Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight
Abstract
1. Introduction
2. Results
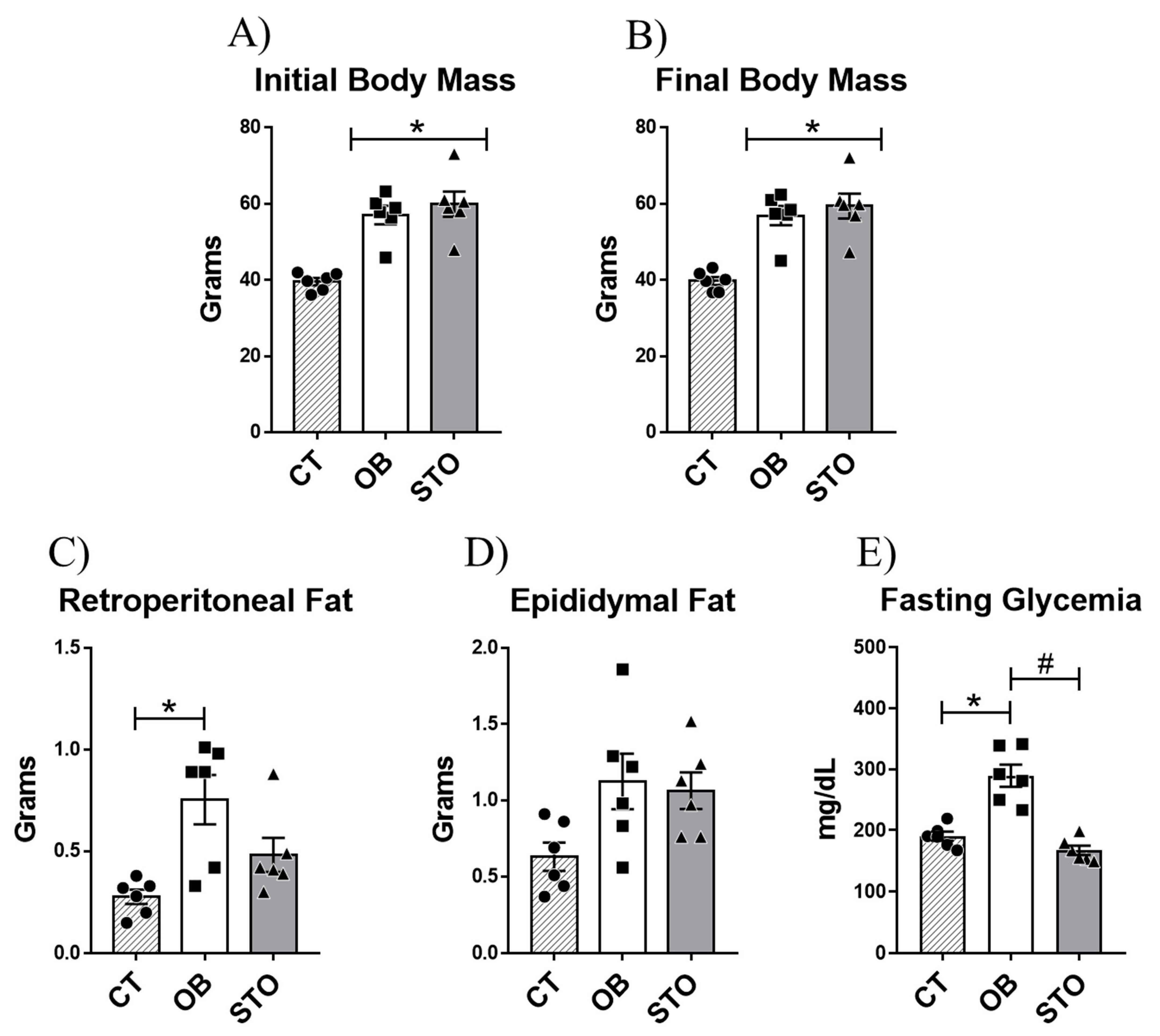
2.1. Short-Term Strength Exercise Ameliorates Glucose Sensitivity without Reducing Body Weight and Fat Depots
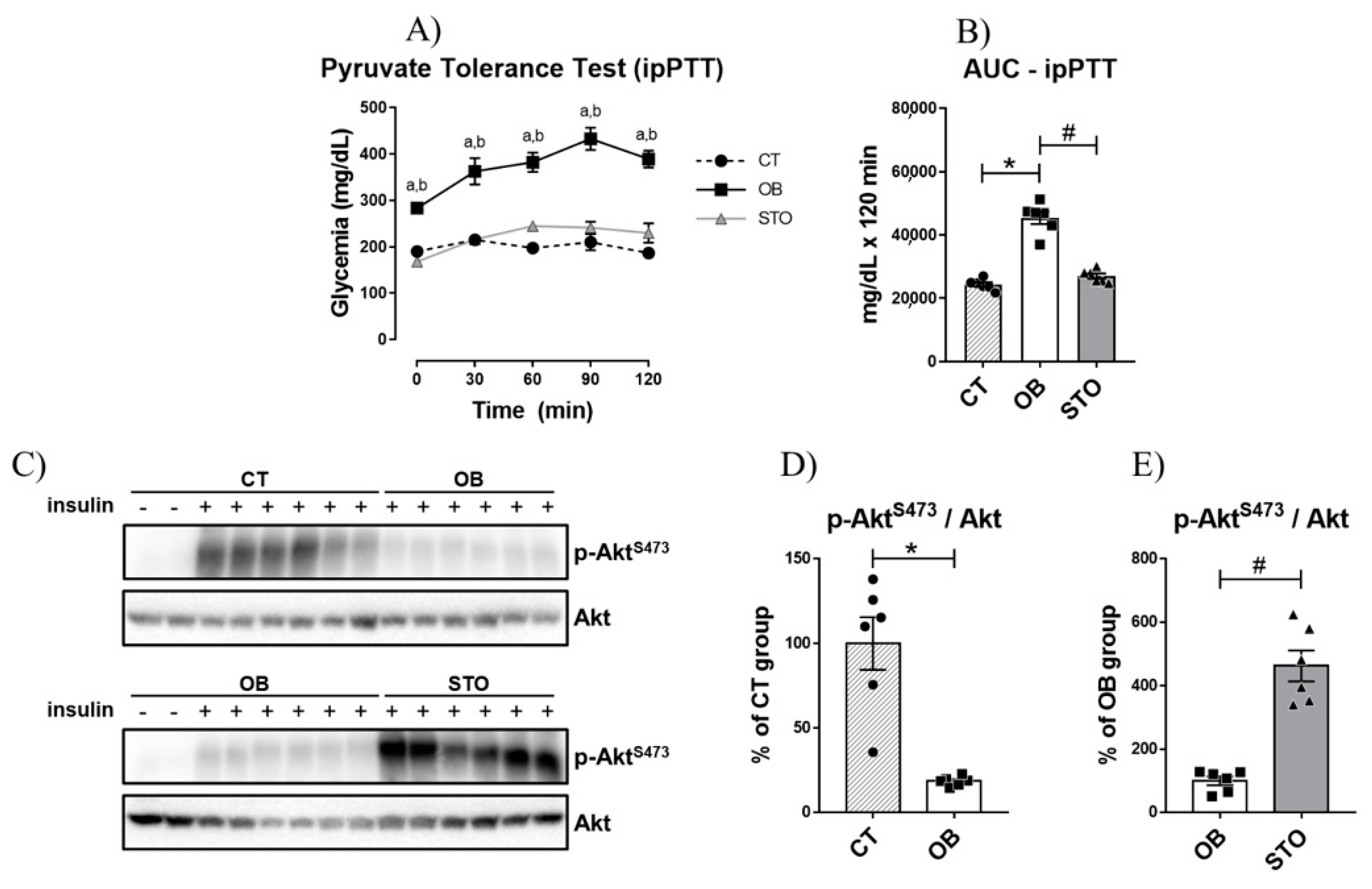
2.2. Short-Term Strength Exercise Improves Insulin Sensitivity and Reduces Hepatic Glucose Production in Obese Mice
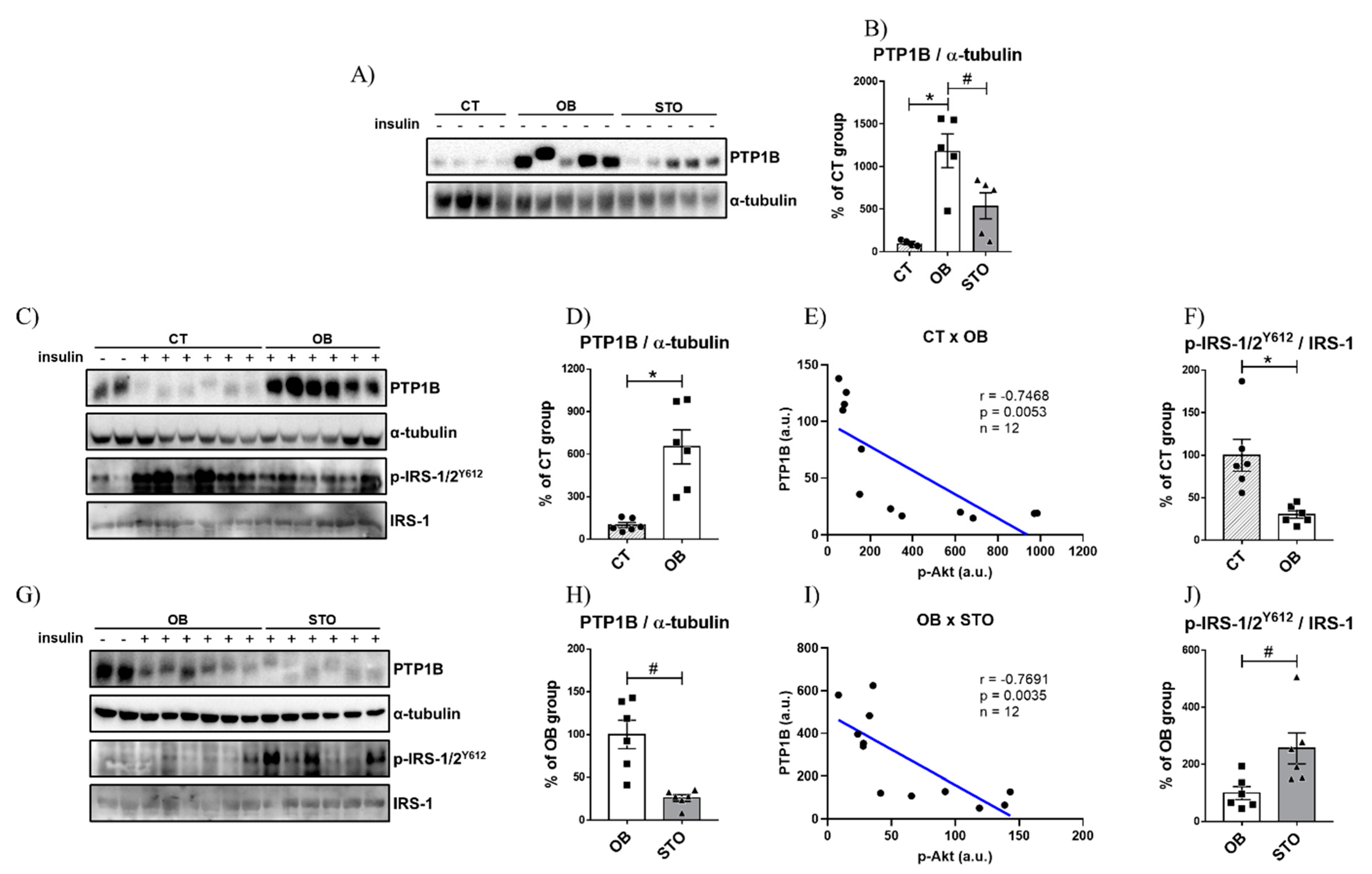
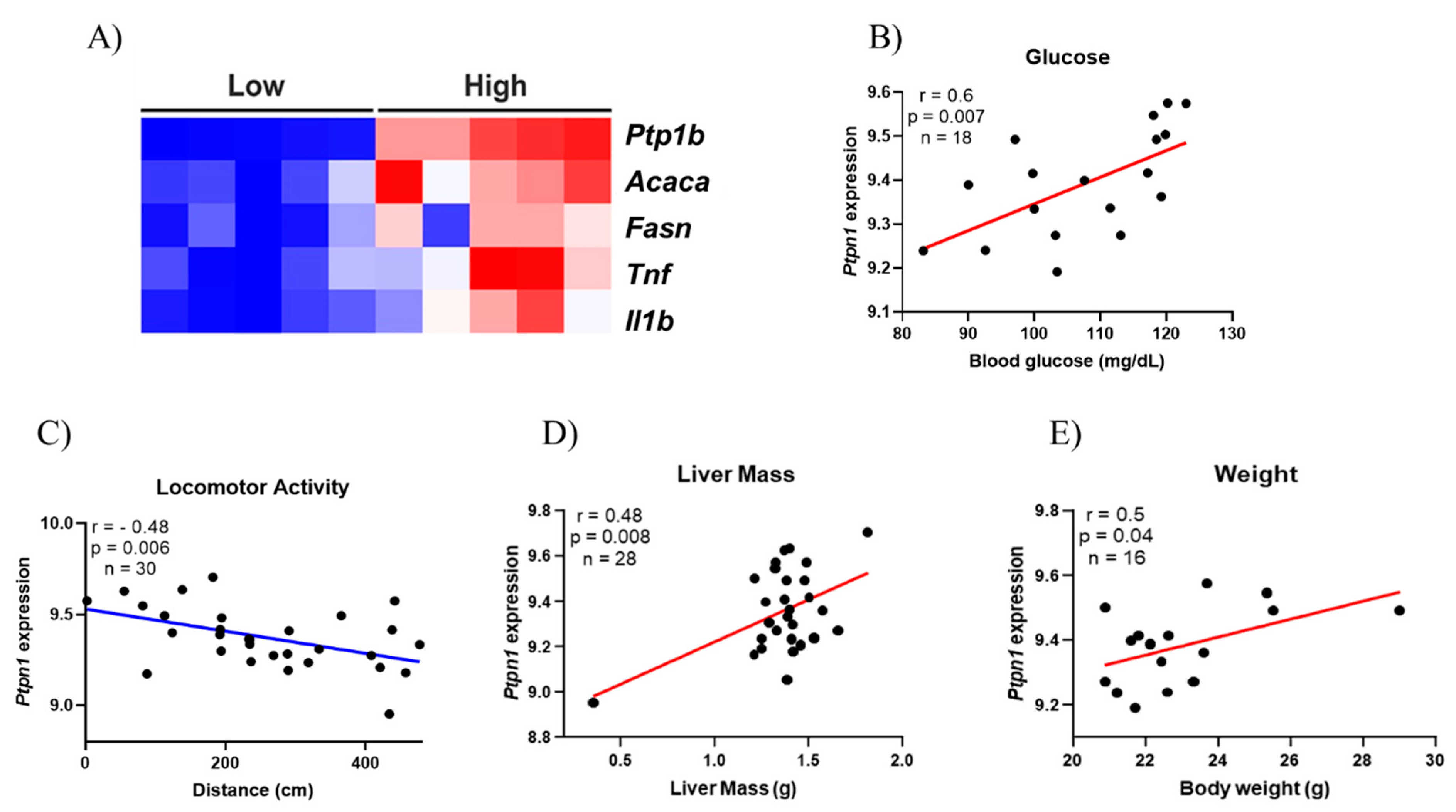
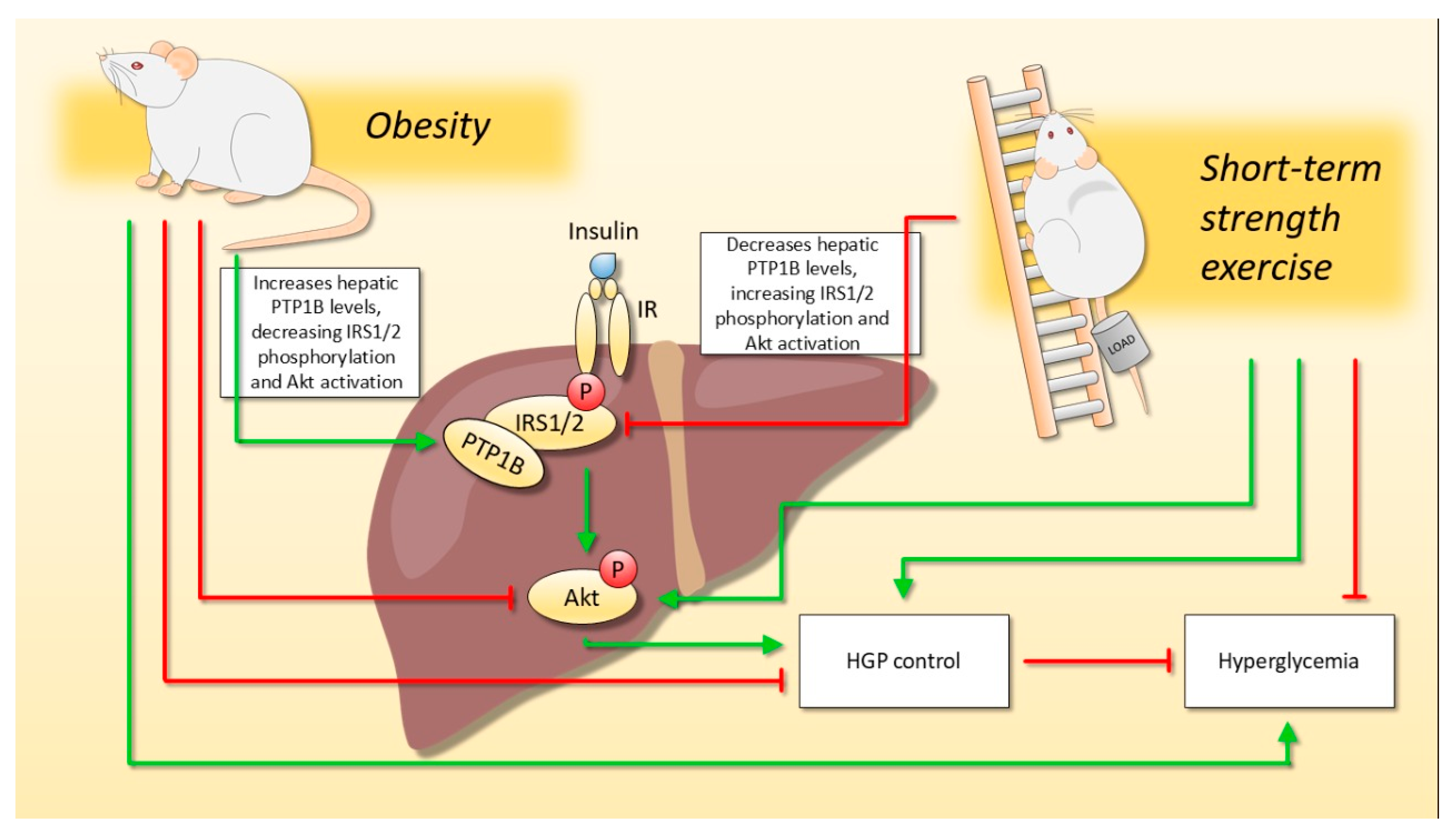
2.3. Strength Exercise Reduces PTP1B Content in the Liver of Obese Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
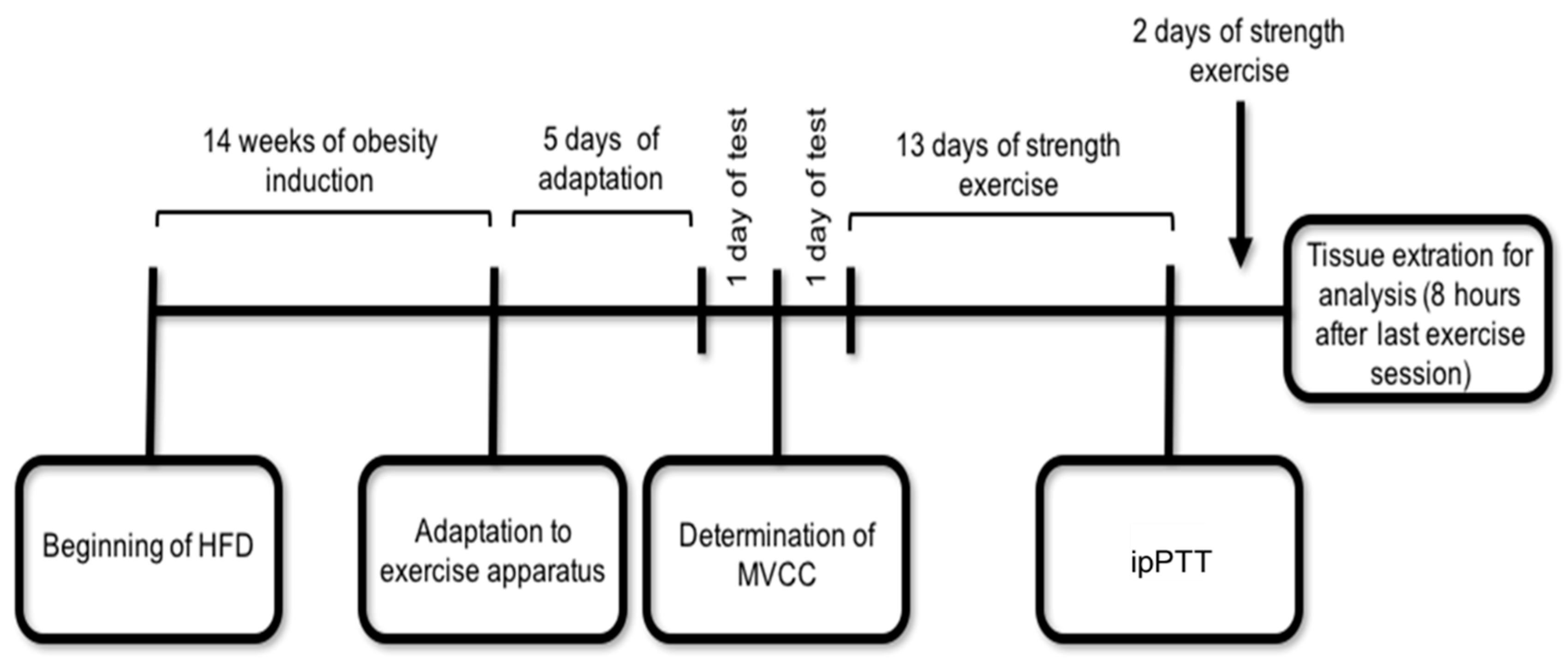
4.2. Experimental Design and Exercise Protocol
4.3. Intraperitoneal Pyruvate Tolerance Test (ipPTT)
4.4. Fasting Glycemia Assay
4.5. Tissue Extraction and Immunoblotting Analysis
4.6. Bioinformatics Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| FOXO1 | Forkhead Box Protein O1 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 Alpha |
| PTP1B | Protein-Tyrosine Phosphatase 1B |
| PTP | Protein Tyrosine Phosphatase |
| IR | Insulin Receptor |
| IRS | Insulin Receptor Substrate |
| TNF-α | Tumor Necrosis Factor-alpha |
| HGP | Hepatic Glucose Production |
| STSE | Short-term strength exercise |
| ipPTT | intraperitoneal Pyruvate Tolerance Test |
| AUC | Area Under the Curve |
| ACC | Acetyl-CoA Carboxylase |
| FAS | Fatty Acid Synthase |
| IL1β | Interleukin 1β |
| OLETF | Otsuka Long-Evans Tokushima Fatty |
| CEMIB | Unicamp Central Animal Facility |
| CEUA | Ethics Committee on Animal Use |
| CT | Control Lean |
| HFD | High-fat diet |
| OB | Sedentary Obese |
| STO | Strength Training Obese |
| AIN-93 | American Institute of Nutrition |
| MVCC | Maximal Voluntary Carrying Capacity |
| SEM | Standard error of the mean |
| ANOVA | Analysis of Variance |
References
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Matsumoto, M.; Pocai, A.; Rossetti, L.; DePinho, R.A.; Accili, D. Impaired Regulation of Hepatic Glucose Production in Mice Lacking the Forkhead Transcription Factor Foxo1 in Liver. Cell Metab. 2007, 6, 208–216. [Google Scholar] [CrossRef]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef]
- Haj, F.G.; Markova, B.; Klaman, L.D.; Bohmer, F.D.; Neel, B.G. Regulation of Receptor Tyrosine Kinase Signaling by Protein Tyrosine Phosphatase-1B. J. Biol. Chem. 2003, 278, 739–744. [Google Scholar] [CrossRef]
- Koren, S.; Fantus, I.G. Inhibition of the protein tyrosine phosphatase PTP1B: Potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 621–640. [Google Scholar] [CrossRef]
- Tiganis, T. PTP1B and TCPTP—Nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013, 280, 445–458. [Google Scholar] [CrossRef]
- Zabolotny, J.M.; Haj, F.G.; Kim, Y.-B.; Kim, H.-J.; Shulman, G.I.; Kim, J.K.; Neel, B.G.; Kahn, B.B. Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action. J. Biol. Chem. 2004, 279, 24844–24851. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Mas Gutierrez, J.A.; Sanz-González, S.; Ros, M.; Burks, D.J.; Valverde, A.M. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 2010, 59, 588–599. [Google Scholar] [CrossRef]
- Delibegovic, M.; Zimmer, D.; Kauffman, C.; Rak, K.; Hong, E.-G.; Cho, Y.-R.; Kim, J.K.; Kahn, B.B.; Neel, B.G.; Bence, K.K. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 2009, 58, 590–599. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Ropelle, E.R.; Flores, M.B.; Cintra, D.E.; Rocha, G.Z.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Moraes, J.C.; Prada, P.O.; Guadagnini, D.; et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 2010, 8, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- de Moura, L.P.; Souza Pauli, L.S.; Cintra, D.E.; de Souza, C.T.; da Silva, A.S.R.; Marinho, R.; de Melo, M.A.R.; Ropelle, E.R.; Pauli, J.R. Acute exercise decreases PTP-1B protein level and improves insulin signaling in the liver of old rats. Immun. Ageing 2013, 10, 1–9. [Google Scholar] [CrossRef]
- Passos, E.; Pereira, C.D.; Gonçalves, I.O.; Rocha-Rodrigues, S.; Silva, N.; Guimarães, J.T.; Neves, D.; Ascensão, A.; Magalhães, J.; Martins, M.J. Role of physical exercise on hepatic insulin, glucocorticoid and inflammatory signaling pathways in an animal model of non-alcoholic steatohepatitis. Life Sci. 2015, 123, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Rodrigues, K.C. da C.; Anaruma, C.P.; Sant’Ana, M.R.; de Campos, T.D.P.; Gaspar, R.S.; Canciglieri, R.D.S.; de Melo, D.G.; Mekary, R.A.; da Silva, A.S.R.; et al. Short-term strength training reduces gluconeogenesis and NAFLD in obese mice. J. Endocrinol. 2019, 241, 59–70. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.J.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Shinozaki, S.; Nakamoto, H.; Kaneki, M.; Goto, S.; Shimokado, K.; Kobayashi, H.; Naito, H. Voluntary Exercise Can Ameliorate Insulin Resistance by Reducing iNOS-Mediated S-Nitrosylation of Akt in the Liver in Obese Rats. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Xu, Z.; Hua, T.; Sun, Q. Exercise ameliorates insulin resistance via regulating TGFβ-activated kinase 1 (TAK1)-mediated insulin signaling in liver of high-fat diet-induced obese rats. J. Cell. Physiol. 2019, 234, 7467–7474. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; del Rincon, J.P.; Huang, H.; Kirwan, J.P. Exercise-induced lowering of fetuin-A may increase hepatic insulin sensitivity. Med. Sci. Sports Exerc. 2014, 46, 2085–2090. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef]
- Panzhinskiy, E.; Ren, J.; Nair, S. Protein Tyrosine Phosphatase 1B and Insulin Resistance: Role of Endoplasmic Reticulum Stress/Reactive Oxygen Species/Nuclear Factor Kappa B Axis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Owen, C.; Lees, E.K.; Grant, L.; Zimmer, D.J.; Mody, N.; Bence, K.K.; Delibegović, M. Inducible liver-specific knockdown of protein tyrosine phosphatase 1B improves glucose and lipid homeostasis in adult mice. Diabetologia 2013, 56, 2286–2296. [Google Scholar] [CrossRef]
- Ravichandran, L.V.; Chen, H.; Li, Y.; Quon, M.J. Phosphorylation of PTB1B at Ser50 by Akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 2001, 15, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Botezelli, J.D.; Coope, A.; Ghezzi, A.C.; Cambri, L.T.; Moura, L.P.; Scariot, P.P.M.; Gaspar, R.S.; Mekary, R.A.; Ropelle, E.R.; Pauli, J.R. Strength Training Prevents Hyperinsulinemia, Insulin Resistance, and Inflammation Independent of Weight Loss in Fructose-Fed Animals. Sci. Rep. 2016, 6, 31106. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Oliveira, V.; Marinho, R.; Vitorino, D.; Santos, G.A.; Moraes, J.C.; Dragano, N.; Sartori-Cintra, A.; Pereira, L.; Catharino, R.R.; da Silva, A.S.R.; et al. Diets Containing α-Linolenic (ω3) or Oleic (ω9) Fatty Acids Rescues Obese Mice From Insulin Resistance. Endocrinology 2015, 156, 4033–4046. [Google Scholar] [CrossRef]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. Br. Med. J. 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cruz Rodrigues, K.C.; Martins Pereira, R.; Peruca, G.F.; Torres Barbosa, L.W.; Ramos Sant’Ana, M.; Rosetto Muñoz, V.; Morelli, A.P.; Moreira Simabuco, F.; Sanchez Ramos da Silva, A.; Esper Cintra, D.; et al. Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight. Int. J. Mol. Sci. 2021, 22, 6402. https://doi.org/10.3390/ijms22126402
da Cruz Rodrigues KC, Martins Pereira R, Peruca GF, Torres Barbosa LW, Ramos Sant’Ana M, Rosetto Muñoz V, Morelli AP, Moreira Simabuco F, Sanchez Ramos da Silva A, Esper Cintra D, et al. Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight. International Journal of Molecular Sciences. 2021; 22(12):6402. https://doi.org/10.3390/ijms22126402
Chicago/Turabian Styleda Cruz Rodrigues, Kellen Cristina, Rodrigo Martins Pereira, Guilherme Francisco Peruca, Lucas Wesley Torres Barbosa, Marcella Ramos Sant’Ana, Vitor Rosetto Muñoz, Ana Paula Morelli, Fernando Moreira Simabuco, Adelino Sanchez Ramos da Silva, Dennys Esper Cintra, and et al. 2021. "Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight" International Journal of Molecular Sciences 22, no. 12: 6402. https://doi.org/10.3390/ijms22126402
APA Styleda Cruz Rodrigues, K. C., Martins Pereira, R., Peruca, G. F., Torres Barbosa, L. W., Ramos Sant’Ana, M., Rosetto Muñoz, V., Morelli, A. P., Moreira Simabuco, F., Sanchez Ramos da Silva, A., Esper Cintra, D., Rochete Ropelle, E., Pauli, J. R., & de Moura, L. P. (2021). Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight. International Journal of Molecular Sciences, 22(12), 6402. https://doi.org/10.3390/ijms22126402







