Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal
Abstract
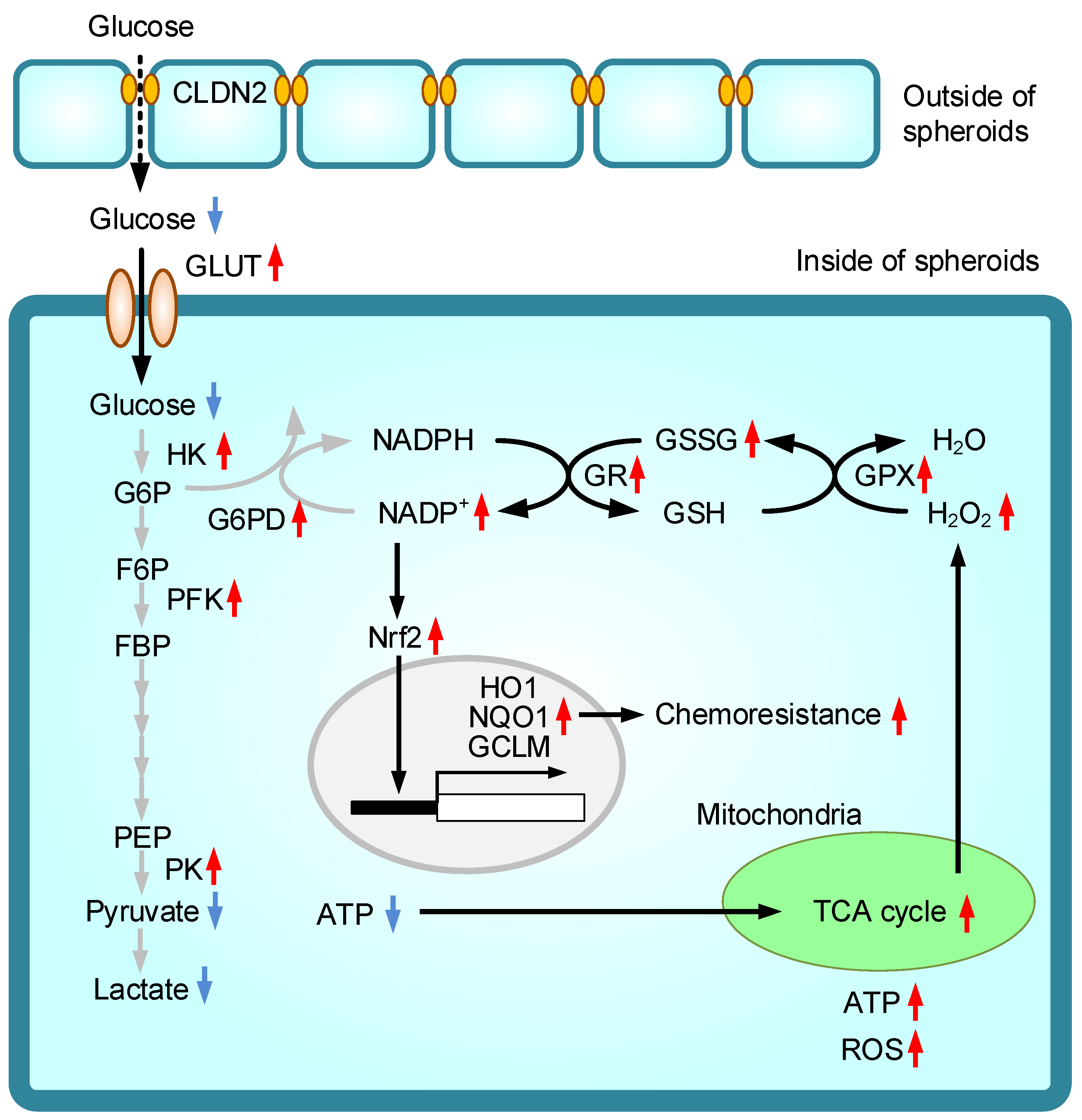
1. Introduction
2. Results
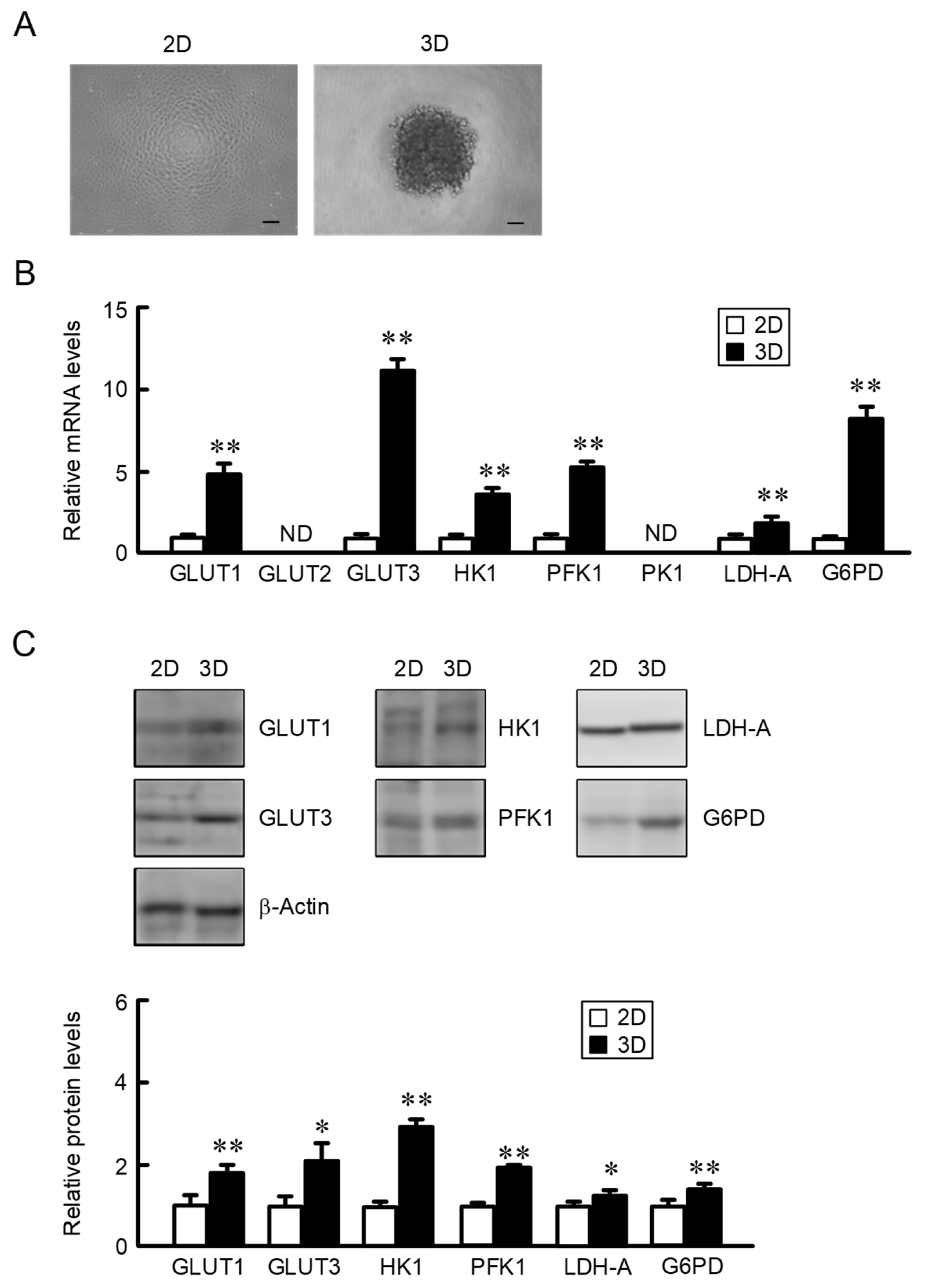
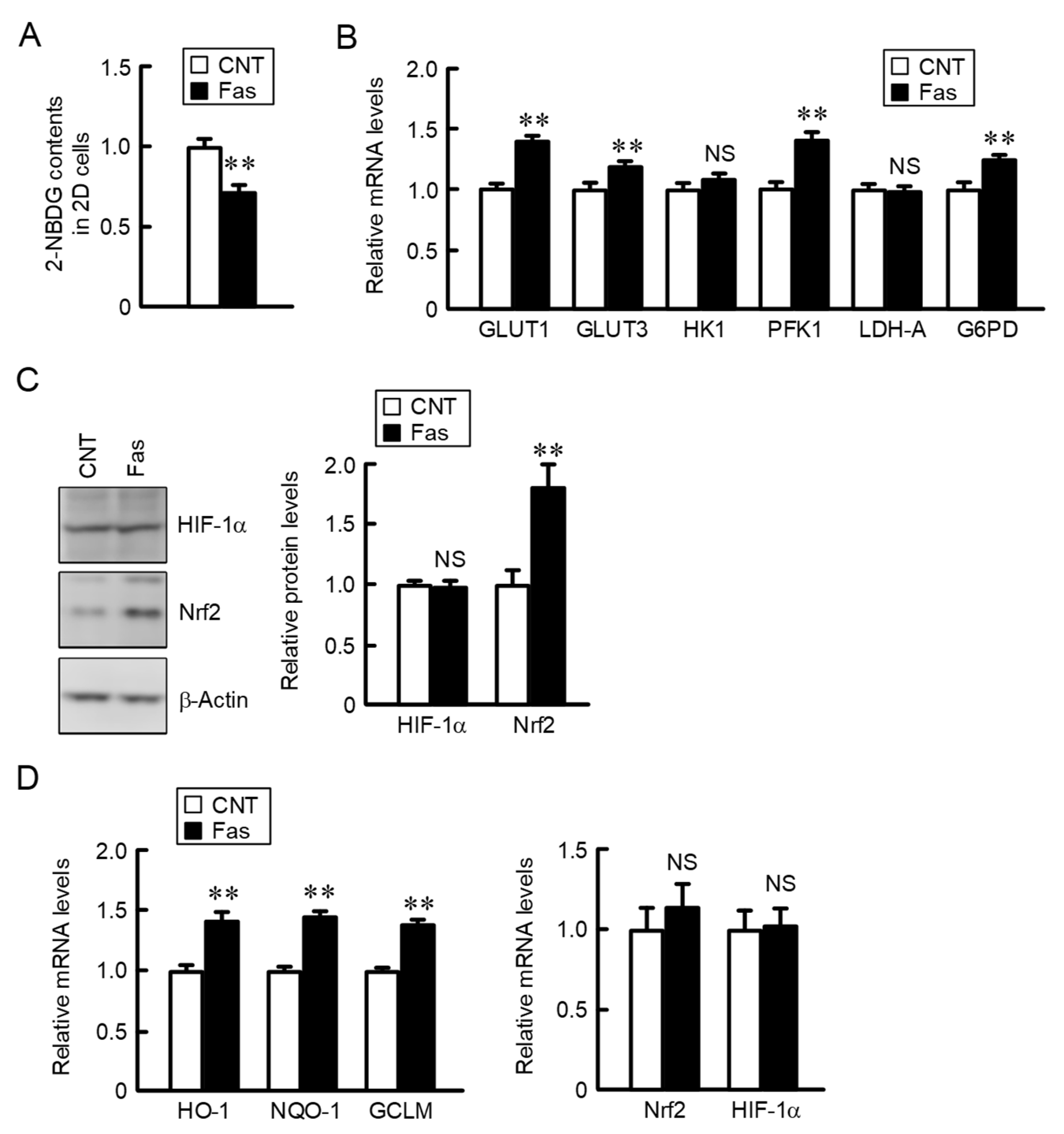
2.1. Increase in the Expression of Glucose Transporters and Metabolic Enzymes in A549 Spheroid Cells
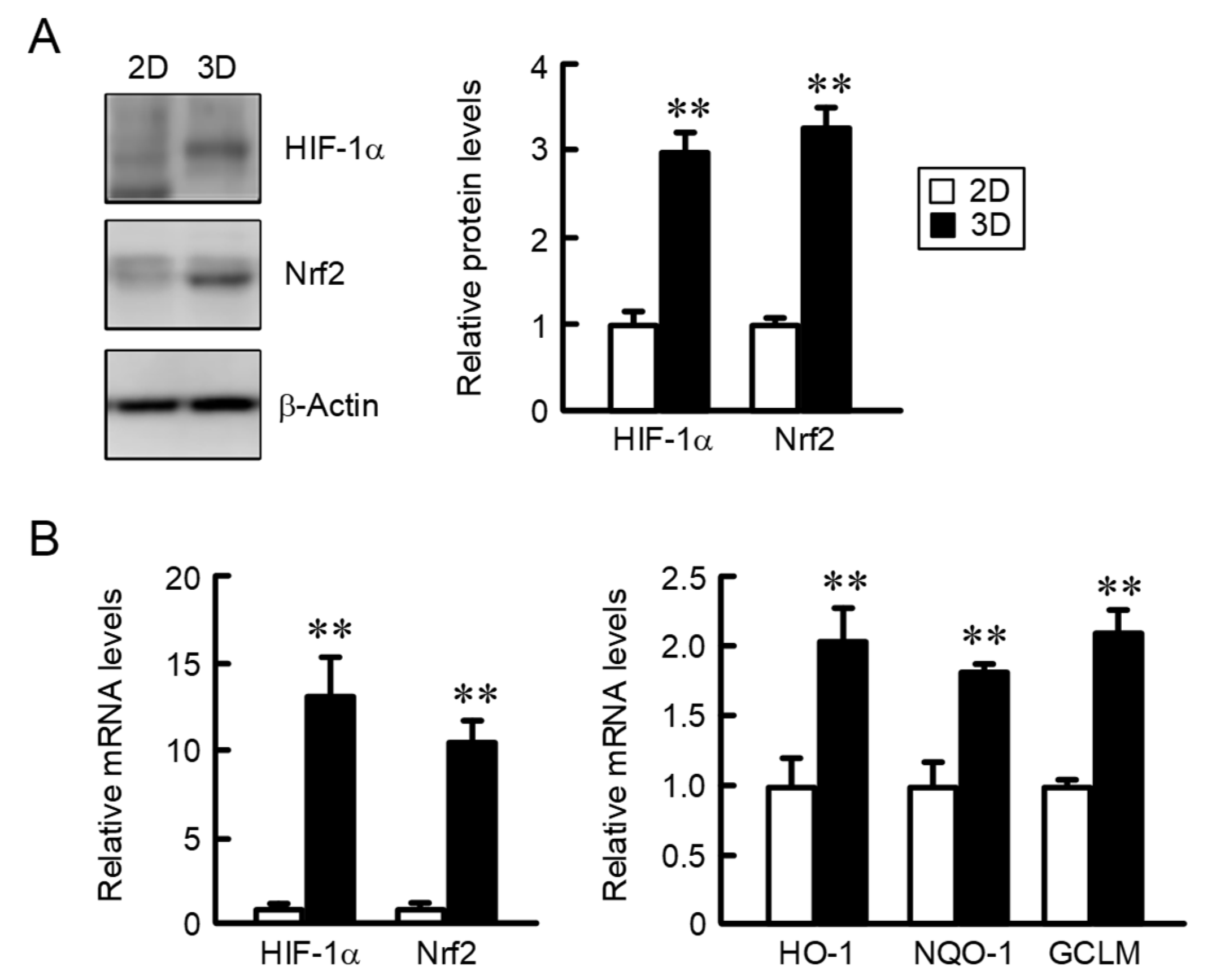
2.2. Comparison of the Expression of HIF-1α and Nrf2 in 2D Monolayer and Three-Dimensional (3D) Spheroid Cells
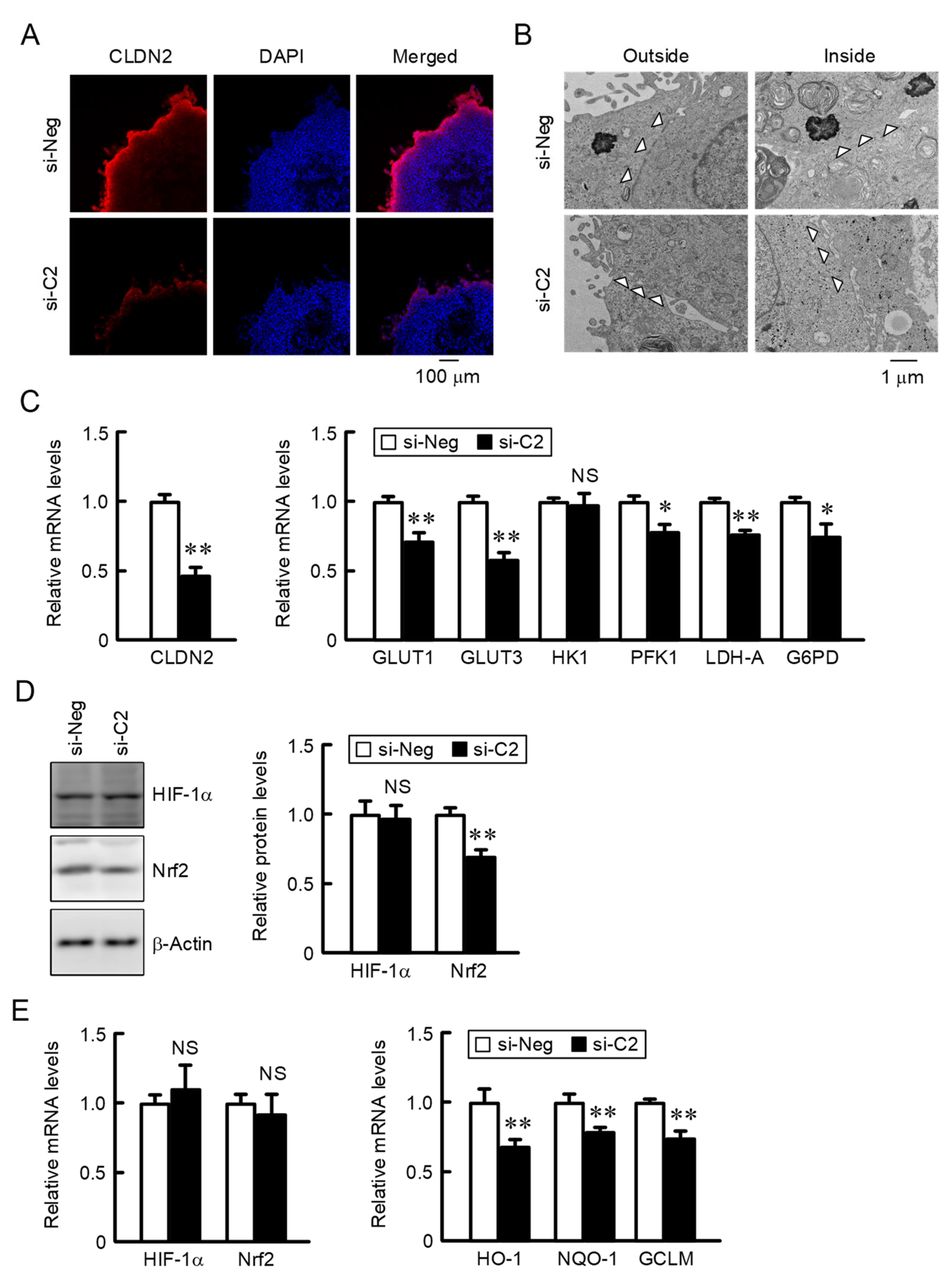
2.3. Decreases in Nrf2 and the Nrf2-Targeted Genes by CLDN2 Knockdown
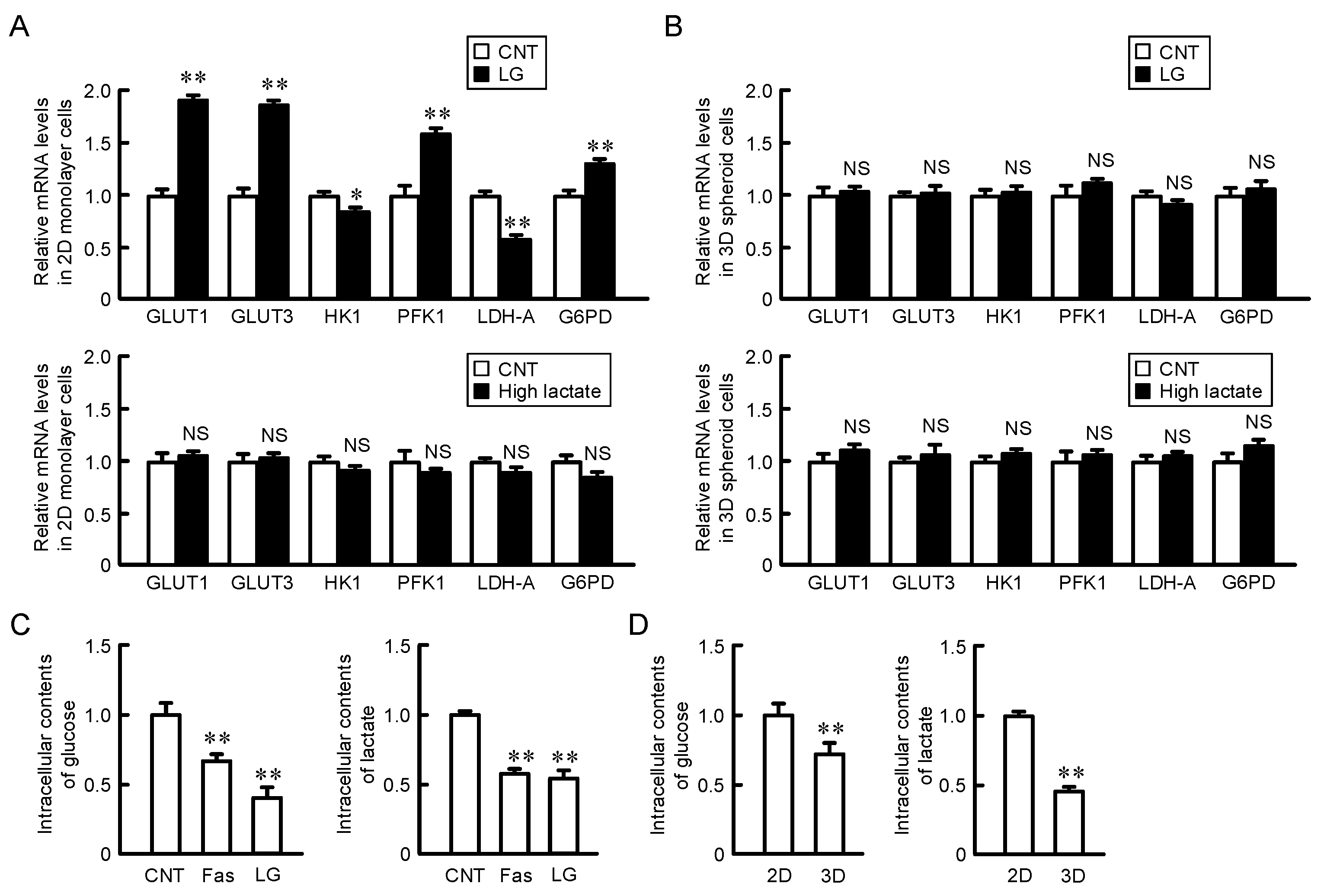
2.4. Effects of Glucose and Lactate Concentration on the Expression of Glucose Transporters and Metabolic Enzymes in 2D Monolayer and 3D Spheroid Cells
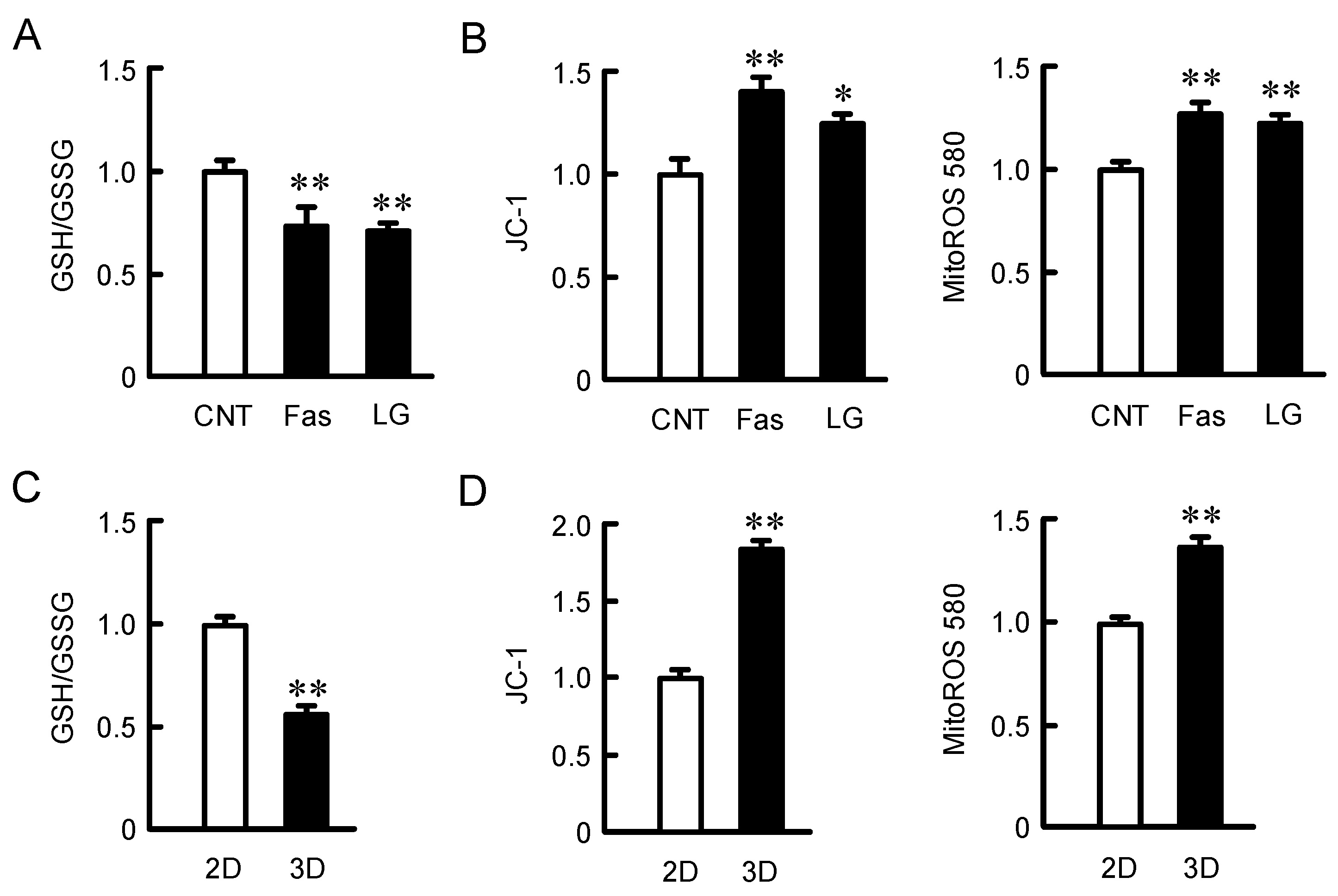
2.5. Effects of Fasentin and Low Glucose Concentration on Mitochondria Activity
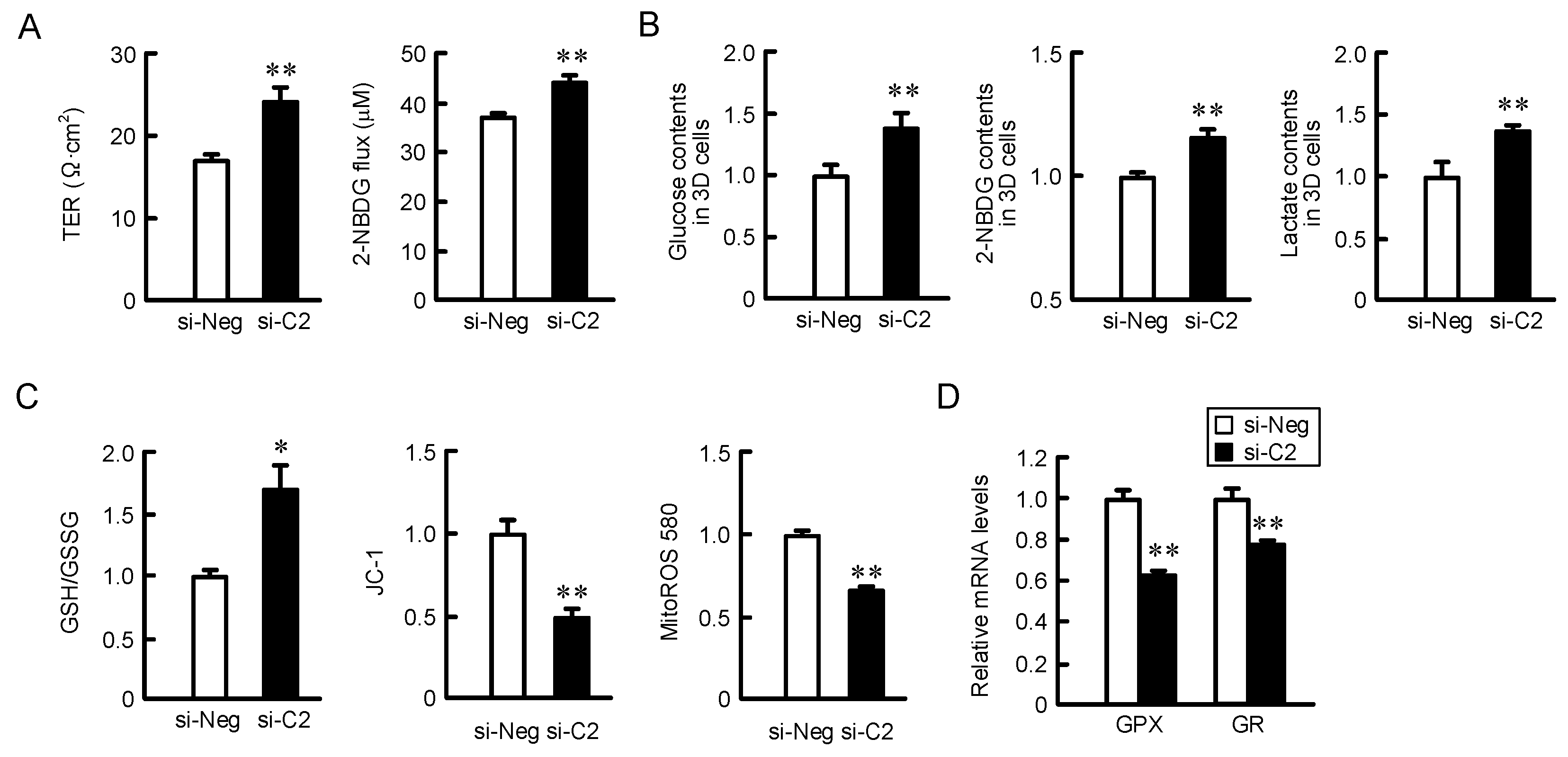
2.6. Effect of CLDN2 Knockdown on Paracellular Permeability in Monolayer Cells, Accumulation of Glucose and Oxidation-Reduction Balance in 3D Spheroid Cells
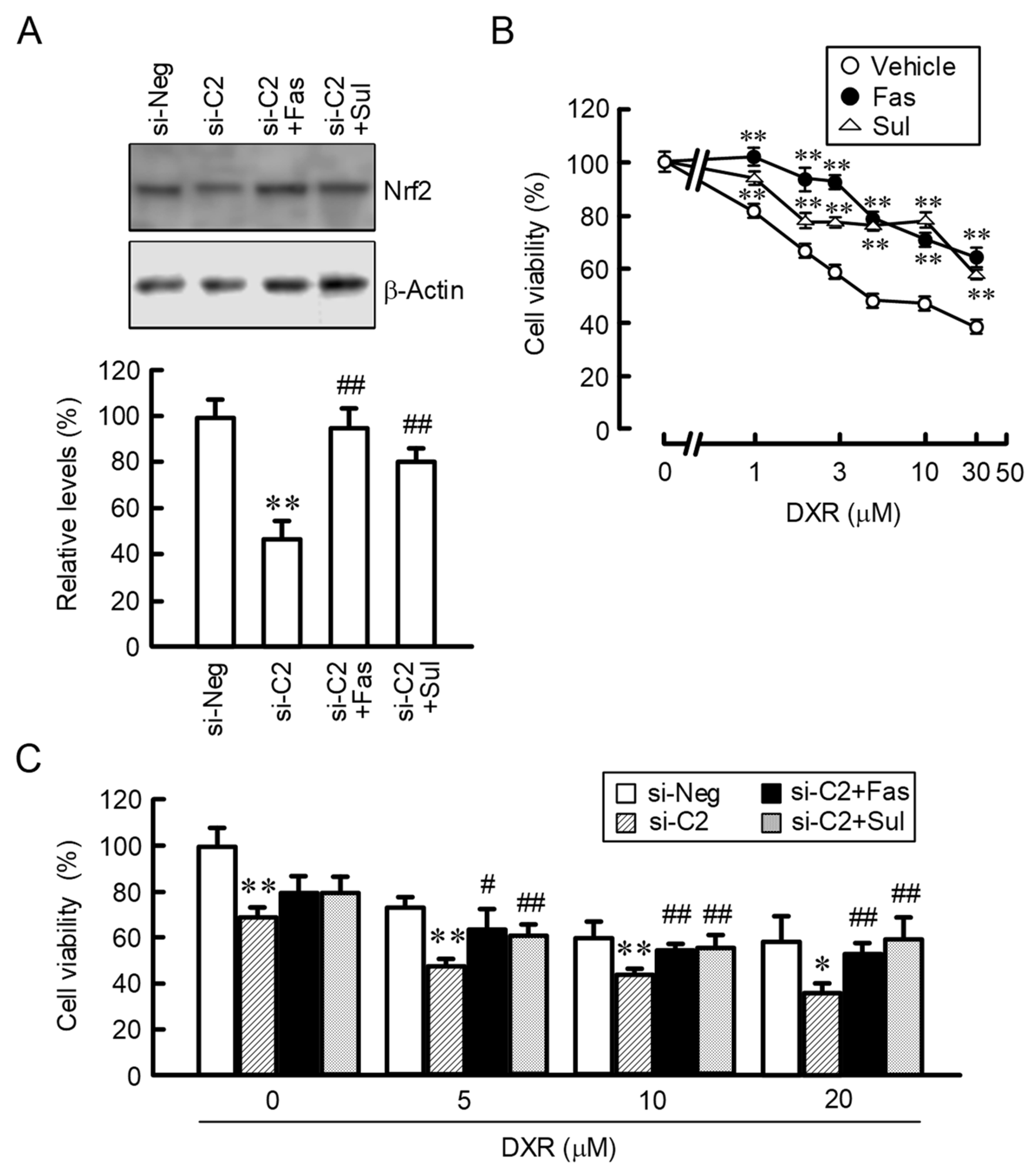
2.7. Effects of Fasentin and Sulforaphane on DXR-Induced Cytotoxicity in 2D Monolayer and 3D Spheroid Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reverse Transcription and Quantitative Real-Time PCR
4.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
4.4. Microarray Analysis
4.5. Immunocytochemistry
4.6. Electron Microscopy
4.7. Intracellular Glutathione, Glucose and Lactate Contents
4.8. Mitochondrial ROS Production and Membrane Potential
4.9. Measurement of Barrier Function of TJs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine tri-phosphate |
| CLDN | claudin |
| DAPI | 4′6-diamidino-2-phenylindole |
| DXR | doxorubicin |
| 3D | three-dimensional |
| GCLM | glutamate-cysteine ligase modifier subunit |
| G6PD | glucose-6-phosphate dehydrogenase |
| GLUT | sodium-independent glucose transporter |
| GPX | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| HIF-1 | hypoxia-inducible factor-1 |
| HK1 | hexokinase 1 |
| HO-1 | heme oxygenase-1 |
| Keap1 | Kelch-like ECH-associated protein |
| LDH-A | lactate dehydrogenase-A |
| 2-NBDG | 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose |
| NQO1 | NAD(P)H: quinone oxidoreductase-1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PCR | polymerase chain reaction |
| PFK1 | phosphofructokinase 1 |
| PFKFB4 | 6-phosphofructo-2-kinase/Fructose-2,6-biphosphatase 4 |
| PFKL | 6-phosphofructokinase |
| PK1 | pyruvate kinase 1 |
| ROS | reactive oxygen species |
| SGLT | sodium-dependent glucose transporter |
| TER | transepithelial electrical resistance |
| TJ | tight junction |
References
- Yeldag, G.; Rice, A.; Del Rio Hernandez, A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2020, 30202–30209. [Google Scholar] [CrossRef]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef]
- Katt, W.P.; Lukey, M.J.; Cerione, R.A. Starving the Devourer: Cutting Cancer off from Its Favorite Foods. Cell Chem. Biol. 2019, 26, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Seino, Y.; Fukumoto, H.; Koh, G.; Yano, H.; Inagaki, N.; Yamada, Y.; Inoue, K.; Manabe, T.; Imura, H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun. 1990, 170, 223–230. [Google Scholar] [CrossRef]
- Sultan, P.; Gutierrez, M.C.; Carvalho, B. Neuraxial morphine and respiratory depression: Finding the right balance. Drugs 2011, 71, 1807–1819. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Sato, T.; Watanabe, R.; Yamazaki, Y.; Sugatani, J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim. Biophys. Acta 2012, 1823, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Watanabe, R.; Sato, T.; Taga, S.; Shimobaba, S.; Yamaguchi, M.; Yamazaki, Y.; Endo, S.; Matsunaga, T.; Sugatani, J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim. Biophys. Acta 2014, 1843, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Ikari, A.; Sato, T.; Takiguchi, A.; Atomi, K.; Yamazaki, Y.; Sugatani, J. Claudin-2 knockdown decreases matrix metalloproteinase-9 activity and cell migration via suppression of nuclear Sp1 in A549 cells. Life Sci. 2011, 88, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, R.; Akizuki, R.; Sato, T.; Matsunaga, T.; Endo, S.; Yamaguchi, M.; Yamazaki, Y.; Sakai, H.; Ikari, A. Elevation of sensitivity to anticancer agents of human lung adenocarcinoma A549 cells by knockdown of claudin-2 expression in monolayer and spheroid culture models. Biochim. Biophys. Acta 2018, 1865, 470–479. [Google Scholar] [CrossRef]
- Airley, R.; Evans, A.; Mobasheri, A.; Hewitt, S.M. Glucose transporter Glut-1 is detectable in peri-necrotic regions in many human tumor types but not normal tissues: Study using tissue microarrays. Ann. Anat. Anat. Anz. 2010, 192, 133–138. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Li, S.; Yan, T.; Yang, J.Q.; Oberley, T.D.; Oberley, L.W. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000, 60, 3927–3939. [Google Scholar]
- Xue, D.; Zhou, X.; Qiu, J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. Pharmacother. 2020, 131, 110676. [Google Scholar] [CrossRef]
- Nasako, H.; Akizuki, R.; Takashina, Y.; Ishikawa, Y.; Shinoda, T.; Shirouzu, M.; Asai, T.; Matsunaga, T.; Endo, S.; Ikari, A. Claudin-2 binding peptides, VPDSM and DSMKF, down-regulate claudin-2 expression and anticancer resistance in human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118642. [Google Scholar] [CrossRef]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin alphavbeta5 Axis. Cell Stem Cell 2020, 26, 187–204.e110. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Palorini, R.; Simonetto, T.; Cirulli, C.; Chiaradonna, F. Mitochondrial complex I inhibitors and forced oxidative phosphorylation synergize in inducing cancer cell death. Int. J. Cell Biol. 2013, 2013, 243876. [Google Scholar] [CrossRef]
- Pollak, M. Potential applications for biguanides in oncology. J. Clin. Investig. 2013, 123, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Misra, V.; Thimmulappa, R.K.; Lee, H.; Ames, S.; Hoque, M.O.; Herman, J.G.; Baylin, S.B.; Sidransky, D.; Gabrielson, E.; et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006, 3, e420. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Iijima, K.; Miyamoto, M.; Nakahara, I.; Tanaka, H.; Ohtsuji, M.; Suzuki, T.; Kobayashi, A.; Yokota, J.; Sakiyama, T.; et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008, 68, 1303–1309. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Kwak, M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Choi, B.H.; Ku, S.K.; Kwak, M.K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef]
- Walker, A.; Singh, A.; Tully, E.; Woo, J.; Le, A.; Nguyen, T.; Biswal, S.; Sharma, D.; Gabrielson, E. Nrf2 signaling and autophagy are complementary in protecting breast cancer cells during glucose deprivation. Free Radic. Biol. Med. 2018, 120, 407–413. [Google Scholar] [CrossRef]
- Momcilovic, M.; Bailey, S.T.; Lee, J.T.; Fishbein, M.C.; Magyar, C.; Braas, D.; Graeber, T.; Jackson, N.J.; Czernin, J.; Emberley, E.; et al. Targeted Inhibition of EGFR and Glutaminase Induces Metabolic Crisis in EGFR Mutant Lung Cancer. Cell Rep. 2017, 18, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.J.; Gorski, S.M. Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer. Cancers 2019, 11, 1775. [Google Scholar] [CrossRef]
- Akizuki, R.; Maruhashi, R.; Eguchi, H.; Kitabatake, K.; Tsukimoto, M.; Furuta, T.; Matsunaga, T.; Endo, S.; Ikari, A. Decrease in paracellular permeability and chemosensitivity to doxorubicin by claudin-1 in spheroid culture models of human lung adenocarcinoma A549 cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Manabe, A.; Furukawa, C.; Endo, S.; Marunaka, K.; Nishiyama, T.; Fujii, N.; Tabuchi, Y.; Matsunaga, T.; Ikari, A. Chlorpheniramine Increases Paracellular Permeability to Marker Fluorescein Lucifer Yellow Mediated by Internalization of Occludin in Murine Colonic Epithelial Cells. Biol. Pharm. Bull. 2017, 40, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
| Genes | Direction | Sequence (5′-----3′) |
|---|---|---|
| GLUT1 | Sense | CAGCTACCCTGGATGTCCTATC |
| Antisense | AATTTGAGGTCCAGTTGGAGAA | |
| GLUT3 | Sense | GCTGCTACTGGGTTTTACCATC |
| Antisense | CTTGTGACATCCTTGCACTCTC | |
| HK1 | Sense | GAAAAAGAGAACGGTGGAAATG |
| Antisense | GTCAGAGATGCAGGAGACAATG | |
| PFK1 | Sense | CTTGTCACCTCTCTGTCCTGTG |
| Antisense | ACAGTAACCCGGGTGTCATATC | |
| PK1 | Sense | TGGGTCTCTAAGTCCCAAAGAG |
| Antisense | AATGTCCAGTAGGCAGAGGTGT | |
| LDH-A | Sense | GGAGTTCACCCATTAAGCTGTC |
| Antisense | GTGAACCTCTTTCCACTGTTCC | |
| G6PD | Sense | CGTGTACACCAAGATGATGACC |
| Antisense | GGGAGCTTCACGTTCTTGTATC | |
| HO-1 | Sense | AAGATTGCCCAGAAAGCCCTGGAC |
| Antisense | AACTGTCGCCACCAGAAAGCTGAG | |
| NQO-1 | Sense | GAAGAGCACTGATCGTACTGGC |
| Antisense | GGATACTGAAAGTTCGCAGGG | |
| GCLM | Sense | TGTCTTGGAATGCACTGTATCTC |
| Antisense | CCCAGTAAGGCTGTAAATGCTC | |
| GPX1 | Sense | TGCGGGGCAAGGTACTACTTAT |
| Antisense | GGACGTACTTGAGGGAATTCAG | |
| GR | Sense | GAGCTTTACCCCGATGTATCAC |
| Antisense | GACTGTGTTGTCAAAGTCTGCC | |
| CLDN2 | Sense | ATTGTGACAGCAGTTGGCTT |
| Antisense | CTATAGATGTCACACTGGGTGATG | |
| β-Actin | Sense | CCTGAGGCACTCTTCCAGCCTT |
| Antisense | TGCGGATGTCCACGTCACACTTC |
| Name | Catalog Number | Supplier | Address |
|---|---|---|---|
| GLUT1 | 10-7548 | Abeomics | San Diego, CA, USA |
| GLUT3 | 20403-1-AP | ProteinTech | Rosemont, IL, USA |
| HK1 | 200-4159S | Rockland Immunochemicals | Limerick, PA, USA |
| PFK1 | 200-1156S | Rockland Immunochemicals | Limerick, PA, USA |
| PK1 | 200-1178S | Rockland Immunochemicals | Limerick, PA, USA |
| LDH-A | sc-137243 | Santa Cruz Biotechnology | Santa Cruz, CA, USA |
| G6PD | 25413-1-AP | ProteinTech | Rosemont, IL, USA |
| β-Actin | sc-1615 | Santa Cruz Biotechnology | Santa Cruz, CA, USA |
| HIF-1α | bs-0737R | Bioss Antibodies | Woburn, MA, USA |
| Nrf2 | GTX102572 | GeneTex | Irvine, CA, USA |
| CLDN2 | 51-6100 | Thermo Fisher Scientific | Rockford, IL, USA |
| 2-Fold or Greater | Half or Less | |||
|---|---|---|---|---|
| EPB41 | CTAG1B | AGPAT1 | GNB2 | PTOV1 |
| LGALS8 | KRTAP5-6 | ANKRD54 | GOLT1A | RFXANK |
| SV2A | MRGPRD | ANKZF1 | GPX1 | RGL2 |
| LPGAT1 | SYTL2 | AP4M1 | HINFP | RHBDD2 |
| PLD5 | IL18 | APOL2 | HIST1H2AB | RNASEH2A |
| TNFRSF25 | LYRM5 | ASIC1 | HMOX1 | RNF123 |
| GCKR | UBE3B | BDKRB2 | HPCAL1 | RTN4RL2 |
| C2orf71 | FGF6 | C20orf96 | HSD17B14 | S100A13 |
| CCNL1 | KRT6B | CA9 | HSPBP1 | SERINC2 |
| PARM1 | KCNA6 | CCDC102A | ID3 | SIPA1 |
| GAR1 | LINC00452 | CCM2 | ILVBL | SLC16A3 |
| TRIM2 | SAMD4A | CD151 | KCNK5 | SLC29A4 |
| TMEM167A | TMOD3 | CDC42EP4 | LAMTOR1 | SLX1A |
| CLPS | ADAMTS17 | CDH16 | LIMK1 | SPAG4 |
| DLD | COL1A1 | CFD | LINC01588 | SPNS1 |
| CSPP1 | SEZ6 | CLDN2 | LTBR | SRXN1 |
| BRF2 | SPIRE1 | COX5B | LYPLA2 | SUMF1 |
| JAK2 | TCF4 | COX7A2 | MCM5 | TBC1D16 |
| IL11RA | ANGPTL8 | CRIP1 | MIF | TCEB2 |
| PGRMC1 | ZNF253 | CUEDC2 | MIF4GD | TEX264 |
| CYB5R1 | MRPS12 | TIMM13 | ||
| DEDD2 | MYL6B | TMEM205 | ||
| DNAL4 | NDRG1 | TMEM219 | ||
| ECI1 | NDUFA13 | TMEM222 | ||
| EFNA1 | NT5C | TMEM8A | ||
| EIF4EBP1 | OTUD7B | TNFRSF12A | ||
| EIF6 | PFKFB4 | TPI1 | ||
| ETFB | PFKL | TPRA1 | ||
| EXOC7 | PGAP2 | TRAF4 | ||
| FAM134C | PLPPR2 | TRAPPC5 | ||
| FAM96B | PMM1 | TSC22D4 | ||
| FIBP | POLE4 | UQCRC1 | ||
| FIZ1 | PQBP1 | VPS28 | ||
| FLOT1 | PRDX5 | ZMYM3 | ||
| GANC | PREB | ZNF358 | ||
| GC | PSMB5 | ZNF428 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, A.; Nasako, H.; Akizuki, R.; Takashina, Y.; Eguchi, H.; Matsunaga, T.; Yoshino, Y.; Endo, S.; Ikari, A. Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal. Int. J. Mol. Sci. 2021, 22, 6582. https://doi.org/10.3390/ijms22126582
Ito A, Nasako H, Akizuki R, Takashina Y, Eguchi H, Matsunaga T, Yoshino Y, Endo S, Ikari A. Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal. International Journal of Molecular Sciences. 2021; 22(12):6582. https://doi.org/10.3390/ijms22126582
Chicago/Turabian StyleIto, Ayaka, Haruka Nasako, Risa Akizuki, Yui Takashina, Hiroaki Eguchi, Toshiyuki Matsunaga, Yuta Yoshino, Satoshi Endo, and Akira Ikari. 2021. "Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal" International Journal of Molecular Sciences 22, no. 12: 6582. https://doi.org/10.3390/ijms22126582
APA StyleIto, A., Nasako, H., Akizuki, R., Takashina, Y., Eguchi, H., Matsunaga, T., Yoshino, Y., Endo, S., & Ikari, A. (2021). Elevation of Chemosensitivity of Lung Adenocarcinoma A549 Spheroid Cells by Claudin-2 Knockdown through Activation of Glucose Transport and Inhibition of Nrf2 Signal. International Journal of Molecular Sciences, 22(12), 6582. https://doi.org/10.3390/ijms22126582







