Is PTSD-Phenotype Associated with HPA-Axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats
Abstract
1. Introduction
2. Results
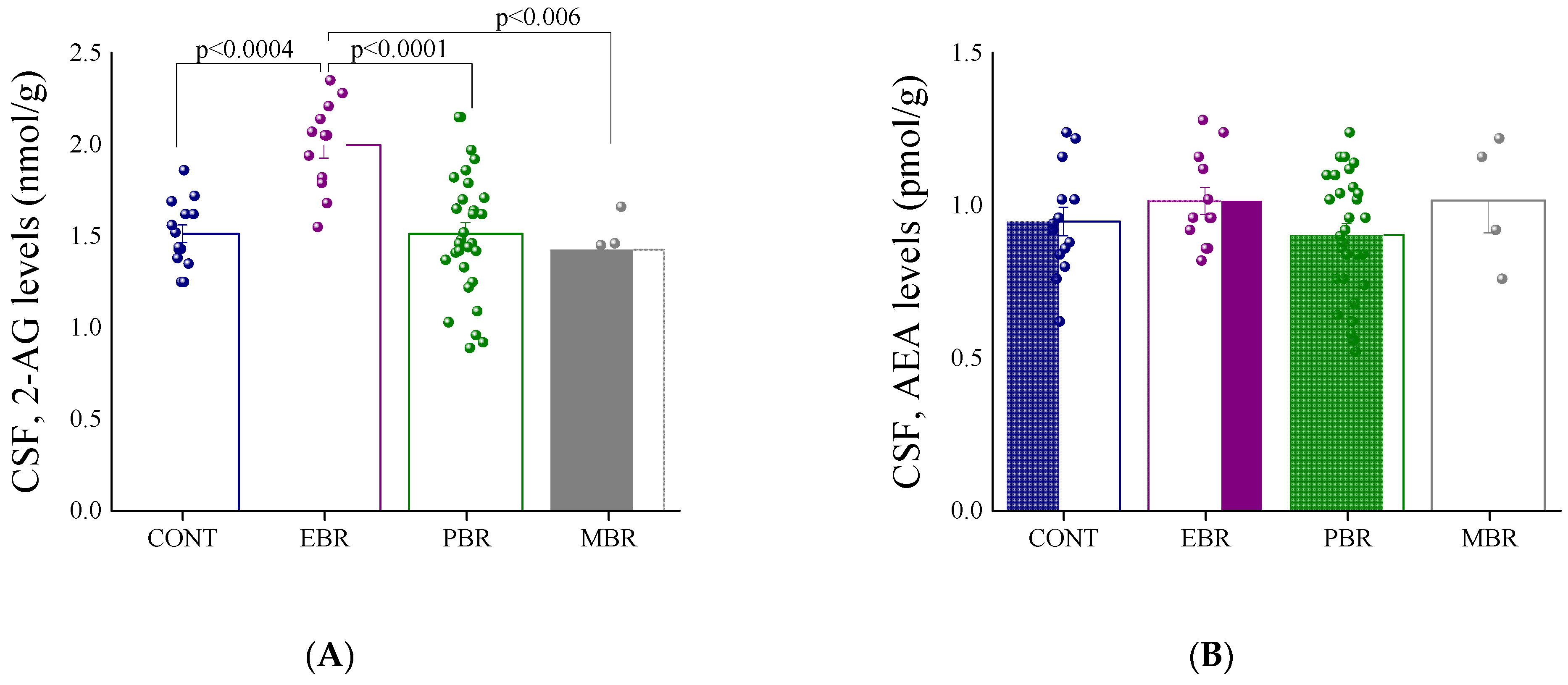
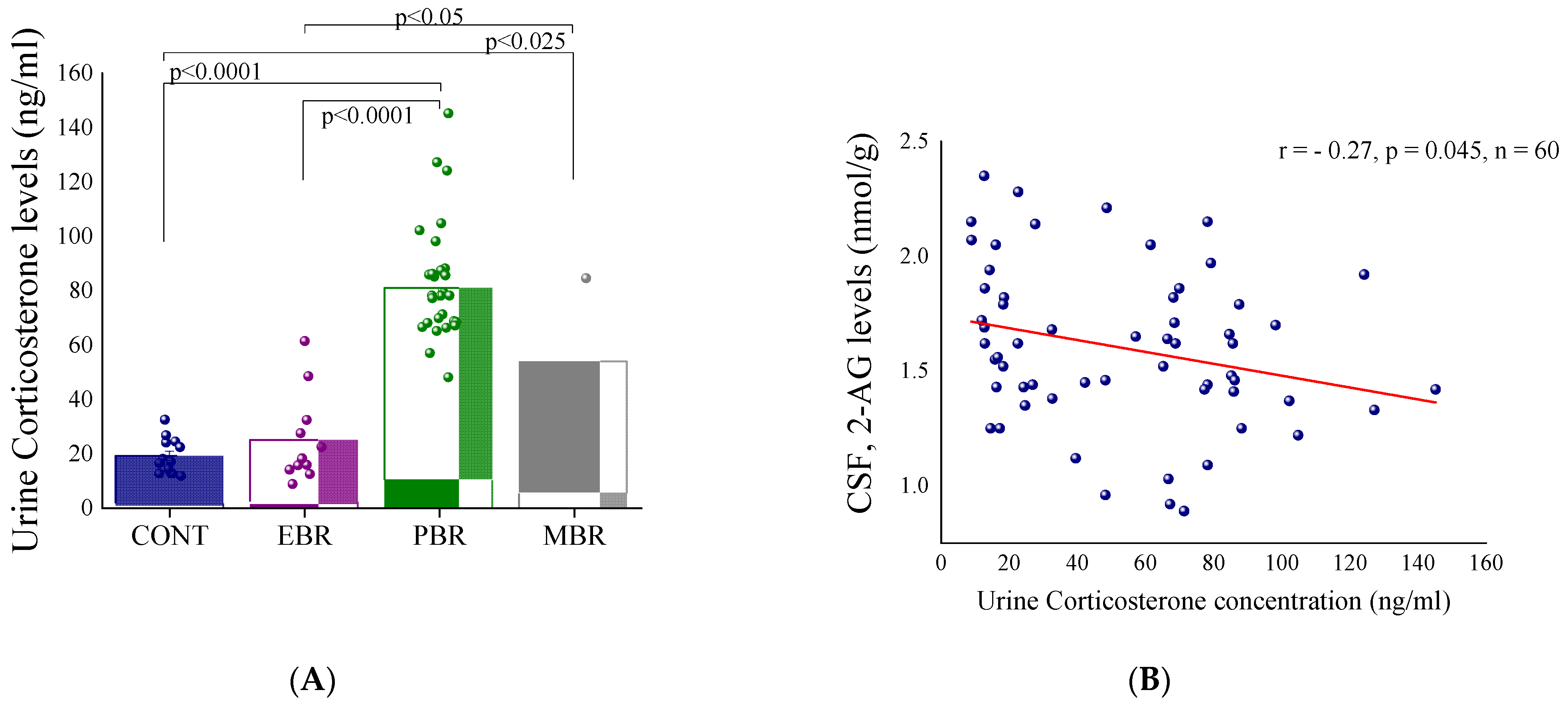
2.1. Shortly after PSS Exposure-CSF Endocannabinoids and Urine Corticosterone Concentrations
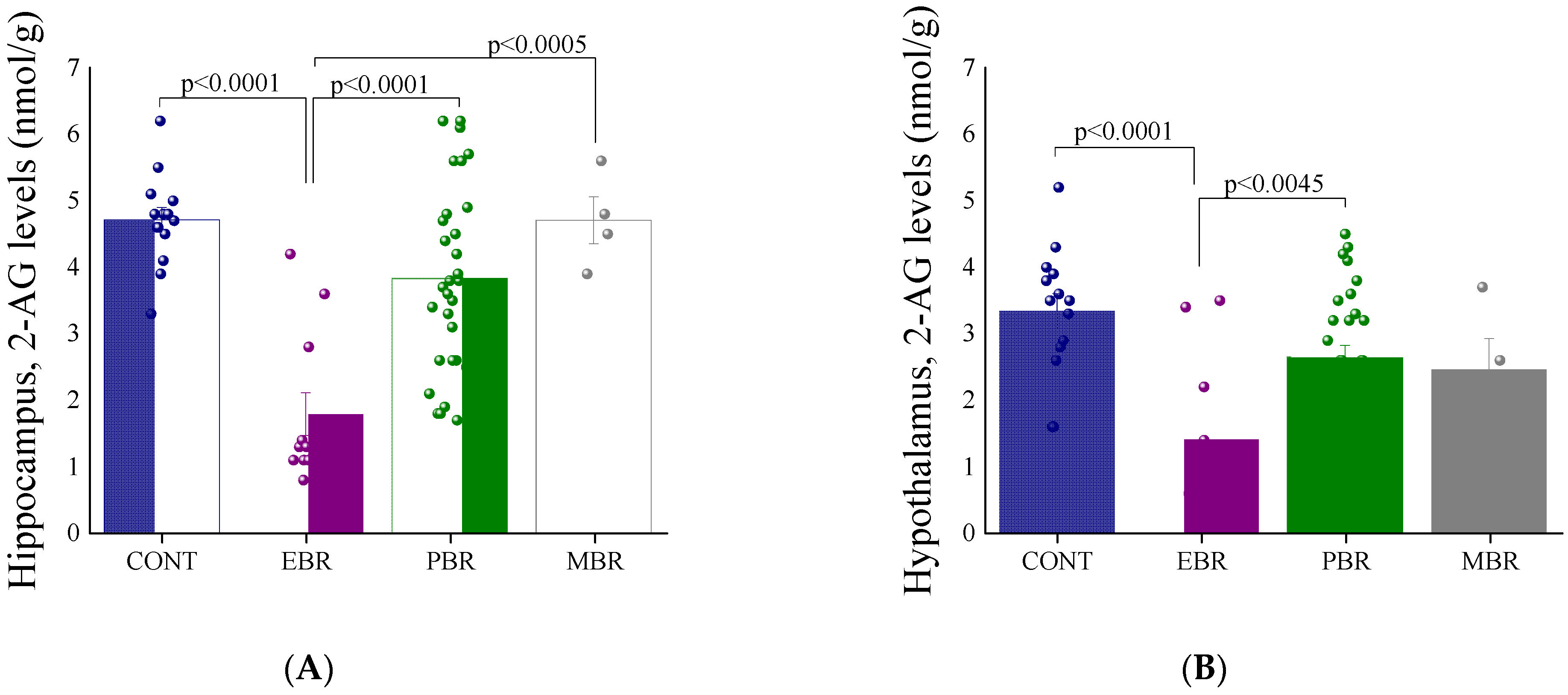
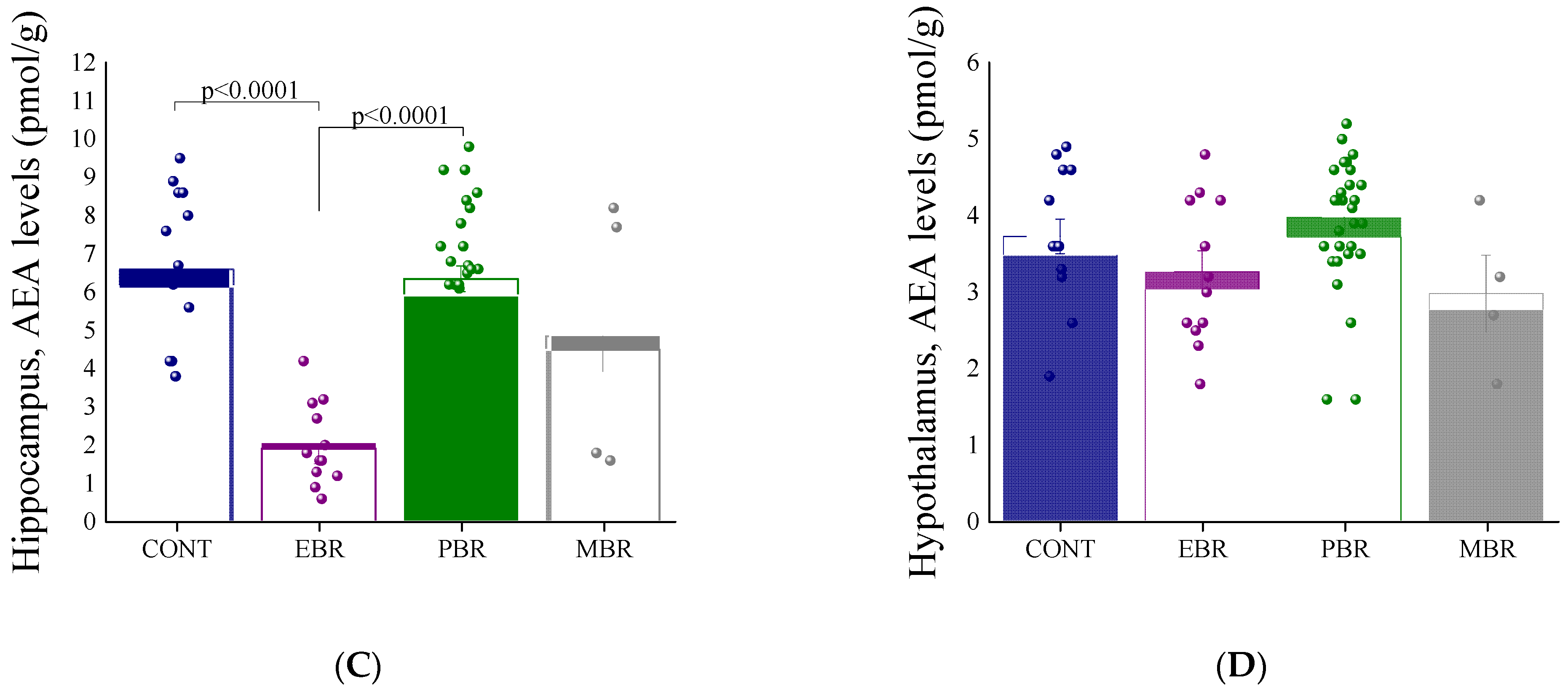
2.2. 8 Days Post-PSS Exposure-Brain Endocannabinoid Levels
3. Discussion
4. Materials and Methods
4.1. Animals
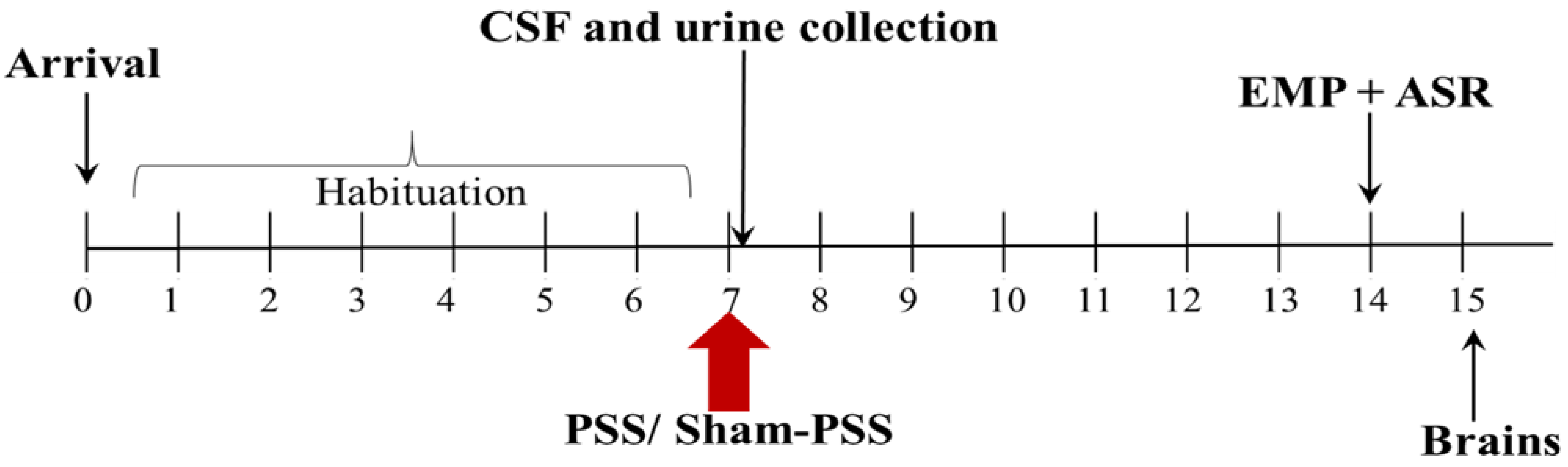
4.2. Experimental Design
4.3. Predator Scent Stress (PSS)
4.4. Behavioral Measurements
4.5. Cut-Off Behavioral Criteria (CBC) Model
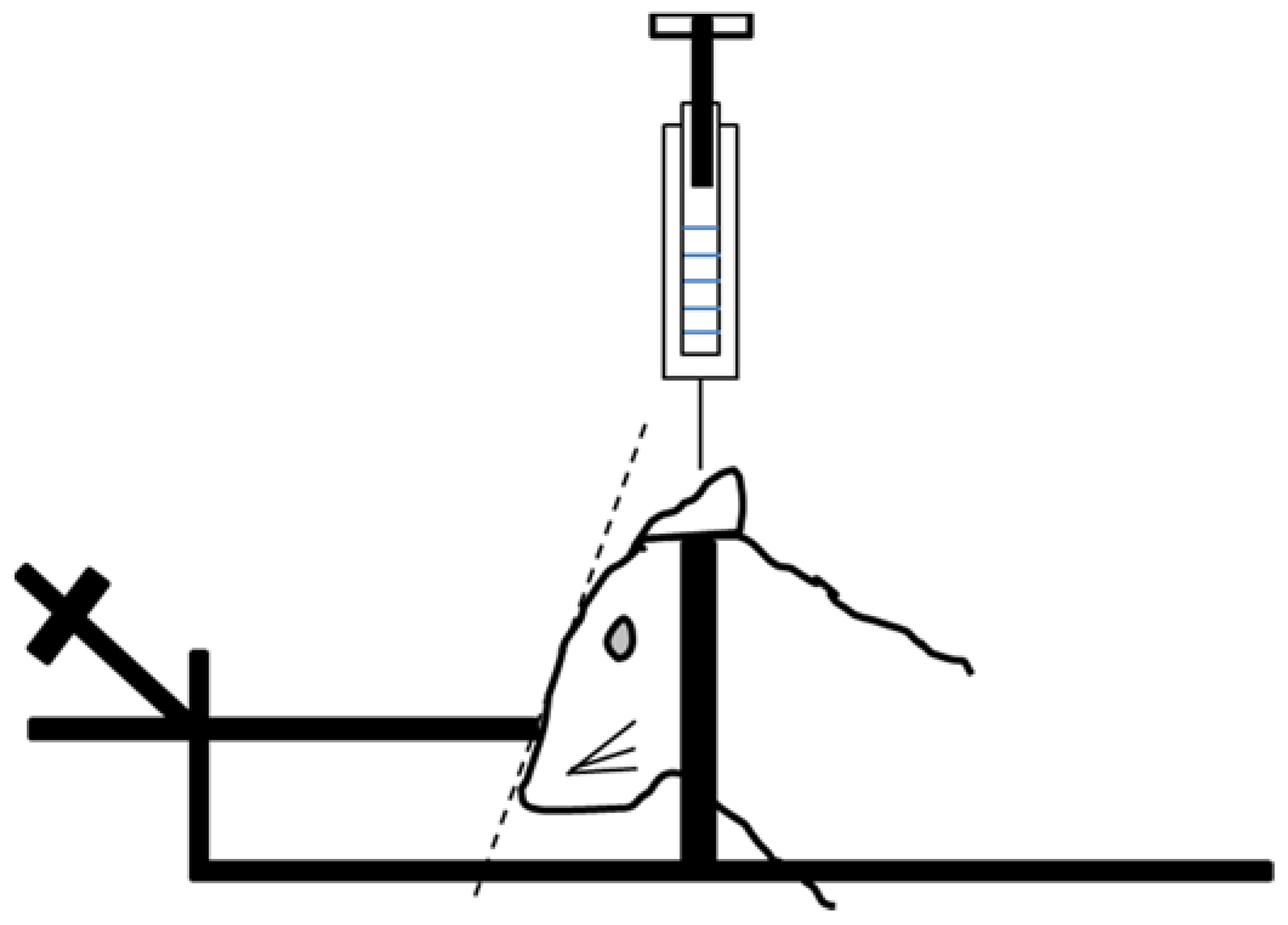
4.6. Cerebrospinal Fluid (CSF) Collection Procedure
4.7. Urine Corticosterone Sampling
4.8. Analysis of Endocannabinoid Levels
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEA | Anandamide |
| 2-AG | 2-arachidonoyl glycerol |
| ANOVA | Analysis of variance |
| CSF | Cerebrospinal fluid |
| ECS | Endocannabinoid system |
| EBR | Extreme behavioral response |
| EPM | Elevated plus-maze |
| GC | Glucocorticoid |
| HPA | Hypothalamus-pituitary-adrenal |
| MBR | Minimal behavioral response |
| PBR | Partial behavioral response |
| PSS | Predator scent stress |
| PTSD | Posttraumatic stress disorder |
| PVN | Paraventricular nucleus of the hypothalamus |
References
- Di, S.; Malcher-Lopes, R.; Marcheselli, V.L.; Bazan, N.G.; Tasker, J.G. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 2005, 146, 4292–4301. [Google Scholar] [CrossRef]
- Di, S.; Itoga, C.A.; Fisher, M.O.; Solomonow, J.; Roltsch, E.A.; Gilpin, N.W.; Tasker, J.G. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J. Neurosci. 2016, 36, 8461–8470. [Google Scholar] [CrossRef]
- Wamsteeker, J.I.; Kuzmiski, J.B.; Bains, J.S. Repeated Stress Impairs Endocannabinoid Signaling in the Paraventricular Nucleus of the Hypothalamus. J. Neurosci. 2010, 30, 11188–11196. [Google Scholar] [CrossRef]
- Di, S.; Malcher-Lopes, R.; Halmos, K.C.; Tasker, J.G. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J. Neurosci. 2003, 23, 4850–4857. [Google Scholar] [CrossRef]
- Malcher-Lopes, R.; Di, S.; Marcheselli, V.S.; Weng, F.-J.; Stuart, C.T.; Bazan, N.G.; Tasker, J.G. Opposing Crosstalk between Leptin and Glucocorticoids Rapidly Modulates Synaptic Excitation via Endocannabinoid Release. J. Neurosci. 2006, 26, 6643–6650. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.P.; Karatsoreos, I.N.; Li, X.; Vemuri, V.K.; Wood, J.A.; Li, Z.; Tamashiro, K.L.; Schwartz, G.J.; Makriyannis, A.M.; Kunos, G. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Bierer, L.M.; Makotkine, I.; Golier, J.A.; Galea, S.; McEwen, B.S.; Hillard, C.J.; Yehuda, R. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 2013, 38, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Balsevich, G.; Petrie, G.N.; Hill, M.N. Endocannabinoids: Effectors of glucocorticoid signaling. Front. Neuroendocrinol. 2017, 47, 86–108. [Google Scholar] [CrossRef]
- Buwembo, A.; Long, H.; Walker, C.-D. Participation of endocannabinoids in rapid suppression of stress responses by glucocorticoids in neonates. Neuroscience 2013, 249, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Cullinan, W.E.; Hillard, C.J. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 2004, 145, 5431–5438. [Google Scholar] [CrossRef]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Hillard, C.J. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur. J. Neurosci. 2005, 21, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Bingham, B.; Shrestha, L.; Lee, T.T.Y.; Gray, J.M.; Hillard, C.J.; Gorzalka, B.B.; Viau, V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 9406–9411. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Miller, G.E.; Carrier, E.J.; Gorzalka, B.B.; Hillard, C.J. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 2009, 34, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Choukèr, A.; Kaufmann, I.; Kreth, S.; Hauer, D.; Feuerecker, M.; Thieme, D.; Vogeser, M.; Thiel, M.; Schelling, G. Motion Sickness, Stress and the Endocannabinoid System. PLoS ONE 2010, 5, e10752. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Hartley, N.D.; Neale, E.; Gaulden, A.D.; Patrick, T.A.; Kingsley, P.J.; Uddin, M.J.; Plath, N.; Marnett, L.J.; Patel, S. Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol. Psychiatry 2017, 82, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Stuhr, K.L.; Hutz, M.J.; Raff, H.; Hillard, C.J. Endocannabinoid signaling in hypothalamic-pituitary-adrenocortical axis recovery following stress: Effects of indirect agonists and comparison of male and female mice. Pharmacol. Biochem. Behav. 2014, 117, 17–24. [Google Scholar] [CrossRef]
- Bluett, R.J.; Báldi, R.; Haymer, A.; Gaulden, A.D.; Hartley, N.D.; Parrish, W.P.; Baechle, J.; Marcus, D.J.; Mardam-Bey, R.; Shonesy, B.; et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat. Commun. 2017, 8, 14782. [Google Scholar] [CrossRef]
- Bosch-Bouju, C.; Larrieu, T.; Linders, L.; Manzoni, O.J.; Layé, S. Endocannabinoid-Mediated Plasticity in Nucleus Accumbens Controls Vulnerability to Anxiety after Social Defeat Stress. Cell Rep. 2016, 16, 1237–1242. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nat. Cell Biol. 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Suzuki, A.; Josselyn, S.A.; Frankland, P.W.; Masushige, S.; Silva, A.J.; Kida, S. Memory Reconsolidation and Extinction Have Distinct Temporal and Biochemical Signatures. J. Neurosci. 2004, 24, 4787–4795. [Google Scholar] [CrossRef]
- Chhatwal, J.P.; Davis, M.; Maguschak, K.A.; Ressler, K. Enhancing Cannabinoid Neurotransmission Augments the Extinction of Conditioned Fear. Neuropsychopharmacology 2004, 30, 516–524. [Google Scholar] [CrossRef]
- Kamprath, K.; Romo-Parra, H.; Häring, M.; Gaburro, S.; Doengi, M.; Lutz, B.; Pape, H.C. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 2011, 36, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hillard, C.J. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur. J. Neurosci. 2008, 27, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 2018, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Cavener, V.S.; Gaulden, A.; Pennipede, D.; Jagasia, P.; Uddin, J.; Marnett, L.J.; Patel, S. Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice. Front. Neurosci. 2018, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Berzal, A.; Terzian, A.L.B.; Di Marzo, V.; Micale, V.; Viveros, M.P.; Wotjak, C.T. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology 2015, 232, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Hartley, N.D.; Gunduz-Cinar, O.; Halladay, L.; Bukalo, O.; Holmes, A.; Patel, S. 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl. Psychiatry 2016, 6, e749. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Yehuda, R. Biology of posttraumatic stress disorder. J. Clin. Psychiatry 2000, 1, 14–21. [Google Scholar]
- Morris, M.C.; Compas, B.E.; Garber, J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clin. Psychol. Rev. 2012, 32, 301–315. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neurosci. Biobehav. Rev. 2013, 37, 860–895. [Google Scholar] [CrossRef]
- McEwen, B.S. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin. N. Am. 2002, 25, 469–494. [Google Scholar] [CrossRef]
- Danan, D.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Blunted basal corticosterone pulsatility predicts post-exposure susceptibility to PTSD phenotype in rats. Psychoneuroendocrinology 2018, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Tasker, J.G.; Hill, M.N.; Hillard, C.J.; Herman, J.P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010, 151, 4811–4819. [Google Scholar] [CrossRef]
- Russell, A.L.; Tasker, J.G.; Lucion, A.B.; Fiedler, J.; Munhoz, C.D.; Wu, T.-Y.J.; Deak, T. Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. J. Neuroendocr. 2018, 30, e12641. [Google Scholar] [CrossRef]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal models of post-traumatic stress disorder. Curr. Protoc. Neurosci. 2013, 64, 9–45. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J.; Matar, M.; Joseph, Z.; Matar, M. The relevance of differential response to trauma in an animal model of post-traumatic stress disorder. Biol Psychiatry 2003, 53, 463–473. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M.A.; Kaplan, Z.; Geva, A.B. Unsupervised Fuzzy Clustering Analysis Supports Behavioral Cutoff Criteria in an Animal Model of Posttraumatic Stress Disorder. Biol. Psychiatry 2005, 58, 640–650. [Google Scholar] [CrossRef]
- Cohen, H.; Zohar, J.; Matar, M.A.; Zeev, K.; Loewenthal, U.; Richter-Levin, G. Setting Apart the Affected: The Use of Behavioral Criteria in Animal Models of Post Traumatic Stress Disorder. Neuropsychopharmacology 2004, 29, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; McEwen, B. Allostasis, Homeostats, and the Nature of Stress. Stress 2002, 5, 55–58. [Google Scholar] [CrossRef]
- Yehuda, R.; McFarlane, A.; Shalev, A. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol. Psychiatry 1998, 44, 1305–1313. [Google Scholar] [CrossRef]
- Keller-Wood, M. Hypothalamic-Pituitary-Adrenal axis-feedback control. Compr. Physiol. 2015, 5, 1161–1182. [Google Scholar]
- Osterlund, C.D.; Rodriguez-Santiago, M.; Woodruff, E.R.; Newsom, R.J.; Chadayammuri, A.P.; Spencer, R.L. Glucocorticoid fast feedback inhibition of stress-induced ACTH secretion in the male rat: Rate independence and stress-state resistance. Endocrinology 2016, 157, 2785–2798. [Google Scholar] [CrossRef]
- Walker, J.J.; Spiga, F.; Gupta, R.; Zhao, Z.; Lightman, S.L.; Terry, J.R. Rapid intra-adrenal feedback regulation of glucocorticoid synthesis. J. R. Soc. Interface 2015, 12, 20140875. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, D.L.; Raimonde, A.J.; Spoonster, E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatry 2000, 48, 940–947. [Google Scholar] [CrossRef]
- Ehring, T.; Ehlers, A.; Cleare, A.J.; Glucksman, E. Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Res. 2008, 161, 67–75. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.C. The prevalence and longitudinal course of PTSD. Implications for the neurobiological models of PTSD. Ann. N. Y. Acad. Sci. 1997, 821, 10–23. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.C.; Barton, C.A.; Yehuda, R.; Wittert, G. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology 2011, 36, 720–727. [Google Scholar] [CrossRef]
- de Kloet, C.S.; Vermetten, E.; Geuze, E.; Kavelaars, A.; Heijnen, C.J.; Westenberg, H.G. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 2006, 40, 550–567. [Google Scholar] [CrossRef]
- Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 2002, 346, 108–114. [Google Scholar] [CrossRef]
- Yehuda, R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann. N. Y. Acad. Sci. 2009, 1179, 56–69. [Google Scholar] [CrossRef]
- Rainville, J.R.; Weiss, G.L.; Evanson, N.; Herman, J.P.; Vasudevan, N.; Tasker, J.G. Membrane-initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids 2019, 142, 55–64. [Google Scholar] [CrossRef]
- Tasker, J.G.; Herman, J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011, 14, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Tasker, J.G. Nongenomic glucocorticoid suppression of a postsynaptic potassium current via emergent autocrine endocannabinoid signaling in hypothalamic neuroendocrine cells following chronic dehydration. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Otte, C.; Kumsta, R.; Kok, L.; Hillegers, M.H.J.; Hasselmann, H.; Kliegel, D.; Joels, M. Stress and Depression: A Crucial Role of the Mineralocorticoid Receptor. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, N.; Akirav, I. The effects of cannabinoid receptors activation and glucocorticoid receptors deactivation in the amygdala and hippocampus on the consolidation of a traumatic event. Neurobiol. Learn. Mem. 2017, 144, 248–258. [Google Scholar] [CrossRef]
- Wang, S.-S.; Mu, R.-H.; Li, C.-F.; Dong, S.-Q.; Geng, D.; Liu, Q.; Yi, L.-T. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 79, 417–425. [Google Scholar] [CrossRef]
- Di, S.; Maxson, M.M.; Franco, A.; Tasker, J.G. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J. Neurosci. 2009, 29, 393–401. [Google Scholar] [CrossRef]
- Neumeister, A.; Normandin, M.; Pietrzak, R.H.; Piomelli, D.; Zheng, M.Q.; Anton, A.I.G.; Potenza, M.N.; Bailey, C.R.; Lin, S.F.; Najafzadeh, S.; et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol. Psychiatry 2013, 18, 1034–1040. [Google Scholar] [CrossRef]
- Furini, C.; Myskiw, J.; Izquierdo, I. The learning of fear extinction. Neurosci. Biobehav. Rev. 2014, 47, 670–683. [Google Scholar] [CrossRef]
- Segev, A.; Korem, N.; Zer-Aviv, T.M.; Abush, H.; Lange, R.; Sauber, G.; Hillard, C.J.; Akirav, I. Role of endocannabinoids in the hippocampus and amygdala in emotional memory and plasticity. Neuropsychopharmacology 2018, 43, 2017–2027. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Ecagniard, B.; Eyamazaki, M.; Enakayama, J.; Esakimura, K.; Ekirino, Y.; Ekano, M. Task-specific enhancement of hippocampus-dependent learning in mice deficient in monoacylglycerol lipase, the major hydrolyzing enzyme of the endocannabinoid 2-arachidonoylglycerol. Front. Behav. Neurosci. 2015, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of Endocannabinoid Signaling in the Hippocampus Following Chronic Unpredictable Stress. Neuropsychopharmacology 2004, 30, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Valverde, O.; Barbaccia, M.L.; Castañé, A.; Maldonado, R.; Ledent, C.; Parmentier, M.; Finazzi-Agro, A. Age-related changes of anandamide metabolism in CB 1 cannabinoid receptor knockout mice: Correlation with behaviour. Eur. J. Neurosci. 2002, 15, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Abush, H.; Akirav, I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 2009, 20, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef]
- Atkinson, H.C.; Leggett, J.D.; Wood, S.A.; Castrique, E.S.; Kershaw, Y.M.; Lightman, S.L. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology 2010, 151, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Vainer, E.; Matar, M.A.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A.; Cohen, H. Diurnal Fluctuations in HPA and Neuropeptide Y-ergic Systems Underlie Differences in Vulnerability to Traumatic Stress Responses at Different Zeitgeber Times. Neuropsychopharmacology 2015, 40, 774–790. [Google Scholar] [CrossRef]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnology 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, S.V.; Blinov, D.V.; Petrov, S.V. Spatial Characteristics of Cisterna Magna in Rats and Novel Technique for Puncture with a Stereotactic Manipulator. Bull. Exp. Biol. Med. 2004, 137, 635–638. [Google Scholar] [CrossRef]
- Mahat, M.Y.A.; Ahamed, N.F.A.; Chandrasekaran, S.; Rajagopal, S.; Narayanan, S.; Surendran, N. An improved method of transcutaneous cisterna magna puncture for cerebrospinal fluid sampling in rats. J. Neurosci. Methods 2012, 211, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Pegg, C.C.; He, C.; Stroink, A.R.; Kattner, K.A.; Wang, C.X. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J. Neurosci. Methods 2010, 187, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Zohar, J.; Cohen, H. Post-Exposure Sleep Deprivation Facilitates Correctly Timed Interactions between Glucocorticoid and Adrenergic Systems, which Attenuate Traumatic Stress Responses. Neuropsychopharmacology 2012, 37, 2388–2404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, J.; Igarashi, M.; Jung, K.-M.; Butini, S.; Campiani, G.; Piomelli, D. Endocannabinoid Modulation of Predator Stress-Induced Long-Term Anxiety in Rats. Neuropsychopharmacology 2015, 41, 1329–1339. [Google Scholar] [CrossRef]
- Fu, J.; Astarita, G.; Gaetani, S.; Kim, J.; Cravatt, B.F.; Mackie, K.; Piomelli, D. Food Intake Regulates Oleoylethanolamide Formation and Degradation in the Proximal Small Intestine. J. Biol. Chem. 2007, 282, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Fu, J.; Astarita, G.; Li, X.; Gaetani, S.; Campolongo, P.; Cuomo, V.; Piomelli, D. The Lipid Messenger OEA Links Dietary Fat Intake to Satiety. Cell Metab. 2008, 8, 281–288. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danan, D.; Todder, D.; Zohar, J.; Cohen, H. Is PTSD-Phenotype Associated with HPA-Axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats. Int. J. Mol. Sci. 2021, 22, 6416. https://doi.org/10.3390/ijms22126416
Danan D, Todder D, Zohar J, Cohen H. Is PTSD-Phenotype Associated with HPA-Axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats. International Journal of Molecular Sciences. 2021; 22(12):6416. https://doi.org/10.3390/ijms22126416
Chicago/Turabian StyleDanan, Dor, Doron Todder, Joseph Zohar, and Hagit Cohen. 2021. "Is PTSD-Phenotype Associated with HPA-Axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats" International Journal of Molecular Sciences 22, no. 12: 6416. https://doi.org/10.3390/ijms22126416
APA StyleDanan, D., Todder, D., Zohar, J., & Cohen, H. (2021). Is PTSD-Phenotype Associated with HPA-Axis Sensitivity?: The Endocannabinoid System in Modulating Stress Response in Rats. International Journal of Molecular Sciences, 22(12), 6416. https://doi.org/10.3390/ijms22126416




