A.; Coca, S.G.; Parikh, C.R. Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI) Consortium. Plasma Monocyte Chemotactic Protein-1 Is Associated with Acute Kidney Injury and Death After Cardiac Operations. Ann. Thorac. Surg. 2017, 104, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Gregg, L.P.; Tio, M.C.; Li, X.; Adams-Huet, B.; de Lemos, J.A.; Hedayati, S.S. Association of Monocyte Chemoattractant Protein-1 with Death and Atherosclerotic Events in Chronic Kidney Disease. Am. J. Nephrol. 2018, 47, 395–405. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Urinary MCP-1 as a biomarker for lupus nephritis: A meta-analysis. Z. Rheumatol. 2017, 76, 357–363. [Google Scholar] [CrossRef]
- Hojs, R.; Ekart, R.; Bevc, S.; Hojs, N. Biomarkers of Renal Disease and Progression in Patients with Diabetes. J. Clin. Med. 2015, 4, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Lipka, D.; Boratyński, J. Metalloproteinases. Structure and function. Postepy Hit. Med. Dosw. 2008, 62, 328–336. [Google Scholar]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press Res. 2019, 44, 298–330. [Google Scholar] [CrossRef]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Ren. Physiol. 2012, 302, 1351–1361. [Google Scholar] [CrossRef]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Ren. Physiol. 2007, 292, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, K.; Koyanagi, I.; Ohara, I.; Ito, T.; Saitoh, A.; Horikoshi, S.; Tomino, Y. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2004, 18, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.C.; Silva, G.B.D., Jr.; Tôrres, P.P.B.F.; Castro, V.Q.; Lopes, R.L.; Martins, A.M.C.; Daher, E.F. Novel kidney injury biomarkers in tropical infections: A review of the literature. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, 14. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.M.; Waikar, S.S. Biomarkers in nephrology: Core Curriculum 2013. Am. J. Kidney Dis. 2013, 62, 165–178. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef]
- Oberbauer, R. Biomarkers—A potential route for improved diagnosis and management of ongoing renal damage. Transplant. Proc. 2008, 40, 44–47. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Silva, G.B., Jr.; Sampaio, A.M.; Montenegro, B.L.; Alves, M.P.; Henn, G.A.; Rocha, H.A.L.; Meneses, G.C.; Martins, A.M.C.; Daher, E.F. Preliminary study on tubuloglomerular dysfunction and evidence of renal inflammation in patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2014, 91, 908–911. [Google Scholar] [CrossRef]
- Al-Kaysi, A.M.; Eid, R.A.A.; Fahmy, B.G.A. Biochemical studies on the effect of Toxoplasma infection on liver and kidney functions in mice. Egypt J. Comp. Path Clin. Pathol. 2010, 23, 174–185. [Google Scholar]
- Kudo, M.; Aosai, F.; Mun, H.S.; Norose, K.; Akira, S.; Iwakura, Y.; Yano, A. The role of IFN-gamma and Toll-like receptors in nephropathy induced by Toxoplasma gondii infection. Microbiol. Immunol. 2004, 48, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Kot, K.; Pilarczyk, B.; Tomza-Marciniak, A.; Kabat-Koperska, J.; Kosik-Bogacka, D. Biochemical profile, liver and kidney selenium (Se) status during acanthamoebiasis in a mouse model. Folia Biol. 2018, 66, 33–40. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Xu, S.; Li, Q.; Sun, Y.; Han, R.; Jiang, C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press Res. 2016, 41, 680–700. [Google Scholar] [CrossRef]
- Tesch, G.H. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2008, 294, 697–701. [Google Scholar] [CrossRef]
- Yin, C.; Wang, N. Kidney injury molecule-1 in kidney disease. Ren. Fail. 2016, 38, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.C.; De Daher, E.F.; da Silva, G.B., Jr.; Bezerra, G.F.; da Rocha, T.P.; de Azevedo, I.E.P.; Libório, A.B.; Martins, A.M.C. Visceral leishmaniasis-associated nephropathy in hospitalised Brazilian patients: New insights based on kidney injury biomarkers. Trop. Med. Int. Health 2018, 23, 1046–1057. [Google Scholar] [CrossRef]
- van Wolfswinkel, M.E.; Koopmans, L.C.; Hesselink, D.A.; Hoorn, E.J.; Koelewijn, R.; van Hellemond, J.J.; van Genderen, P.J.J. Neutrophil gelatinase-associated lipocalin (NGAL) predicts the occurrence of malaria-induced acute kidney injury. Malar J. 2016, 15, 464. [Google Scholar] [CrossRef]
- Punsawad, C.; Viriyavejakul, P. Increased expression of kidney injury molecule-1 and matrix metalloproteinase-3 in severe Plasmodium falciparum malaria with acute kidney injury. Int. J. Clin. Exp. Pathol. 2017, 10, 7856–7864. [Google Scholar]
- Pianta, T.J.; Pickering, J.W.; Succar, L.; Chin, M.; Davidson, T.; Buckley, N.A.; Mohamed, F.; Endre, Z.H. Dexamethasone Modifies Cystatin C-Based Diagnosis of Acute Kidney Injury During Cisplatin-Based Chemotherapy. Kidney Blood Press Res. 2017, 42, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Koperska, J.; Kolasa-Wołosiuk, A.; Baranowska-Bosiacka, I.; Safranow, K.; Kosik-Bogacka, D.; Gutowska, I.; Pilutin, A.; Gołembiewska, E.; Kędzierska, K.; Ciechanowski, K. The influence of exposure to immunosuppressive treatment during pregnancy on renal function and rate of apoptosis in native kidneys of female Wistar rats. Apoptosis 2016, 21, 1240–1248. [Google Scholar] [CrossRef]
- Peris, M.P.; Morales, M.; Ares-Gómez, S.; Esteban-Gil, A.; Gómez-Ochoa, P.; Gascón, M.; Moreno, B.; Castillo, J.A. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs. Microorganisms 2020, 8, 1966. [Google Scholar] [CrossRef]
- Plewes, K.; Royakkers, A.A.; Hanson, J.; Hasan, M.M.; Alam, S.; Ghose, A.; Maude, R.J.; Stassen, P.M.; Charunwatthana, P.; Lee, S.J.; et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J. 2014, 13, 91. [Google Scholar] [CrossRef]
- Imamaki, H.; Ishii, A.; Yokoi, H.; Kasahara, M.; Kuwabara, T.; Mori, K.P.; Kato, Y.; Kuwahara, T.; Satoh, M.; Nakatani, K.; et al. Low Serum Neutrophil Gelatinase-associated Lipocalin Level as a Marker of Malnutrition in Maintenance Hemodialysis Patients. PLoS ONE 2015, 10, e0132539. [Google Scholar] [CrossRef]
- Choi, J.W.; Fujii, T. The Prevalence of Low Plasma Neutrophil Gelatinase-Associated Lipocalin Level in Systemic Inflammation and its Relationship with Proinflammatory Cytokines, Procalcitonin, Nutritional Status, and Leukocyte Profiles. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Meneses, A.V.; Sanchez, C.; Alvar, J.; Moreno, J.; Carrillo, E. Monocyte Chemotactic Protein 1 in Plasma from Soluble Leishmania Antigen-Stimulated Whole Blood as a Potential Biomarker of the Cellular Immune Response to Leishmania infantum. Front. Immunol. 2017, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.M.; Correa-Costa, M.; Barreto, C.R.; Silva, R.C.; Hayashida, C.Y.; Castoldi, A.; Gonçalves, G.M.; Braga, T.T.; Barboza, R.; Rios, F.J.; et al. Oxidative stress and modification of renal vascular permeability are associated with acute kidney injury during P. berghei ANKA infection. PLoS ONE 2012, 7, e44004. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Van Aelst, I.; Starckx, S.; Maskos, K.; Opdenakker, G.; Pagenstecher, A. Matrix metalloproteinases, tissue inhibitors of MMPs and TACE in experimental cerebral malaria. Lab. Investig. 2006, 86, 873–888. [Google Scholar] [CrossRef]
- Han, W.K.; Waikar, S.S.; Johnson, A.; Betensky, R.A.; Dent, C.L.; Devarajan, P.; Bonventre, J.V. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008, 73, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 598–611. [Google Scholar] [CrossRef]
- Cheng, Z.; Limbu, M.H.; Wang, Z.; Liu, J.; Liu, L.; Zhang, X.; Chen, P.; Liu, B. MMP-2 and 9 in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 776. [Google Scholar] [CrossRef]
- Li, Z.L.; Liu, B.C. Hypoxia and Renal Tubulointerstitial Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Candido, S.; Abrams, S.L.; Steelman, L.S.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Montalto, G.; Cervello, M.; Polesel, J.; et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy. Biochim. Biophys. Acta 2016, 1863, 438–448. [Google Scholar] [CrossRef]
- Perrin, C.; Patard, J.J.; Jouan, F.; Collet, N.; Théoleyre, S.; Edeline, J.; Zerrouki, S.; Laguerre, B.; Bellaud-Roturaud, M.-A.; Rioux-Leclercq, N.; et al. The neutrophil gelatinase-associated lipocalin, or LCN 2, marker of aggressiveness in clear cell renal cell carcinoma. Prog. Urol. 2011, 21, 851–858. [Google Scholar] [CrossRef]
- Korzeniecka-Kozerska, A.; Wasilewska, A.; Tenderenda, E.; Sulik, A.; Cybulski, K. Urinary MMP-9/NGAL ratio as a potential marker of FSGS in nephrotic children. Dis. Markers 2013, 34, 357–362. [Google Scholar] [CrossRef]
- Mertowski, S.; Lipa, P.; Morawska, I.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Roliński, J.; Załuska, W. Toll-Like Receptor as a Potential Biomarker in Renal Diseases. Int. J. Mol. Sci. 2020, 21, 6712. [Google Scholar] [CrossRef]
- Garibotto, G.; Carta, A.; Picciotto, D.; Viazzi, F.; Verzola, D. Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J. Nephrol. 2017, 30, 719–727. [Google Scholar] [CrossRef]
- Marcato, L.G.; Ferlini, A.P.; Bonfim, R.C.; Ramos-Jorge, M.L.; Ropert, C.; Afonso, L.F.; Vieira, L.Q.; Sobrinho, P.P.R. The role of Toll-like receptors 2 and 4 on reactive oxygen species and nitric oxide production by macrophage cells stimulated with root canal pathogens. Oral Microbiol. Immunol. 2008, 23, 353–359. [Google Scholar] [CrossRef]
- Leemans, J.C.; Butter, L.M.; Pulskens, W.P.; Teske, G.J.; Claessen, N.; van der Poll, T.; Florquin, S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE 2009, 4, e5704. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, M.; Bakker, S.; Vaidya, V.; Bailly, V.; Schuurs, T.; Damman, J.; Stegeman, C.A.; Bonventre, J.V.; van Goor, H. Tubular kidney injury molecule-1 in protein-overload nephropathy. Am. J. Physiol. Ren. Physiol. 2006, 291, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Lanocha, N.; Kosik-Bogacka, D.; Maciejewska, A.; Sawczuk, M.; Wilk, A.; Kuzna-Grygiel, W. The occurrence Acanthamoeba [free-living amoeba] in environmental and respiratory samples in Poland. Acta Protozool. 2009, 48, 271–279. [Google Scholar]
- Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Kot, K.; Gutowska, I.; Kolasa-Wołosiuk, A.; Chlubek, D.; Kosik-Bogacka, D. Expression and Activity of COX-1 and COX-2 in Acanthamoeba sp.-Infected Lungs According to the Host Immunological Status. Int. J. Mol. Sci. 2018, 19, 121. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, K.; Kupnicka, P.; Witulska, O.; Czepan, A.; Łanocha-Arendarczyk, N.A.; Łanocha, A.A.; Kosik-Bogacka, D.I. Potential Biomarkers in Diagnosis of Renal Acanthamoebiasis. Int. J. Mol. Sci. 2021, 22, 6583. https://doi.org/10.3390/ijms22126583
Kot K, Kupnicka P, Witulska O, Czepan A, Łanocha-Arendarczyk NA, Łanocha AA, Kosik-Bogacka DI. Potential Biomarkers in Diagnosis of Renal Acanthamoebiasis. International Journal of Molecular Sciences. 2021; 22(12):6583. https://doi.org/10.3390/ijms22126583
Chicago/Turabian StyleKot, Karolina, Patrycja Kupnicka, Oliwia Witulska, Aleksandra Czepan, Natalia Agnieszka Łanocha-Arendarczyk, Aleksandra Anna Łanocha, and Danuta Izabela Kosik-Bogacka. 2021. "Potential Biomarkers in Diagnosis of Renal Acanthamoebiasis" International Journal of Molecular Sciences 22, no. 12: 6583. https://doi.org/10.3390/ijms22126583
APA StyleKot, K., Kupnicka, P., Witulska, O., Czepan, A., Łanocha-Arendarczyk, N. A., Łanocha, A. A., & Kosik-Bogacka, D. I. (2021). Potential Biomarkers in Diagnosis of Renal Acanthamoebiasis. International Journal of Molecular Sciences, 22(12), 6583. https://doi.org/10.3390/ijms22126583






