Effects of Dihydrotanshinone I on Proliferation and Invasiveness of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells
Abstract
1. Introduction
2. Results
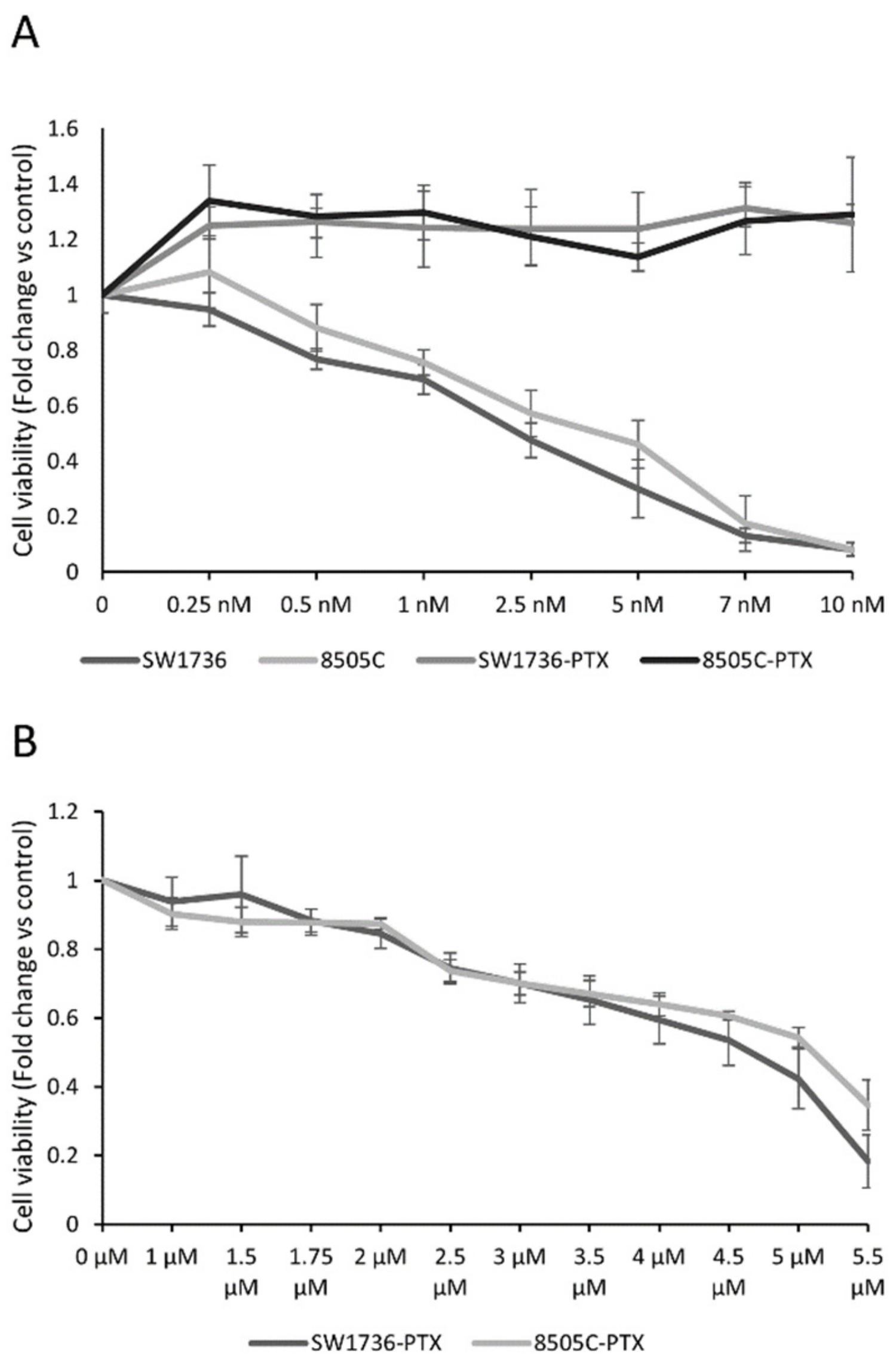
2.1. Generation of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells and Paclitaxel Effects on Cell Viability
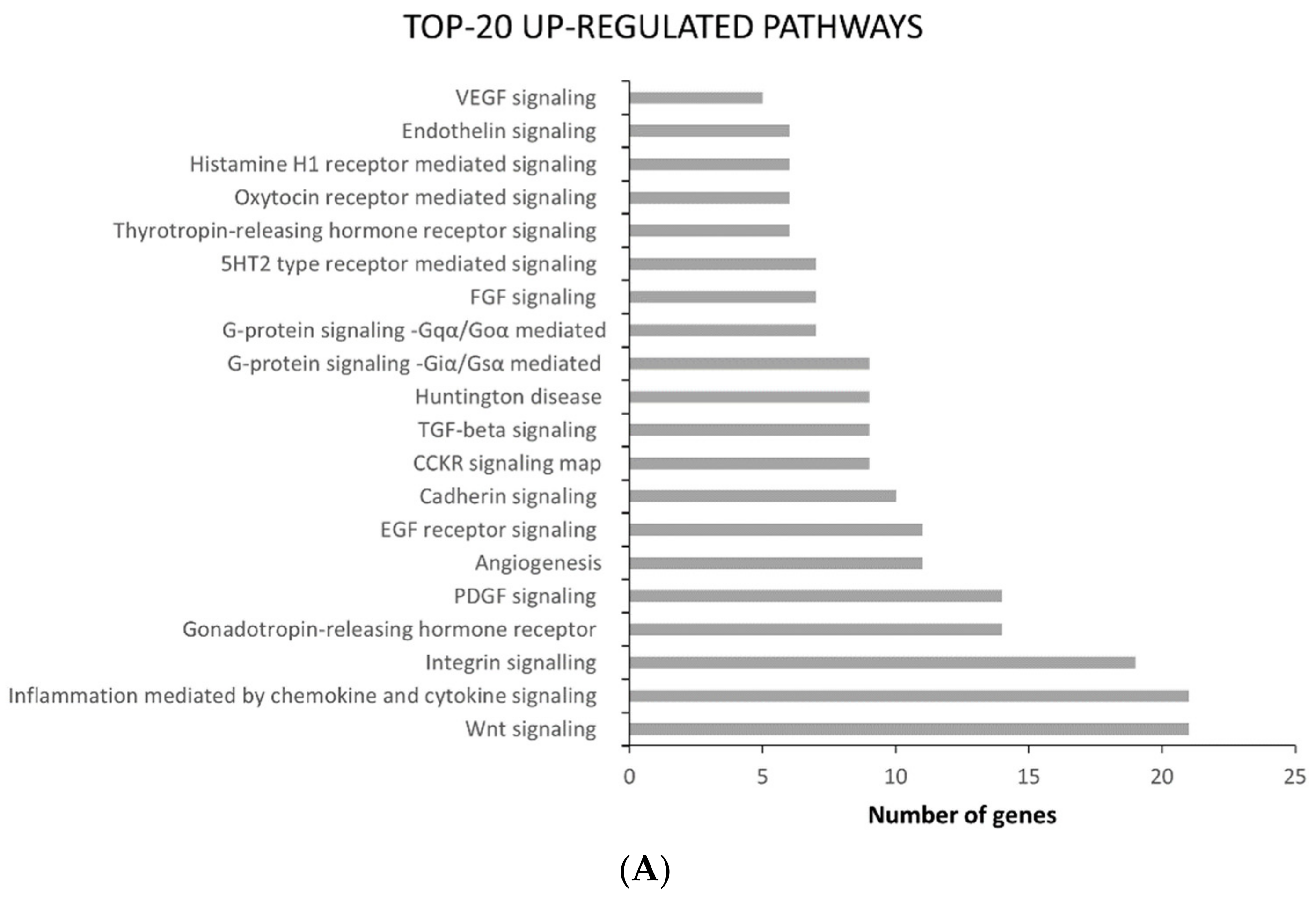
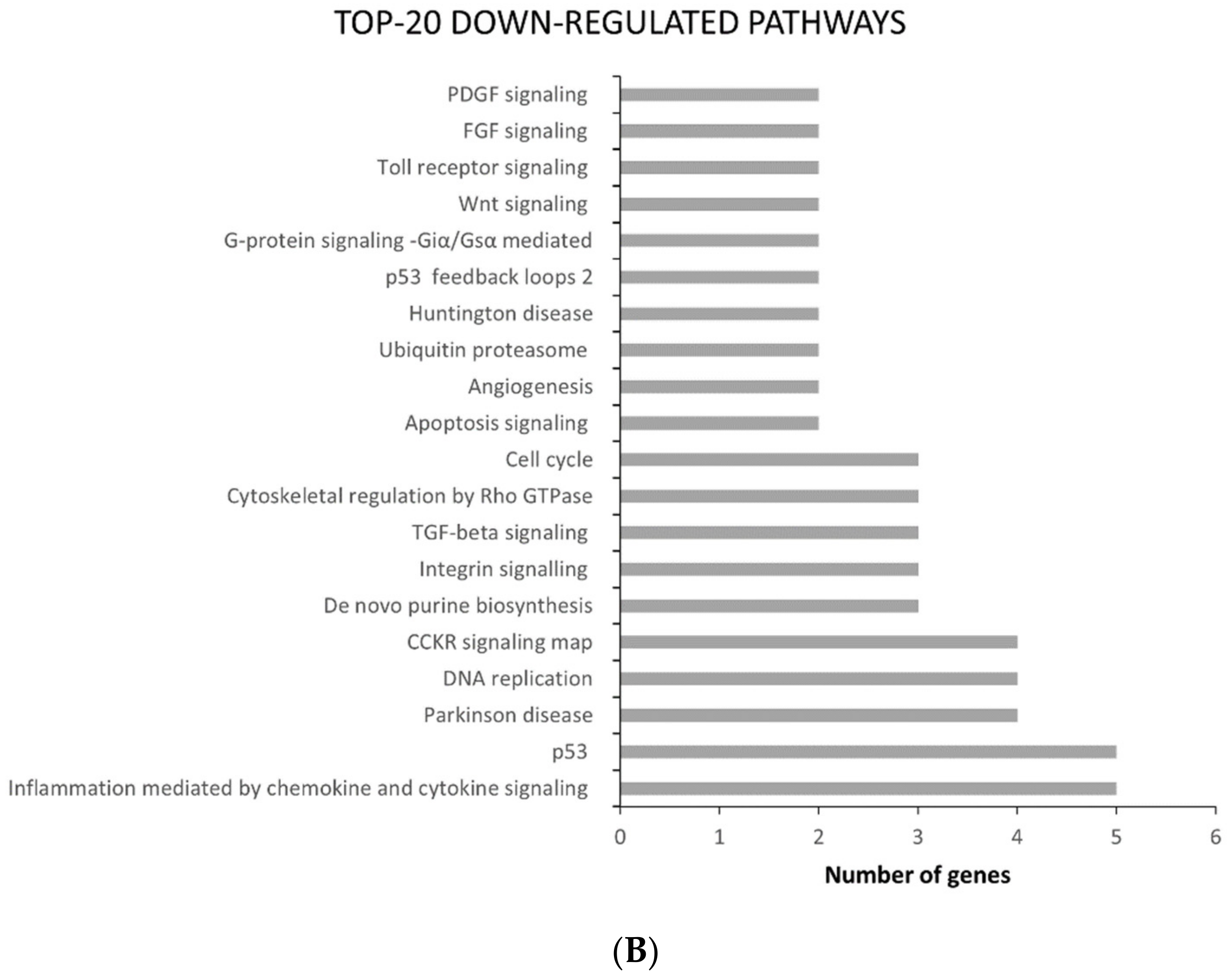
2.2. ATC and ATC-PTX Cell Lines Gene Expression Analysis
2.3. Activation of STAT 3 and ERK in Wild Type and Paclitaxel-Resistant ATC Cells
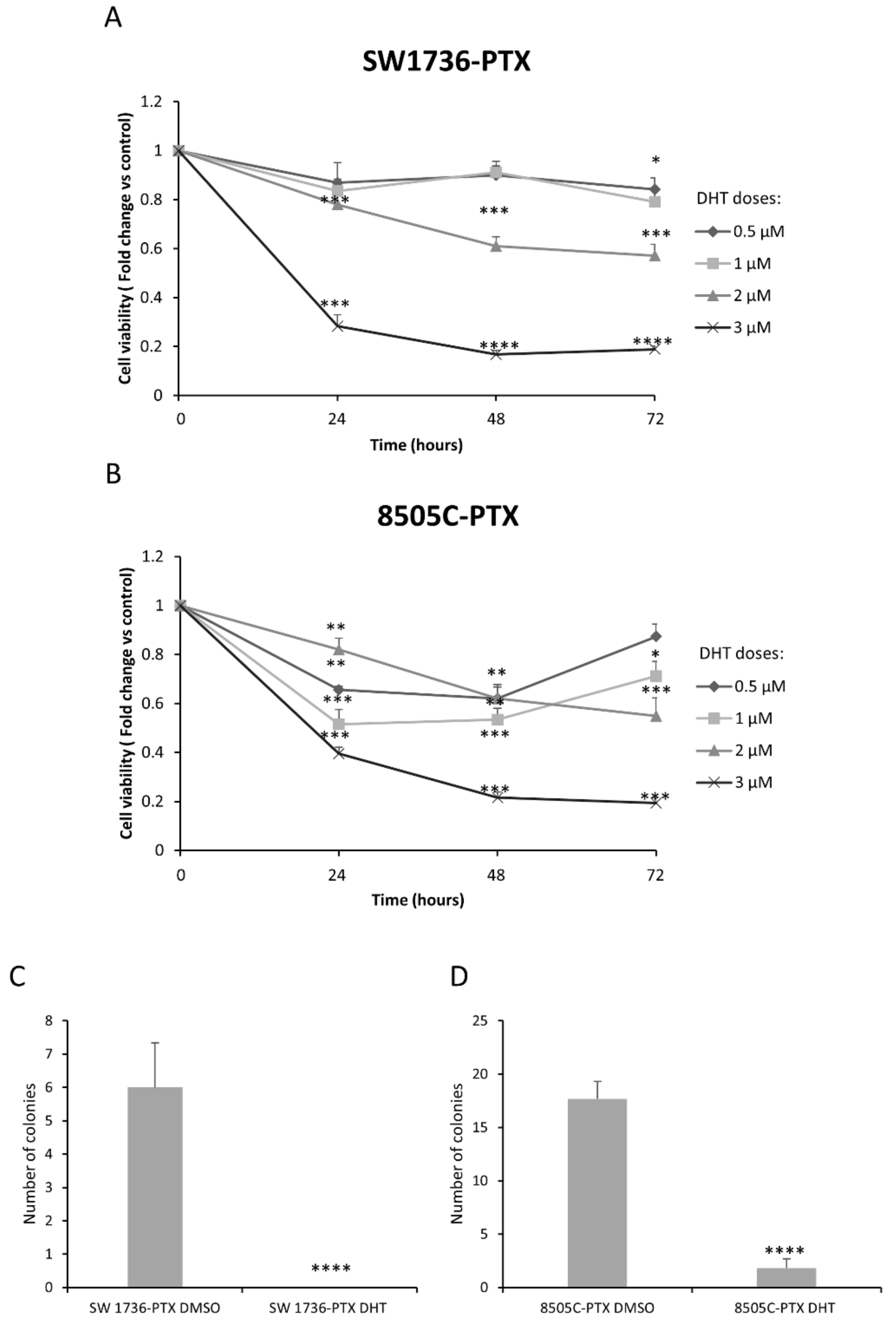
2.4. Effects of DHT on Cell Viability and Cell Colony Formation Ability in ATC-PTX Cell Lines
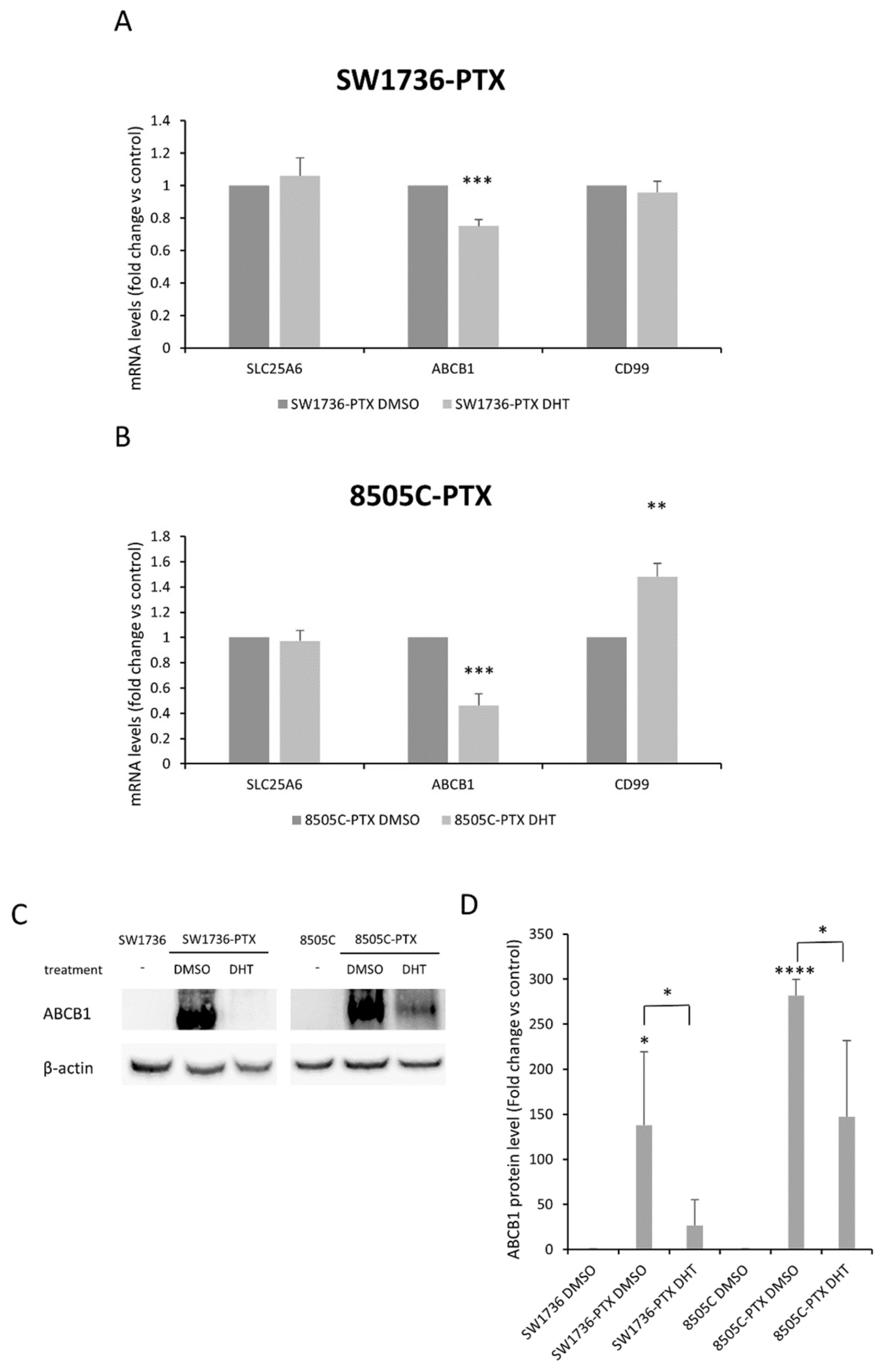
2.5. Effects of DHT on Gene and Protein Expression in ATC-PTX Cells
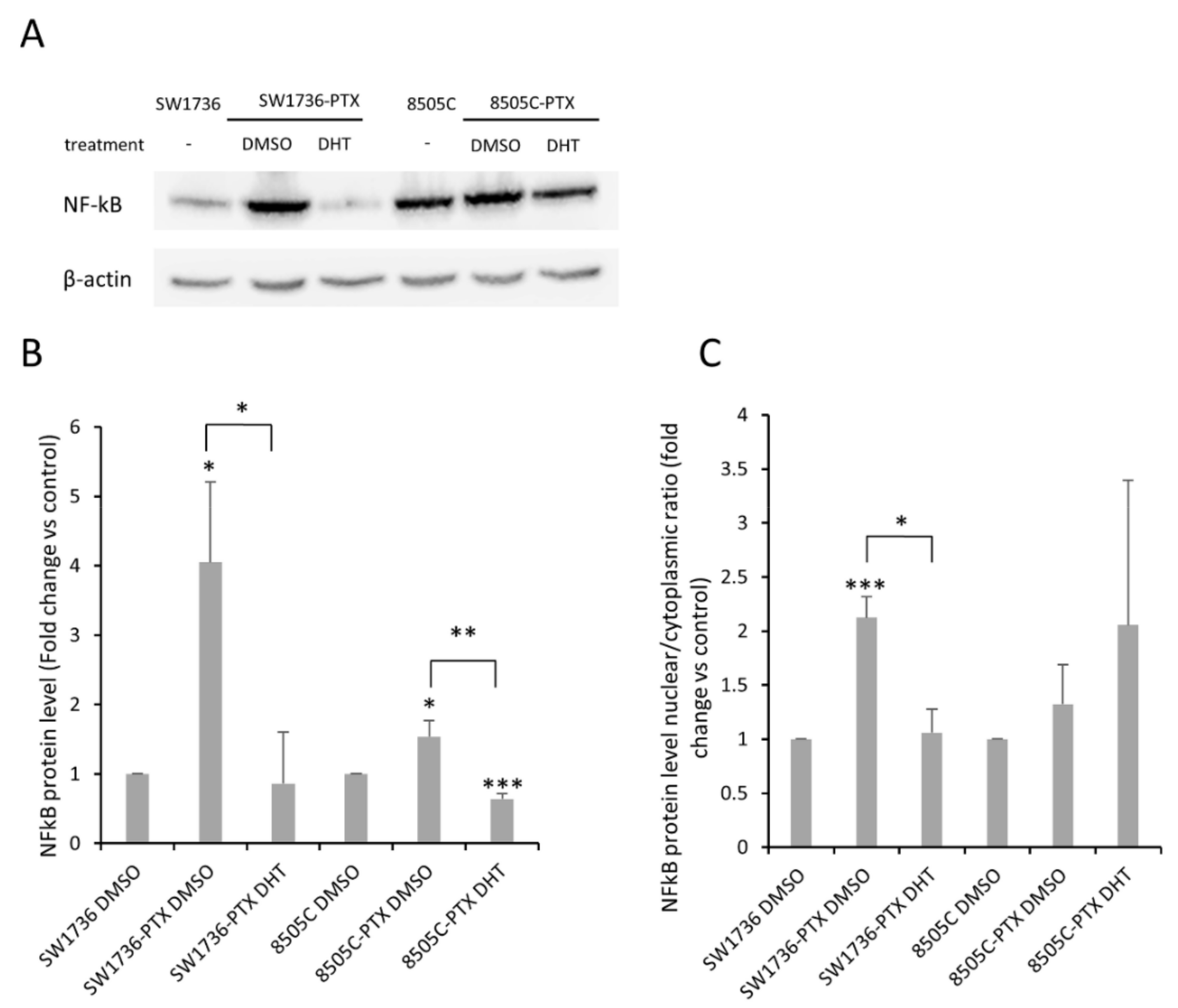
2.6. Effects of DHT on NF-kB Levels and Activation in ATC-PTX Cell Lines
3. Discussion
4. Methods
4.1. Cell Lines
4.2. Cell Viability
4.3. High-Throughput RNA Sequencing and Analysis
4.4. Gene Expression Assay
4.5. Western Blot Analysis
4.6. Soft Agar Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Shaha, A.R. Anaplastic Thyroid Cancer. Oral Oncol. 2013, 49, 702–706. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Wong, D.J. Perspectives on the Treatment of Advanced Thyroid Cancer: Approved Therapies, Resistance Mechanisms, and Future Directions. Front. Oncol. 2021, 10, 592202. [Google Scholar] [CrossRef] [PubMed]
- Alobuia, W.; Gillis, A.; Kebebew, E. Contemporary Management of Anaplastic Thyroid Cancer. Curr. Treat. Options Oncol. 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of Oncogenesis and Cancer Therapy Resistance by the Transcription Factor NF-KappaB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Abdin, S.M.; Tolba, M.F.; Zaher, D.M.; Omar, H.A. Nuclear Factor-κB Signaling Inhibitors Revert Multidrug-Resistance in Breast Cancer Cells. Chem. Biol. Interact. 2021, 340, 109450. [Google Scholar] [CrossRef]
- Pacifico, F.; Leonardi, A. Role of NF-κB in Thyroid Cancer. Mol. Cell. Endocrinol. 2010, 321, 29–35. [Google Scholar] [CrossRef]
- Pacifico, F.; Mauro, C.; Barone, C.; Crescenzi, E.; Mellone, S.; Monaco, M.; Chiappetta, G.; Terrazzano, G.; Liguoro, D.; Vito, P.; et al. Oncogenic and Anti-Apoptotic Activity of NF-κB in Human Thyroid Carcinomas. J. Biol. Chem. 2004, 279, 54610–54619. [Google Scholar] [CrossRef]
- Wu, C.-P.; Ohnuma, S.; Ambudkar, S.V. Discovering Natural Product Modulators to Overcome Multidrug Resistance in Cancer Chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef]
- Sui, H.; Fan, Z.-Z.; Li, Q. Signal Transduction Pathways and Transcriptional Mechanisms of ABCB1/Pgp-Mediated Multiple Drug Resistance in Human Cancer Cells. J. Int. Med. Res. 2012, 40, 426–435. [Google Scholar] [CrossRef]
- Breier, A.; Gibalova, L.; Seres, M.; Barancik, M.; Sulova, Z. New Insight into P-Glycoprotein as a Drug Target. Anti-Cancer Agents Med. Chem. 2013, 13, 159–170. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Gao, Y.; Feng, Y.; Guan, Y.; Zhang, Z.; Mankin, H.; Hornicek, F.J.; Duan, Z. Nsc23925 Prevents the Development of Paclitaxel Resistance by Inhibiting the Introduction of P-Glycoprotein and Enhancing Apoptosis. Int. J. Cancer 2015, 137, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Tiriveedhi, V. Perplexing Role of P-Glycoprotein in Tumor Microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Abbasifarid, E.; Sajjadi-Jazi, S.M.; Beheshtian, M.; Samimi, H.; Larijani, B.; Haghpanah, V. The Role of ATP-Binding Cassette Transporters in the Chemoresistance of Anaplastic Thyroid Cancer: A Systematic Review. Endocrinology 2019, 160, 2015–2023. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, D.; Xu, S.; Brabant, G.; Derwahl, M. Doxorubicin Fails to Eradicate Cancer Stem Cells Derived from Anaplastic Thyroid Carcinoma Cells: Characterization of Resistant Cells. Int. J. Oncol. 2010, 37, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosseini, A.; Ghorbani, A. Cancer Therapy with Phytochemicals: Evidence from Clinical Studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar] [PubMed]
- Celano, M.; Maggisano, V.; Lepore, S.M.; Russo, D.; Bulotta, S. Secoiridoids of Olive and Derivatives as Potential Coadjuvant Drugs in Cancer: A Critical Analysis of Experimental Studies. Pharmacol. Res. 2019, 142, 77–86. [Google Scholar] [CrossRef]
- Allegri, L.; Rosignolo, F.; Mio, C.; Filetti, S.; Baldan, F.; Damante, G. Effects of Nutraceuticals on Anaplastic Thyroid Cancer Cells. J. Cancer Res. Clin. Oncol. 2017, 144, 285–294. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; Bulotta, S.; Allegri, L.; Pecce, V.; Abballe, L.; Damante, G.; Russo, D. Quercetin Improves the Effects of Sorafenib on Growth and Migration of Thyroid Cancer Cells. Endocrine 2020, 67, 496–498. [Google Scholar] [CrossRef]
- Bulotta, S.; Capriglione, F.; Celano, M.; Pecce, V.; Russo, D.; Maggisano, V. Phytochemicals in Thyroid Cancer: Analysis of the Preclinical Studies. Endocrine 2021, 73, 8–15. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Zhong, B.; Lu, J.; Lu, J.-J.; Li, S.; Lu, Y. Pharmacological Activities of Dihydrotanshinone I, a Natural Product from Salvia Miltiorrhiza Bunge. Pharmacol. Res. 2019, 145, 104254. [Google Scholar] [CrossRef]
- Cheng, R.; Chen, J.; Wang, Y.; Ge, Y.; Huang, Z.; Zhang, G. Dihydrotanshinone Induces Apoptosis of SGC7901 and MGC803 Cells via Activation of JNK and P38 Signalling Pathways. Pharm. Biol. 2016, 54, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; He, Y.; Du, H.; Zhan, Z.; Zhao, H.; Shi, J.; Ye, Q.; Hu, J. 15,16-Dihydrotanshinone I Induces Apoptosis and Inhibits the Proliferation, Migration of Human Osteosarcoma Cell Line 143B in Vitro. Anti-Cancer Agents Med. Chem. 2017, 17, 1234–1242. [Google Scholar] [CrossRef]
- Allegri, L.; Domenis, R.; Navarra, M.; Celano, M.; Russo, D.; Capriglione, F.; Damante, G.; Baldan, F. Dihydrotanshinone Exerts Antitumor Effects and Improves the Effects of Cisplatin in Anaplastic Thyroid Cancer Cells. Oncol. Rep. 2021, 46, 204. [Google Scholar] [CrossRef]
- Liu, J.-J.; Wu, H.-H.; Chen, T.-H.; Leung, W.; Liang, Y.-C. 15,16-Dihydrotanshinone I from the Functional Food Salvia Miltiorrhiza Exhibits Anticancer Activity in Human HL-60 Leukemia Cells: In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2015, 16, 19387–19400. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Wang, K.S.; Mi, C.; Lee, J.J.; Jin, X. Blockade of TNF-α-Induced NF-κB Signaling Pathway and Anti-Cancer Therapeutic Response of Dihydrotanshinone I. Int. Immunopharmacol. 2015, 28, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; To, K.K.W.; Wang, L.; Zhang, L.; Lu, L.; Shen, J.; Chan, R.L.Y.; Li, M.; Yeung, J.H.K.; Cho, C.H. Reversal of P-Glycoprotein (P-Gp) Mediated Multidrug Resistance in Colon Cancer Cells by Cryptotanshinone and Dihydrotanshinone of Salvia Miltiorrhiza. Phytomedicine 2014, 21, 1264–1272. [Google Scholar] [CrossRef]
- Han, H.; Cho, J.-W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: An Expanded Reference Database of Human and Mouse Transcriptional Regulatory Interactions. Nucleic Acids Res. 2018, 46, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Costante, G.; Russo, D. Novel Therapeutic Options for Radioiodine-Refractory Thyroid Cancer: Redifferentiation and Beyond. Curr. Opin. Oncol. 2020, 32, 13–19. [Google Scholar] [CrossRef]
- Clémençon, B.; Babot, M.; Trézéguet, V. The Mitochondrial ADP/ATP Carrier (SLC25 Family): Pathological Implications of Its Dysfunction. Mol. Asp. Med. 2013, 34, 485–493. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Li, H.; Wu, S.; Liu, Z. Nrf2 induced cisplatin resistance in ovarian cancer by promoting CD99 expression. Biochem. Biophys. Res. Commun. 2019, 518, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. The P-Glycoprotein Multidrug Transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef]
- Van Waterschoot, R.A.B.; Lagas, J.S.; Wagenaar, E.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Individual and Combined Roles of CYP3A, P-Glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) in the Pharmacokinetics of Docetaxel. Int. J. Cancer 2010, 127, 2959–2964. [Google Scholar] [CrossRef]
- Sharom, F.J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Future Med. 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Sosonkina, N.; Starenki, D.; Park, J.-I. The Role of STAT3 in Thyroid Cancer. Cancers 2014, 6, 526–544. [Google Scholar] [CrossRef]
- Allegri, L.; Baldan, F.; Mio, C.; Puppin, C.; Russo, D.; Kryštof, V.; Damante, G. Effects of BP-14, a Novel Cyclin-Dependent Kinase Inhibitor, on Anaplastic Thyroid Cancer Cells. Oncol. Rep. 2016, 35, 2413–2418. [Google Scholar] [CrossRef]
- Shin, H.-J.; Hwang, K.-A.; Choi, K.-C. Antitumor Effect of Various Phytochemicals on Diverse Types of Thyroid Cancers. Nutrientes 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Allegri, L.; Mio, C.; Russo, D.; Filetti, S.; Baldan, F. Effects of HuR Downregulation on Anaplastic Thyroid Cancer Cells. Oncol. Lett. 2017, 15, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Allegri, L.; Baldan, F.; Roy, S.; Aubé, J.; Russo, D.; Filetti, S.; Damante, G. The HuR CMLD-2 Inhibitor Exhibits Antitumor Effects via MAD2 Downregulation in Thyroid Cancer Cells. Sci. Rep. 2019, 9, 7374. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Lee, S.-H. Biological Activity of Dihydrotanshinone I: Effect on Apoptosis. J. Biosci. Bioeng. 2000, 89, 292–293. [Google Scholar] [CrossRef]
- Meng, Z.; Mitsutake, N.; Nakashima, M.; Starenki, D.; Matsuse, M.; Takakura, S.; Namba, H.; Saenko, V.; Umezawa, K.; Ohtsuru, A.; et al. Dehydroxymethylepoxyquinomicin, a Novel Nuclear Factor-κB Inhibitor, Enhances Antitumor Activity of Taxanes in Anaplastic Thyroid Cancer Cells. Endocrinology 2008, 149, 5357–5365. [Google Scholar] [CrossRef]
- Shiraiwa, K.; Matsuse, M.; Nakazawa, Y.; Ogi, T.; Suzuki, K.; Saenko, V.; Xu, S.; Umezawa, K.; Yamashita, S.; Tsukamoto, K.; et al. JAK/STAT3 and NF-κB Signaling Pathways Regulate Cancer Stem-Cell Properties in Anaplastic Thyroid Cancer Cells. Thyroid 2019, 29, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Lepore, S.M.; Maggisano, V.; Lombardo, G.E.; Maiuolo, J.; Mollace, V.; Bulotta, S.; Russo, D.; Celano, M. Antiproliferative Effects of Cynaropicrin on Anaplastic Thyroid Cancer Cells. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Lee, S.-H.; Yang, M.-K.; Lee, J.-J.; Song, M.-J.; Ryu, S.-Y.; Chung, H.-J.; Won, H.-S.; Lee, C.-S.; Kwon, S.-H.; et al. 15,16-Dihydrotanshinone I, a Major Component from Salvia Miltiorrhiza Bunge (Dansham), Inhibits Rabbit Platelet Aggregation by Suppressing Intracellular Calcium Mobilization. Arch. Pharm. Res. 2008, 31, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Scalabrin, S.; Morgante, M.; Giorgi, F.M. An Extensive Evaluation of Read Trimming Effects on Illumina NGS Data Analysis. PLoS ONE 2013, 8, e85024. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformation 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Mio, C.; Lavarone, E.; Conzatti, K.; Baldan, F.; Toffoletto, B.; Puppin, C.; Filetti, S.; Durante, C.; Russo, D.; Orlacchio, A.; et al. MCM5 as a Target of BET Inhibitors in Thyroid Cancer Cells. Endocr. Relat. Cancer 2016, 23, 335–347. [Google Scholar] [CrossRef]


| Top-10 Up-Regulated Genes | Top-10 Down-Regulated Genes | ||||
|---|---|---|---|---|---|
| Gene ID | SW1736 FPKM | SW1736-PTX FPKM | Gene ID | SW1736 FPKM | SW1736-PTX FPKM |
| SLC25A6 | 0 | 1068.014282 | CRIP1 | 121.2277 | 0 |
| CD99 | 0 | 438.171265 | INHBA | 120.7393 | 0 |
| SFRP1 | 0 | 353.209839 | G0S2 | 110.1006 | 0 |
| ABCB1 | 0 | 302.292755 | TMEM200A | 80.16118 | 0 |
| ASS1 | 0 | 272.908447 | MT1L | 70.32181 | 0 |
| MMP1 | 0 | 253.921478 | CDCA7L | 64.91097 | 0 |
| KISS1 | 0 | 227.140152 | PEG10 | 59.8796 | 0 |
| CD24 | 0 | 161.055496 | CDH13 | 56.48613 | 0 |
| LAMA5 | 0 | 128.902344 | GNG11 | 48.80974 | 0 |
| EPB41L3 | 0 | 111.837128 | LIF | 47.06105 | 0 |
| Top-10 Up-Regulated Genes | Top-10 Down-Regulated Genes | ||||
|---|---|---|---|---|---|
| Gene ID | 8505C FPKM | 8505C-PTX FPKM | Gene ID | 8505C FPKM | 8505C-PTX FPKM |
| SLC25A6 | 0 | 906.9684 | PSMD5 | 4.736901 | 0 |
| ABCB1 | 0 | 497.1158 | STEAP1 | 4.928442 | 0 |
| CD99 | 0 | 344.5431 | VEPH1 | 5.764932 | 0 |
| NUPR1 | 0 | 117.2764 | NLGN4X | 6.481269 | 0 |
| ASMTL | 0 | 44.84652 | IL1RL1 | 7.034636 | 0 |
| ERV3-1 | 0 | 42.39337 | RPL36A-HNRNPH2 | 7.240747 | 0 |
| ALPPL2 | 0 | 36.63164 | CDCP1 | 8.863821 | 0 |
| WFDC21P | 0 | 33.00259 | ZNF702P | 8.940498 | 0 |
| ZNF117 | 0 | 31.36288 | UCHL1 | 24.07678 | 0 |
| PIEZO2 | 0 | 28.97086 | CBWD5 | 67.27538 | 0 |
| Key TF | Description | # of Overlapped Genes |
|---|---|---|
| SP1 | Sp1 transcription factor | 10 |
| NFKB1 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | 8 |
| SPI1 | spleen focus forming virus (SFFV) proviral integration oncogene spi1 | 5 |
| WT1 | Wilms tumor 1 | 4 |
| STAT1 | signal transducer and activator of transcription 1, 91 kDa | 4 |
| JUN | jun proto-oncogene | 4 |
| TP53 | tumor protein p53 | 4 |
| RFXANK | regulatory factor X-associated ankyrin-containing protein | 3 |
| RFXAP | regulatory factor X-associated protein | 3 |
| RFX5 | regulatory factor X, 5 | 3 |
| Key TF | Description | # of Overlapped Genes |
|---|---|---|
| E2F1 | E2F transcription factor 1 | 12 |
| SP1 | Sp1 transcription factor | 10 |
| TP53 | tumor protein p53 | 7 |
| MYCN | v-myc myelocytomatosis viral related oncogene, | 5 |
| MYC | v-myc myelocytomatosis viral oncogene homolog | 5 |
| NFKB1 | nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | 5 |
| SRF | serum response factor | 4 |
| BRCA1 | breast cancer 1, early onset | 4 |
| TFDP1 | transcription factor Dp-1 | 3 |
| RB1 | retinoblastoma 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegri, L.; Capriglione, F.; Maggisano, V.; Damante, G.; Baldan, F. Effects of Dihydrotanshinone I on Proliferation and Invasiveness of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells. Int. J. Mol. Sci. 2021, 22, 8083. https://doi.org/10.3390/ijms22158083
Allegri L, Capriglione F, Maggisano V, Damante G, Baldan F. Effects of Dihydrotanshinone I on Proliferation and Invasiveness of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells. International Journal of Molecular Sciences. 2021; 22(15):8083. https://doi.org/10.3390/ijms22158083
Chicago/Turabian StyleAllegri, Lorenzo, Francesca Capriglione, Valentina Maggisano, Giuseppe Damante, and Federica Baldan. 2021. "Effects of Dihydrotanshinone I on Proliferation and Invasiveness of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells" International Journal of Molecular Sciences 22, no. 15: 8083. https://doi.org/10.3390/ijms22158083
APA StyleAllegri, L., Capriglione, F., Maggisano, V., Damante, G., & Baldan, F. (2021). Effects of Dihydrotanshinone I on Proliferation and Invasiveness of Paclitaxel-Resistant Anaplastic Thyroid Cancer Cells. International Journal of Molecular Sciences, 22(15), 8083. https://doi.org/10.3390/ijms22158083







