Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs
Abstract
1. Introduction
2. Results
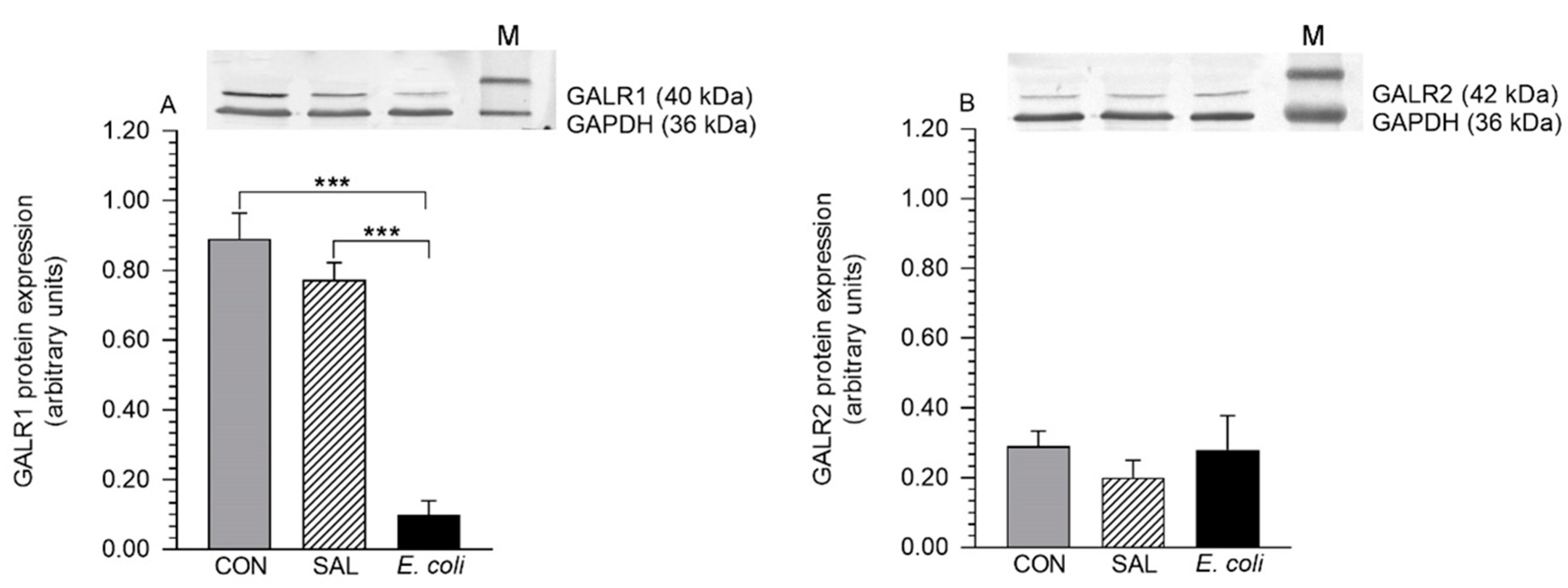
2.1. Expression of GALR1 and GALR2 Proteins
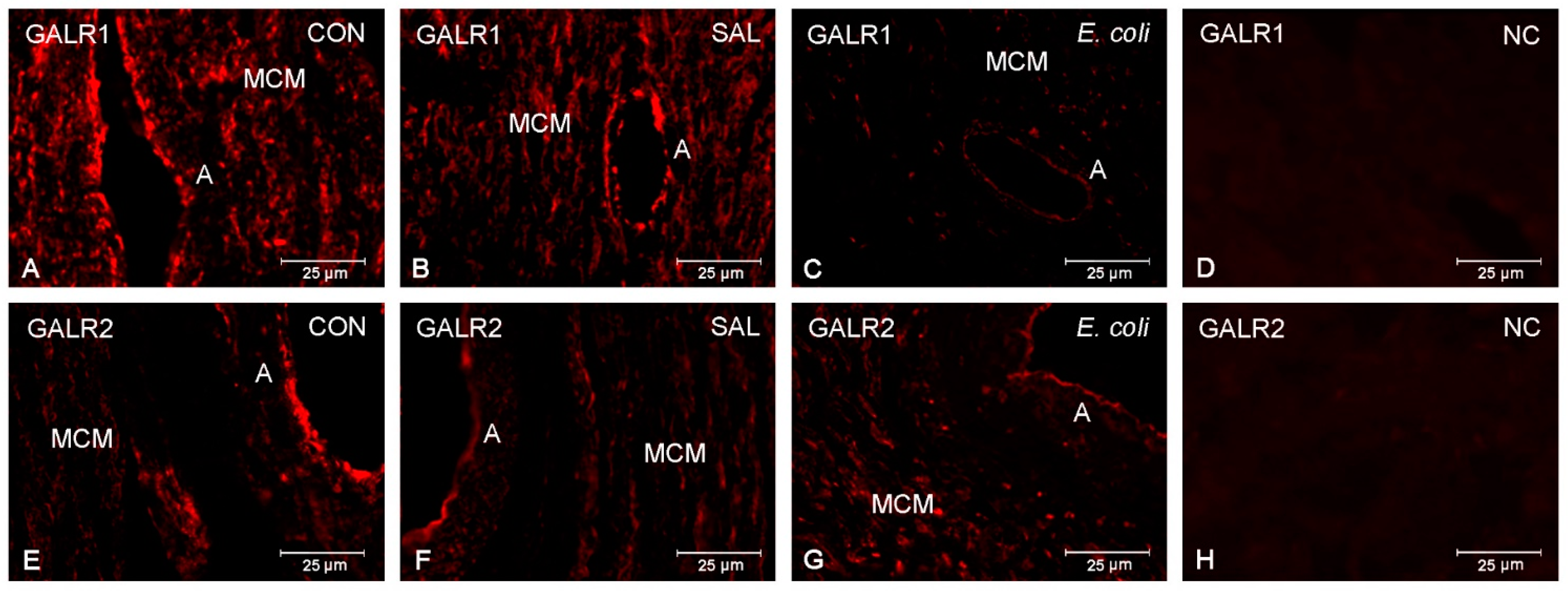
2.2. Distribution of GALR1 and GALR2
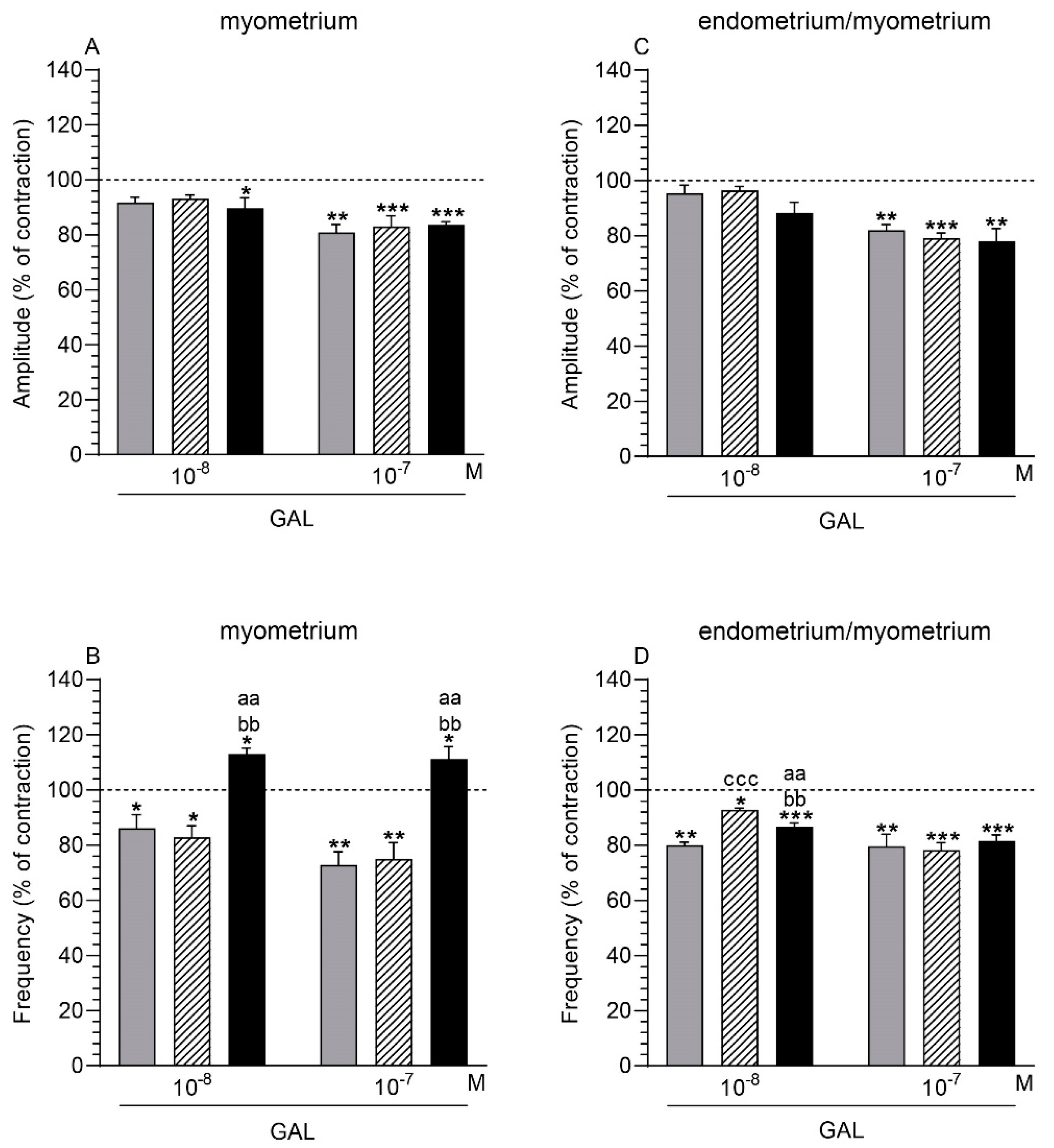
2.3. GAL Influence on the Myometrium Contractility
2.3.1. Comparison of the GAL Influence to the Period before Its Application
2.3.2. Comparison of the GAL Influence between Groups
2.4. GAL Influence on the Endometrium/Myometrium Contractility
2.4.1. Comparison of the GAL Influence to the Period before its Application
2.4.2. Comparison of the GAL Influence between Groups
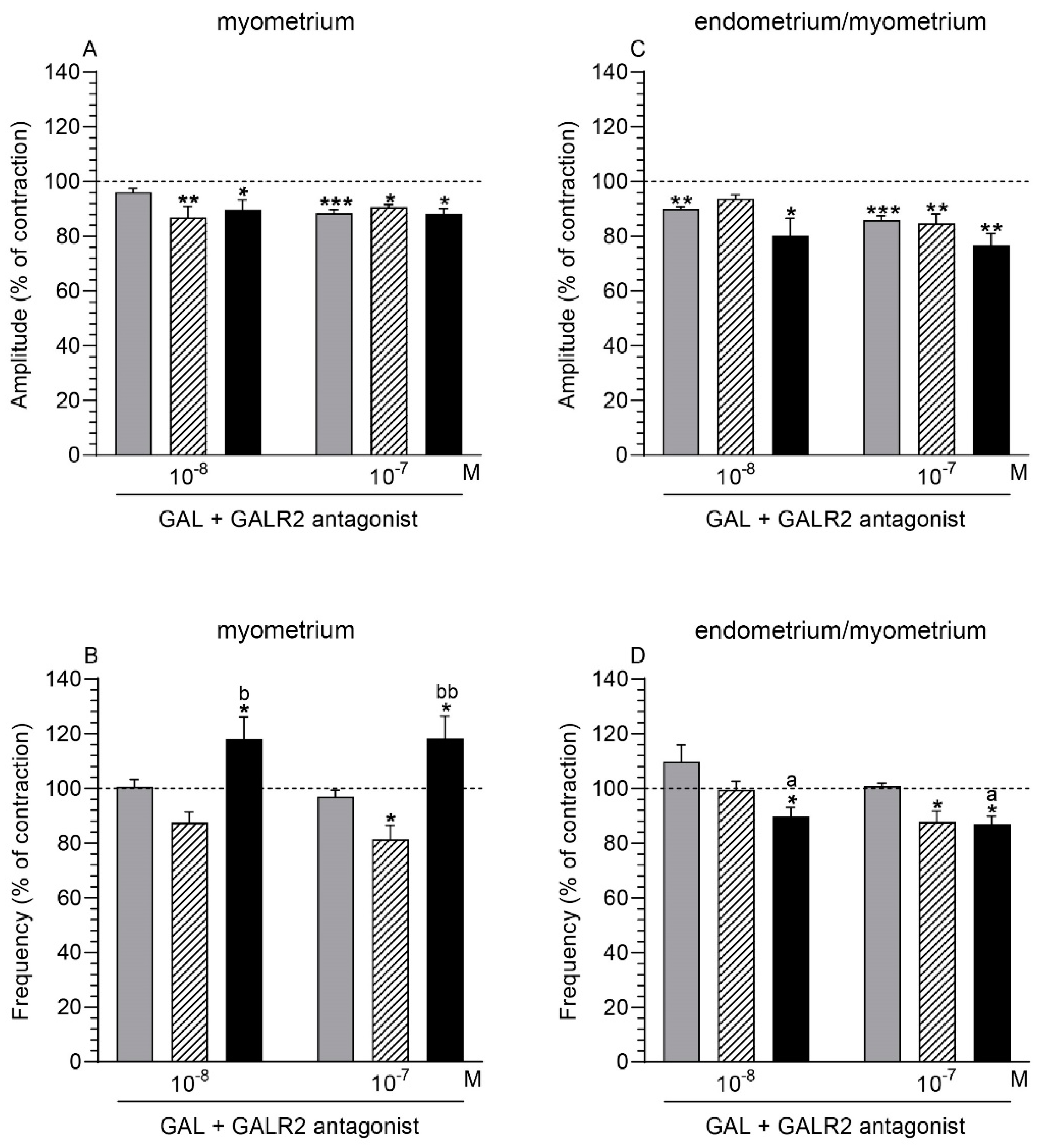
2.5. GALR2 Antagonist and GAL Influence on the Myometrium Contractility
2.5.1. Comparison of the Antagonist and GAL Influence to the Period before Their Application
2.5.2. Comparison of the Antagonist and GAL Influence between Groups
2.6. GALR2 Antagonist and GAL Influence on the Endometrium/Myometrium Contractility
2.6.1. Comparison of the Antagonist and GAL Influence to the Period before Their Application
2.6.2. Comparison of the Antagonist and GAL Influence between Groups
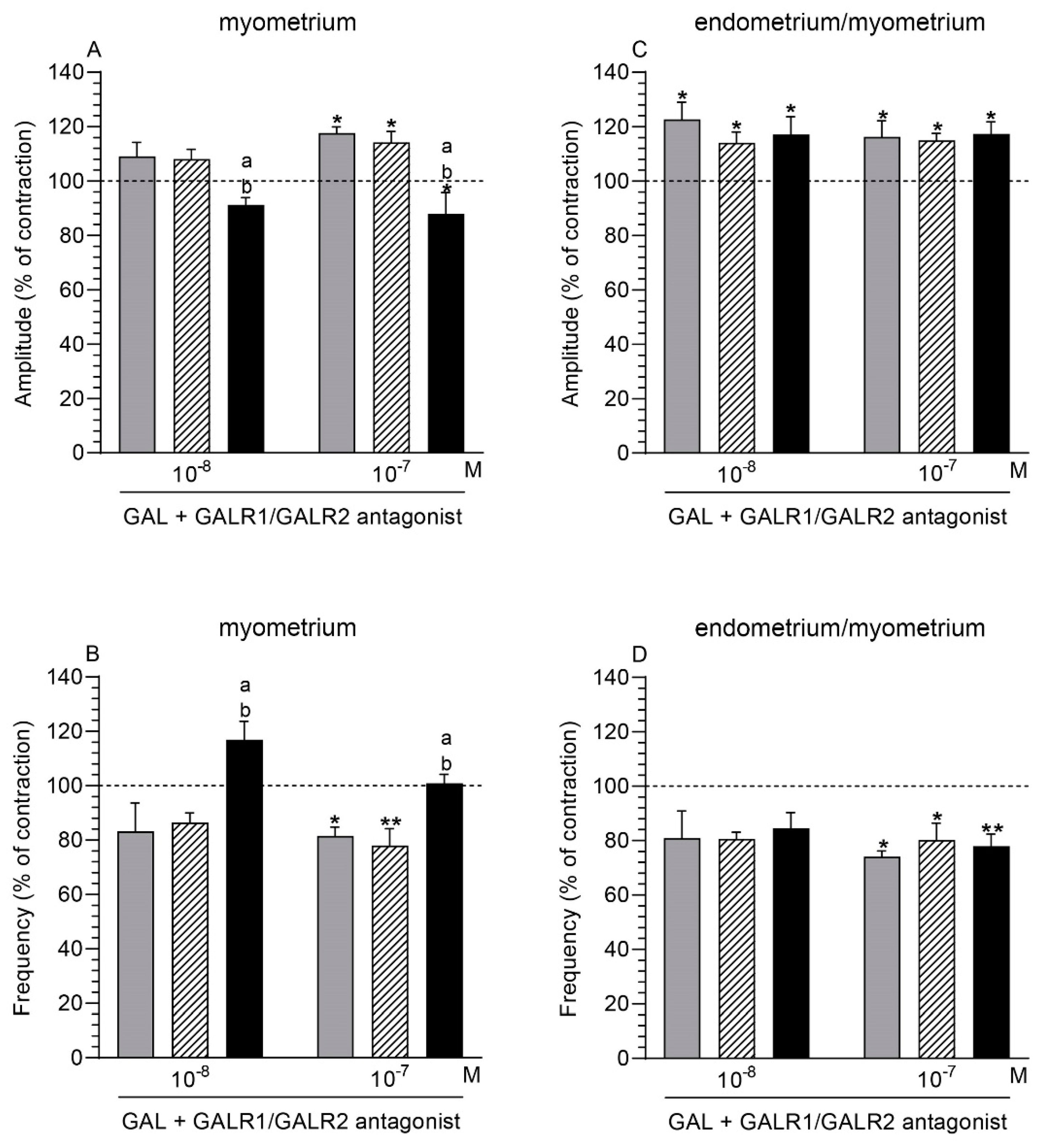
2.7. GALR1/GALR2 Antagonist and GAL Influence on the Myometrium Contractility
2.7.1. Comparison of the Antagonist and GAL Influence to the Period before Their Application
2.7.2. Comparison of the Antagonist and GAL Influence between Groups
2.8. GALR1/GALR2 Antagonist and GAL Influence on the Endometrium/Myometrium Contractility
2.8.1. Comparison of the Antagonist and GAL Influence to the Period before Their Application
2.8.2. Comparison of the Antagonist and GAL Influence between Groups
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Procedures
4.3. Western Blot Analysis
4.4. Immunofluorescence
4.5. Preparation of the Stripes from the Uteri and Measurement of Isometric Contractile Function
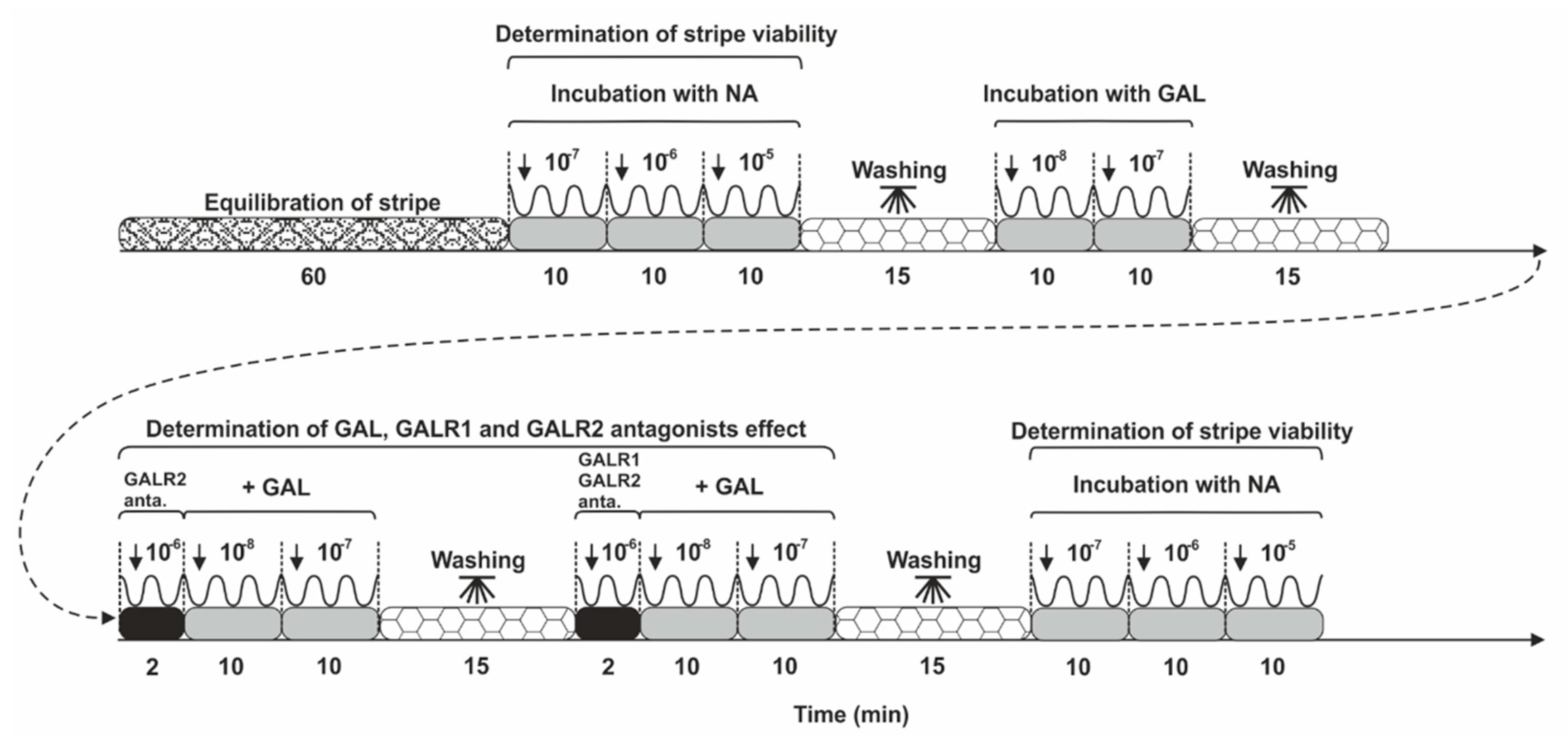
4.6. Contractility Study Schedule
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tummaruk, P.; Kesdangsakonwut, S.; Prapasarakul, N.; Kaeoket, K. Endometritis in gilts: Reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp. Haematol. Int. 2009, 19, 575–584. [Google Scholar] [CrossRef]
- Mateus, L.; Lopes da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerpertal endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Turner, M.; Healey, G.; Sheldon, M. Immunity and Inflammation in the Uterus. Reprod. Domest. Anim. 2012, 47, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Shynlova, O.; Lee, Y.H.; Srikhajon, K.; Lye, S.J. Physiologic uterine inflammation and labor onset: Integration of endocrine and mechanical signals. Reprod. Sci. 2013, 20, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Mordak, R.; Stewart, P.A. Periparturient stress and immune suppression as a potential cause of retained placenta in highly productive dairy cows: Examples of prevention. Acta Vet. Scand. 2015, 57, 1–8. [Google Scholar] [CrossRef]
- Heppelmann, M.; Weinert, M.; Ulbrich, S.; Brömmling, A.; Piechotta, M.; Merbach, S.; Schoon, H.-A.; Hoedemaker, M.; Bollwein, H.; Information, P.E.K.F.C. The effect of puerperal uterine disease on histopathologic findings and mRNA expression of proinflammatory cytokines of the endometrium in dairy cows. Theriogenology 2016, 85, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Owens, S.-E.; Turner, M. Innate immunity and the sensing of infection, damage and danger in the female genital tract. J. Reprod. Immunol. 2017, 119, 67–73. [Google Scholar] [CrossRef]
- Barański, W.; Łukasik, K.; Skarżyński, D.; Sztachańska, M.; Zduńczyk, S.; Janowski, T. Secretion of prostaglandin.ns and leukotrienes by endometrial cells in cows with subclinical and clinical endometritis. Theriogenology 2013, 80, 766–772. [Google Scholar]
- Jana, B.; Kucharski, J.; Dzienis, A.; Deptuła, K. Changes in prostaglandin production and ovarian function in gilts during endometritis induced by Escherichia coli infection. Anim. Reprod. Sci. 2007, 97, 137–150. [Google Scholar] [CrossRef]
- Jana, B.; Czarzasta, J.; Jaroszewski, J. Synthesis of leukotrienes in porcine uteri with endometritis induced by infection with Escherichia coli. Reprod. Fertil. Dev. 2014, 26, 1007–1016. [Google Scholar] [CrossRef]
- Jana, B.; Jaroszewski, J.; Czarzasta, J.; Włodarczyk, M.; Markiewicz, W. Synthesis of prostacyclin and its effect on the contractile activity of the inflamed porcine uterus. Theriogenology 2013, 79, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, J.; Jaroszewski, J.; Jana, B.; Górska, J.; Kozłowska, A.; Markiewicz, W. The influence of inflammatory process on prostaglandin F2α contractile activity in porcine uterus. J. Anim. Feed. Sci. 2007, 16, 654–667. [Google Scholar] [CrossRef]
- Jana, B.; Jaroszewski, J.; Kucharski, J.; Koszykowska, M.; Górska, J.; Markiewicz, W. Participation of Prostaglandin E2 in Contractile Activity of Inflamed Porcine Uterus. Acta Vet. Brno 2010, 79, 249–259. [Google Scholar] [CrossRef]
- Jana, B.; Jaroszewski, J.J.; Czarzasta, J.; Markiewicz, W. The influence of leukotrienes C4 and D4 on the contractility of an inflamed porcine uterus. Theriogenology 2015, 83, 1328–1337. [Google Scholar] [CrossRef]
- Heppelmann, M.; Volland, J.; Pfarrer, C.; Kietzmann, M.; Bäumer, W.; Merbach, S.; Schoon, H.A.; Wellnitz, O.; Schmicke, M.; Hoedemaker, M.; et al. Effects of oxytocin and PGF2α on uterine contractility in cows with and without metri-tis-An in-vitro study. Anim. Reprod. Sci. 2018, 188, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Całka, J.; Bulc, M.; Piotrowska-Tomala, K. Participation of acetylcholine and its receptors in the contractility of inflamed porcine uterus. Theriogenology 2020, 143, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Całka, J.; Palus, K. Inflammation changes the expression of neuropeptide Y receptors in the pig myometrium and their role in the uterine contractility. PLoS ONE 2020, 15, e0236044. [Google Scholar] [CrossRef]
- ana, B.; Całka, J.; Czajkowska, M. The role of somatostatin and its receptors (sstr2, sstr5) in the contractility of gilt inflamed uterus. Res. Vet. Sci. 2020, 133, 163–173. [Google Scholar] [CrossRef]
- Palus, K.; Całka, J.; Jana, B. Alterations in the relative abundance of the vasoactive intestinal peptide receptors (VPAC1 and VPAC2) and functions in uterine contractility during inflammation. Anim. Reprod. Sci. 2021, 225, 106680. [Google Scholar] [CrossRef]
- Meller, K.; Całka, J.; Kaczmarek, M.M.; Jana, B. Expression of alpha and beta adrenergic receptors in the pig uterus during inflammation. Theriogenology 2018, 119, 96–104. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J.; Palus, K.; Sikora, M. Escherichia coli -induced inflammation changes the expression of acetylcholine receptors (M2R, M3R, and α-7 nAChR) in the pig uterus. J. Vet. Res. 2020, 64, 531–541. [Google Scholar] [CrossRef]
- Klukovits, A.; Márki, A.; Páldy, E.; Benyhe, S.; Gálik, M.; Falkay, G.; Gáspár, R. Inflammatory processes enhance cAMP-mediated uterus relaxation in the pregnant rat: The role of TNF-alpha. Naunyn-Schmiedeberg’s Arch. Pharm. 2009, 379, 501–510. [Google Scholar] [CrossRef]
- Šípková, J.; Kramáriková, I.; Hynie, S.; Klenerová, V. The Galanin and Galanin Receptor Subtypes, its Regulatory Role in the Biological and Pathological Functions. Physiol. Res. 2017, 66, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Volpe, C.M.; Vaz, T.; Rocha-Silva, F.; Villar-Delfino, P.H.; Nogueira-Machado, J.A. Is galanin a promising therapeutic resource for neural and nonneural diseases? Curr. Drug Targets 2020, 21, 922–929. [Google Scholar] [CrossRef]
- Wasowicz, K. Uterus-Innervating Neurones in Porcine Inferior Mesenteric Ganglion: An Immunohistochemical Charac-teristic. Anat. Histol. Embryol. 2003, 32, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Całka, J.; Meller, K.; Jana, B. Endometritis affects chemical coding of the dorsal root ganglia neurons supplying uterus in the sexually mature gilts. Res. Vet. Sci. 2019, 124, 417–425. [Google Scholar] [CrossRef]
- Shew, R.; Papka, R.; McNeill, D. Galanin and calcitonin gene-related peptide immunoreactivity in nerves of the rat uterus: Localization, colocalization, and effects on uterine contractility. Peptides 1992, 13, 273–279. [Google Scholar] [CrossRef]
- Rytel, L.; Gonkowski, S.; Janowski, T.; Wojtkiewicz, J.; Pomianowski, A. The neurochemical characterization of para-sympa-thetic nerve fibres in the porcine wall under physiological conditons and after exposure to bisphenol A (BPA). Neurotox. Res. 2019, 35, 867–882. [Google Scholar]
- Niiro, N.; Nishimura, J.; Hirano, K.; Nakano, H.; Kanaide, H. Mechanisms of galanin-induced contraction in the rat myome-trium. Br. J. Pharmacol. 1998, 124, 1623–1632. [Google Scholar] [CrossRef]
- Bek, T.; Ottesen, B.; Fahrenkrug, J. The effect of galanin, CGRP and ANP on spontaneous smooth muscle activity of rat uterus. Peptides 1988, 9, 497–500. [Google Scholar] [CrossRef]
- Sliwiński, W. [Action of galanin on smooth muscle strips isolated from rat and human uterus]. Ginekol. Polska 2003, 74, 210–214. [Google Scholar]
- Li, J.; Micevych, P.; McDonald, J.; Rapkin, A.; Chaban, V. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J. Neurosci. Res. 2008, 86, 2746–2752. [Google Scholar] [CrossRef]
- Jana, B.; Całka, J. Endometritis Changes the Neurochemical Characteristics of the Caudal Mesenteric Ganglion Neurons Supplying the Gilt Uterus. Animals 2020, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, L.; Lakhter, A.; Hirano, A.A.; Tonini, M.; Sternini, C. Expression of galanin receptor messenger RNAs in different regions of the rat gastrointestinal tract. Peptides 2005, 26, 815–819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for pro-teomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Matkowskyj, K.A.; Danilkovich, A.; Marrero, J.; Savkovic, S.D.; Hecht, G.; Benya, R.V. Galanin-1 receptor up-regulation mediates the excess colonic fluid production caused by infection with enteric pathogens. Nat. Med. 2000, 6, 1048–1051. [Google Scholar] [CrossRef]
- Marrero, J.A.; Matkowskyj, K.A.; Yung, K.; Hecht, G.; Benya, R.V. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G797–G804. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Shi, T.-J.; Landry, M.; Hökfelt, T. Evidence for galanin receptors in primary sensory neurones and effect of axotomy and inflammation. NeuroReport 1996, 8, 237–242. [Google Scholar] [CrossRef]
- Shi, T.-J.S.; Zhang, X.; Holmberg, K.; Xu, Z.-Q.D.; Hökfelt, T. Expression and regulation of galanin-R2 receptors in rat primary sensory neurons: Effect of axotomy and inflammation. Neurosci. Lett. 1997, 237, 57–60. [Google Scholar] [CrossRef]
- Blitek, A.; Morawska, E.; Ziecik, A. Regulation of expression and role of leukemia inhibitory factor and interleukin-6 in the uterus of early pregnant pigs. Theriogenology 2012, 78, 951–964. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Troedsson, M.H. Endo-metrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endome-tritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef]
- Hecht, G.; Marrero, J.A.; Danilkovich, A.; Matkowskyj, K.A.; Savkovic, S.D.; Koutsouris, A.; Benya, R.V. Pathogenic Esche-richia coli increase Cl- secretion from intestinal epithelia by upregulating galanin-1 receptor expression. J. Clin. Investig. 1999, 104, 253–262. [Google Scholar] [CrossRef]
- Schmidhuber, S.M.; Santic, R.; Tam, C.W.; Bauer, J.W.; Kofler, B.; Brain, S.D. Galanin-Like Peptides Exert Potent Vasoactive Functions In Vivo. J. Investig. Dermatol. 2007, 127, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Kuteeva, E.; Brumovsky, P.; Kahl, U.; Karlström, H.; Lucas, G.A.; Rodriguez, J.; Westerblad, H.; Hilke, S.; Theodorsson, E.; et al. Generation and phenotypic characterization of a galanin overexpressing mouse. Neuroscience 2005, 133, 59–77. [Google Scholar] [CrossRef]
- Chen, A.; Li, M.; Song, L.; Zhang, Y.; Luo, Z.; Zhang, W.; Chen, Y.; He, B. Effects of the galanin receptor antagonist M40 on cardiac function and remodeling in rats with heart failure. Cardiovasc. Ther. 2015, 33, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.F.; Pradhan, T.K.; Coy, D.H.; Jensen, R.T. Interaction of galanin fragments with galanin receptors on isolated smooth muscle cells from guinea pig stomach: Identification of a novel galanin receptor subtype. J. Pharmacol. Exp. Ther. 1995, 272, 371–378. [Google Scholar]
- Yamato, S.; Hirano, I.; Goyal, R.K. Effect of galanin and galanin antagonists on peristalsis in esophageal smooth muscle in the opossum. Am. J. Physiol. Liver Physiol. 2000, 279, G719–G725. [Google Scholar] [CrossRef] [PubMed]
- Balint, A.; Fehér, E.; Jr, I.K.; Mate, M.; Zelles, T.; Vizi, E.; Varga, G. Functional and immunocytochemical evidence that galanin is a physiological regulator of human jejunal motility. J. Physiol. 2001, 95, 129–135. [Google Scholar] [CrossRef]
- Wang, S.; Ghibaudi, L.; Hashemi, T.; He, C.; Strader, C.; Bayne, M.; Davis, H.; Hwa, J.J. The GalR2 galanin receptor me-diates galanin-induced jejunal contraction, but not feeding behavior, in the rat: Differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett. 1998, 434, 277–282. [Google Scholar] [CrossRef]
- Di Marzo, V.; Tippins, J.R.; Galadari, S.H.; Morris, H.R. Neuropeptides and leukotriene biosynthesis: The effect of calcitonin, peptide histidine valine-42, helodermin, neuropeptide Y and galanin. Neuropeptides 1988, 11, 169–172. [Google Scholar] [CrossRef]
- Delvaux, M.; Botella, A.; Fioramonti, J.; Frexinos, J.; Bueno, L. Galanin induces contraction of isolated cells from circular muscle layer of pig ileum. Regul. Pept. 1991, 32, 369–374. [Google Scholar] [CrossRef]
- Mensah, E.T.; Blanco, A.M.; Donini, A.; Unniappan, S. Galanin decreases spontaneous resting contractions and potentiates acetyl choline-induced contractions of goldfish gut. Neuropeptides 2018, 69, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Anselmi, L.; Guerrini, S.; Cervio, E.; Pham, T.; Balestra, B.; Vicini, R.; Baiardi, P.; D’Agostino, G.-L.; Tonini, M. Role of galanin receptor 1 in peristaltic activity in the guinea pig ileum. Neuroscience 2004, 125, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, S.; Raybould, H.E.; Anselmi, L.; Agazzi, A.; Cervio, E.; Reeve, J.R.; Jr Tonini, M.; Sternini, C. Role of galanin receptor 1 in gastric motility in rat. Neurogastroenterol. Motil. 2004, 16, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jana, B.; Całka, J.; Miciński, B. Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs. Int. J. Mol. Sci. 2021, 22, 6415. https://doi.org/10.3390/ijms22126415
Jana B, Całka J, Miciński B. Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs. International Journal of Molecular Sciences. 2021; 22(12):6415. https://doi.org/10.3390/ijms22126415
Chicago/Turabian StyleJana, Barbara, Jarosław Całka, and Bartosz Miciński. 2021. "Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs" International Journal of Molecular Sciences 22, no. 12: 6415. https://doi.org/10.3390/ijms22126415
APA StyleJana, B., Całka, J., & Miciński, B. (2021). Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs. International Journal of Molecular Sciences, 22(12), 6415. https://doi.org/10.3390/ijms22126415





