Degradation Products of Tryptophan in Cell Culture Media: Contribution to Color and Toxicity
Abstract
1. Introduction
2. Results
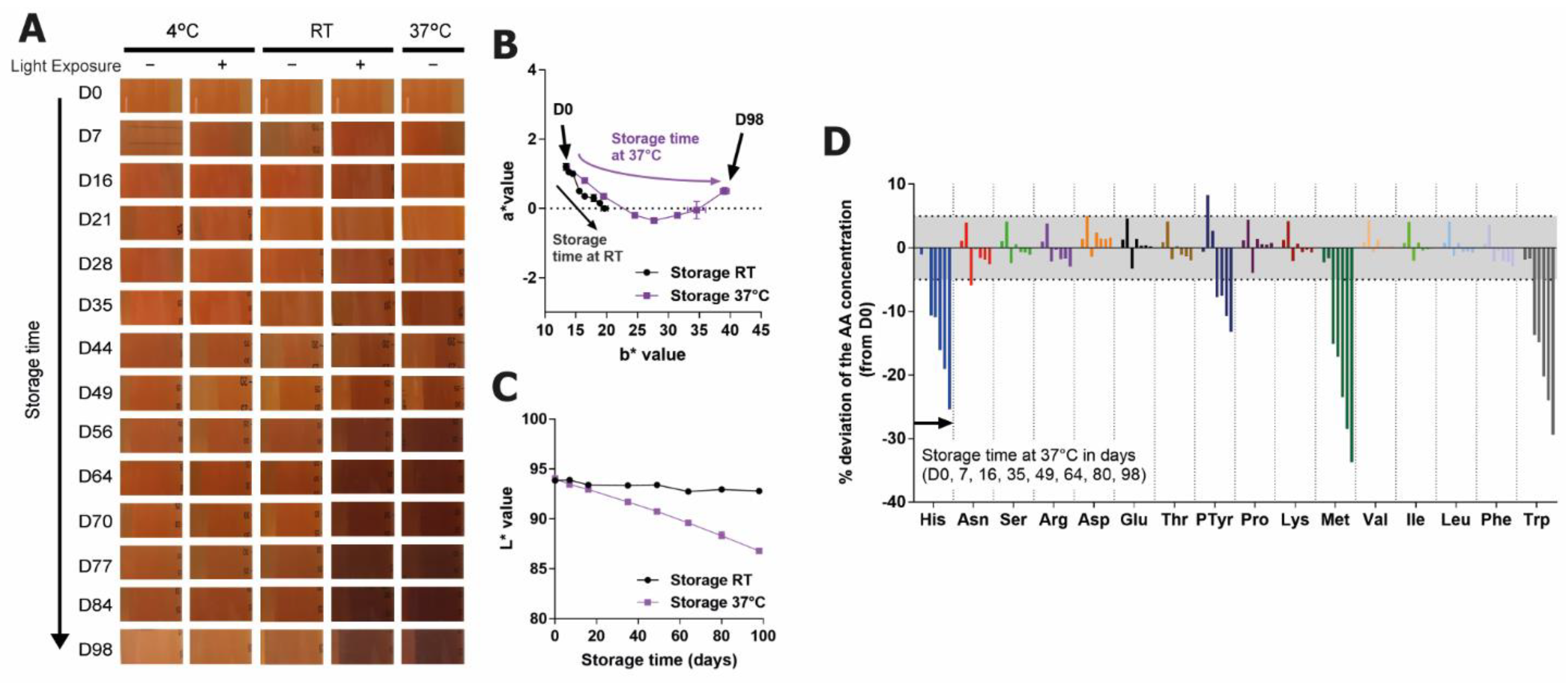
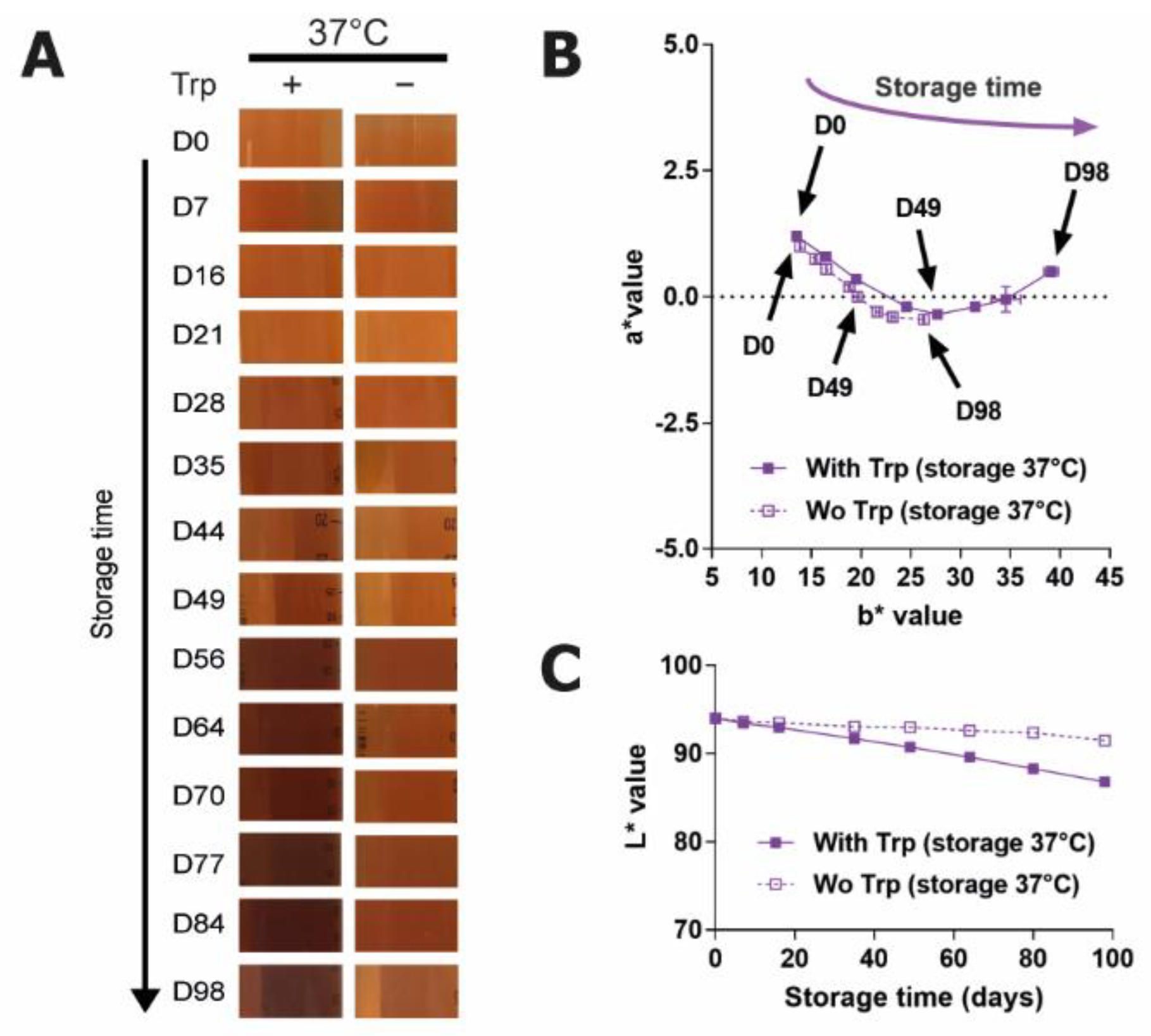
2.1. Tryptophan Is a Major Inducer of Browning in a Feed Used for CHO-Based Bioproduction
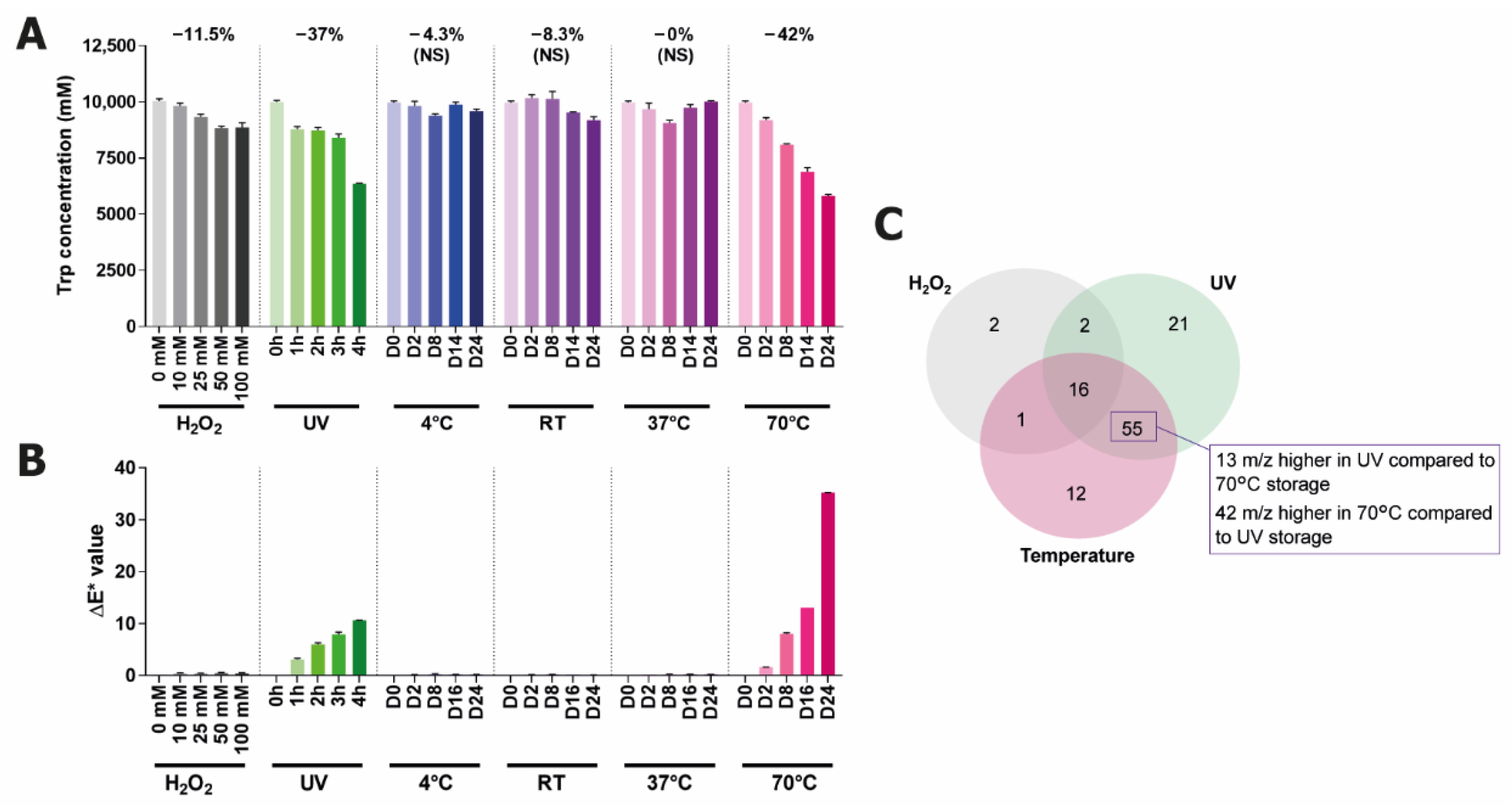
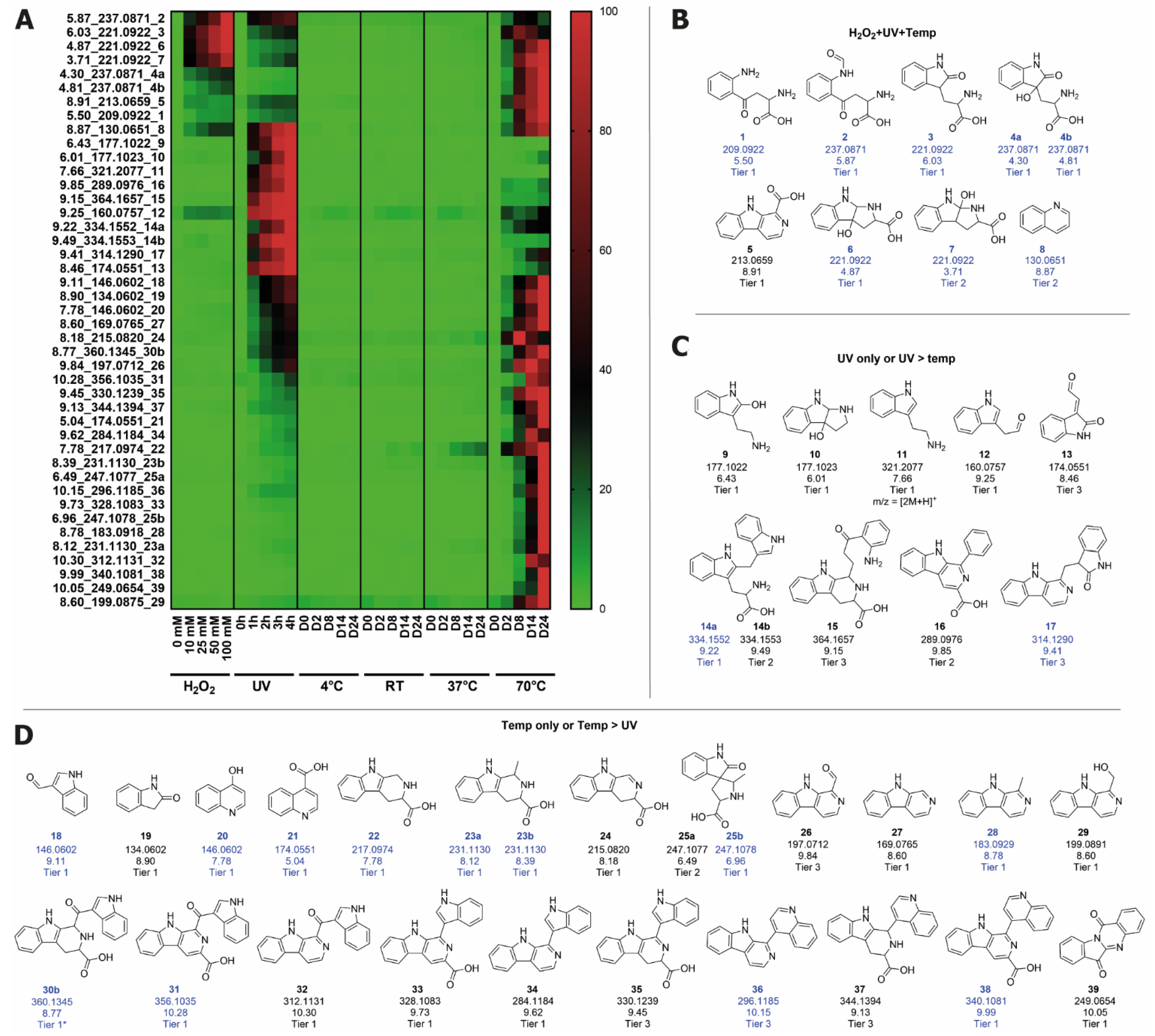
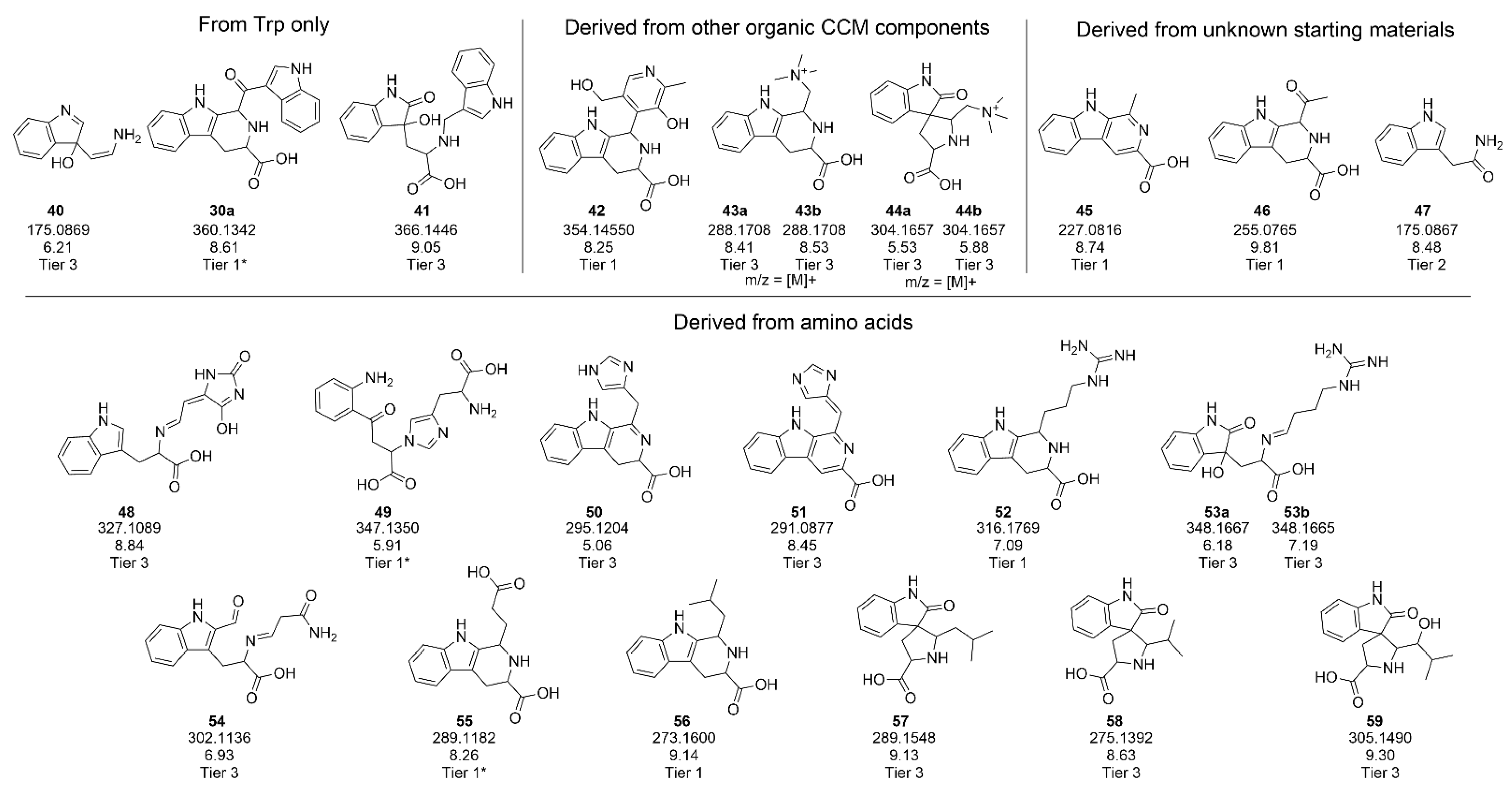
2.2. Identification of Degradation Products from Trp in Water Stored under Stressed Conditions
2.3. Identification of Degradation Products Originating from Trp in Feed Stored at Elevated Temperature
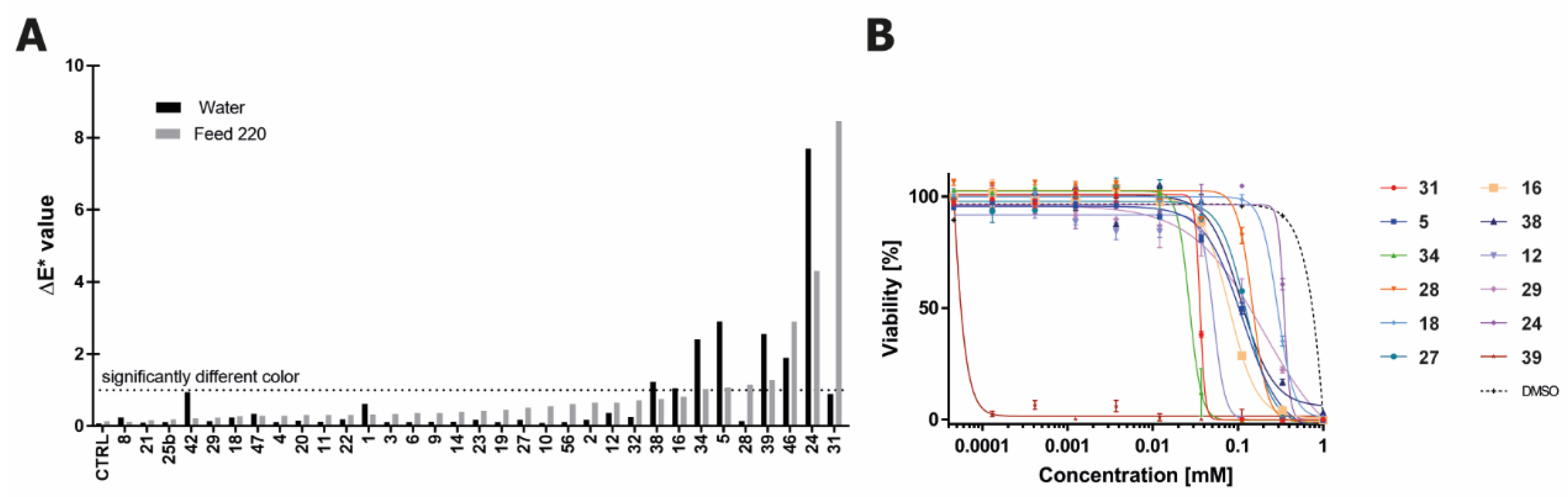
2.4. Color Contribution of Tier 1 Compounds to Water and Feed Solutions
2.5. Toxicity of Trp-Derived Degradation Products in CHO Cells
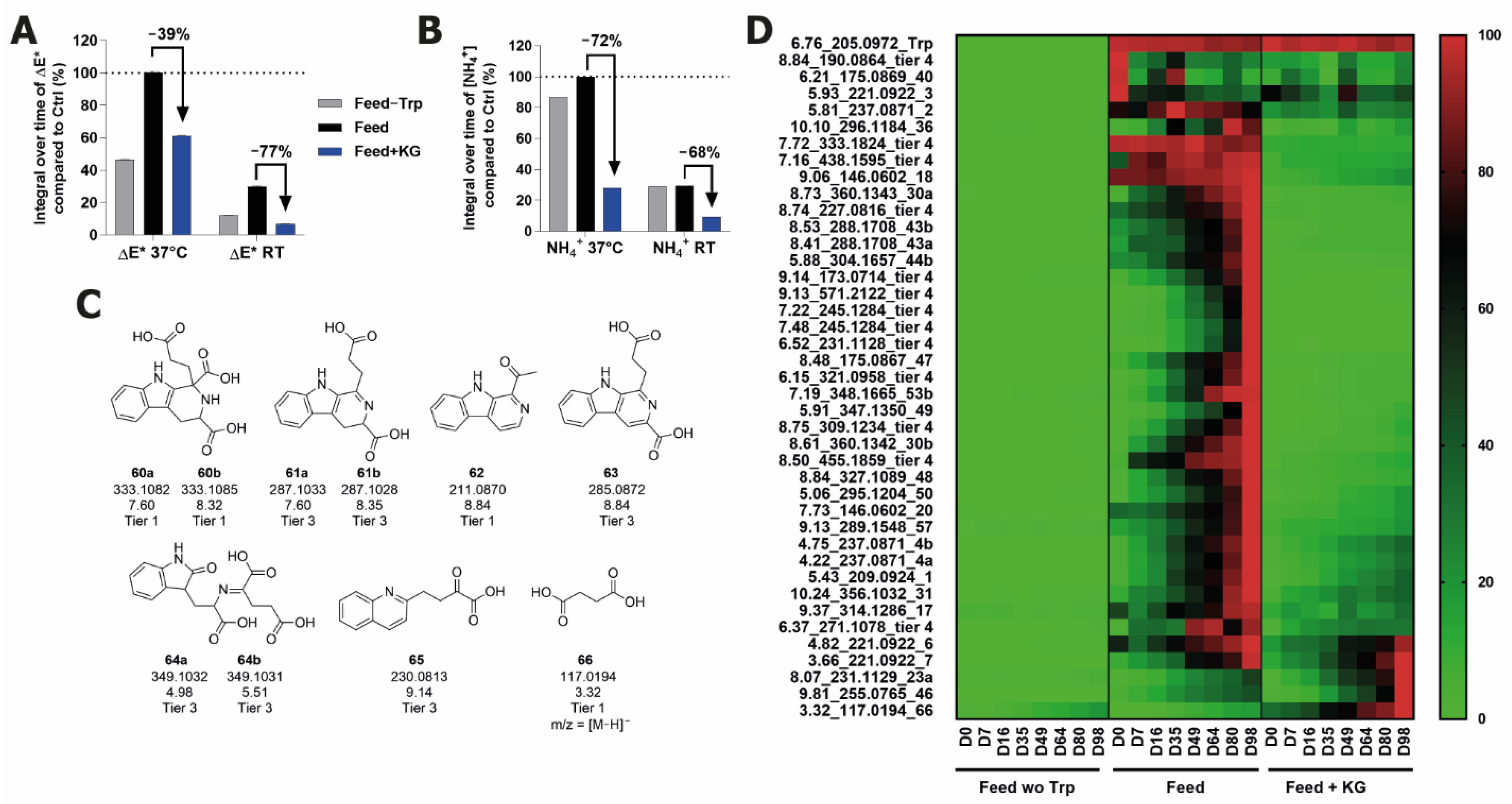
2.6. Inhibition of Browning and Degradation Product Formation Using Alpha-Ketoglutaric Acid
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Stability Studies
4.3. CIELAB Color Analysis
4.4. Amino Acid Analysis
4.5. LC-MS Feature Determination and Structure Elucidation
4.6. Color Measurements of Standards
4.7. Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neutsch, L.; Kroll, P.; Brunner, M.; Pansy, A.; Kovar, M.; Herwig, C.; Klein, T. Media photo-degradation in pharmaceutical biotechnology—Impact of ambient light on media quality, cell physiology, and IgG production in CHO cultures. J. Chem. Technol. Biotechnol. 2018, 93, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Mallaney, M.; Wang, S.-H.; Sreedhara, A. Effect of ambient light on monoclonal antibody product quality during small-scale mammalian cell culture process in clear glass bioreactors. Biotechnol. Prog. 2014, 30, 562–570. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Reinhart, D.; Castan, A.; Kunert, R. Monitoring of heat- and light exposure of cell culture media by RAMAN spectroscopy: Towards an analytical tool for cell culture media quality control. Biochem. Eng. J. 2020, 107845. [Google Scholar] [CrossRef]
- Floris, P.; McGillicuddy, N.; Albrecht, S.; Morrissey, B.; Kaisermayer, C.; Lindeberg, A.; Bones, J. Untargeted LC-MS/MS Profiling of Cell Culture Media Formulations for Evaluation of High Temperature Short Time Treatment Effects. Anal. Chem. 2017, 89, 9953–9960. [Google Scholar] [CrossRef] [PubMed]
- Prentice, K.M.; Gillespie, R.; Lewis, N.; Fujimori, K.; McCoy, R.; Bach, J.; Connell-Crowley, L.; Eakin, C.M. Hydroxocobalamin association during cell culture results in pink therapeutic proteins. MAbs 2013, 5, 974–981. [Google Scholar] [CrossRef][Green Version]
- Vijayasankaran, N.; Varma, S.; Yang, Y.; Mun, M.; Arevalo, S.; Gawlitzek, M.; Swartz, T.; Lim, A.; Li, F.; Zhang, B.; et al. Effect of cell culture medium components on color of formulated monoclonal antibody drug substance. Biotechnol. Prog. 2013, 29, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jin, M.; Song, H.; Huang, C.; Xu, X.; Tian, J.; Qian, N.-X.; Steger, K.; Lewen, N.S.; Tao, L.; et al. Brown drug substance color investigation in cell culture manufacturing using chemically defined media: A case study. Process Biochem. 2014, 49, 130–139. [Google Scholar] [CrossRef]
- Li, Y.; Polozova, A.; Gruia, F.; Feng, J. Characterization of the Degradation Products of a Color-Changed Monoclonal Antibody: Tryptophan-Derived Chromophores. Anal. Chem. 2014, 86, 6850–6857. [Google Scholar] [CrossRef]
- Hofmann, T. Structure, Color, and Formation of Low- and High-Molecular Weight Products Formed by Food-Related Maillard-Type Reactions. In Chemistry and Physiology of Selected Food Colorants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 775, pp. 134–151. [Google Scholar]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Echavarría, A.; Pagán, J.; Ibarz, A. Kinetics of color development in glucose/Amino Acid model systems at different temperatures. Sci. Agropecu. 2016, 7, 15–21. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef]
- Voelker, A.L.; Miller, J.; Running, C.A.; Taylor, L.S.; Mauer, L.J. Chemical stability and reaction kinetics of two thiamine salts (thiamine mononitrate and thiamine chloride hydrochloride) in solution. Food Res. Int. 2018, 112, 443–456. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Unger, N.; Ferraro, A.; Holzgrabe, U. Investigation of tryptophan-related yellowing in parenteral amino acid solution: Development of a stability-indicating method and assessment of degradation products in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2020, 177, 112839. [Google Scholar] [CrossRef] [PubMed]
- Zollinger, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley VCH: Zürich, Switzerland, 2001. [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color: The Fifteen Causes of Color, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Zimmer, A.; Mueller, R.; Wehsling, M.; Schnellbaecher, A.; von Hagen, J. Improvement and simplification of fed-batch bioprocesses with a highly soluble phosphotyrosine sodium salt. J. Biotechnol. 2014, 186, 110–118. [Google Scholar] [CrossRef]
- Maskos, Z.; Rush, J.D.; Koppenol, W.H. The hydroxylation of tryptophan. Arch. Biochem. Biophys. 1992, 296, 514–520. [Google Scholar] [CrossRef]
- Wickern, B.V.; Müller, B.; Simat, T.; Steinhart, H. Determination of γ-radiation induced proudcts in aqueous solutions of tryptophan and synthesis of 4-, 6- and 7-hydroxytryptophan. J. Chromatogr. A 1997, 786, 57–65. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Blaženović, I.; Kind, T.; Sa, M.R.; Ji, J.; Vaniya, A.; Wancewicz, B.; Roberts, B.S.; Torbašinović, H.; Lee, T.; Mehta, S.S.; et al. Structure Annotation of All Mass Spectra in Untargeted Metabolomics. Anal. Chem. 2019, 91, 2155–2162. [Google Scholar] [CrossRef]
- Gracanin, M.; Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Singlet-oxygen-mediated amino acid and protein oxidation: Formation of tryptophan peroxides and decomposition products. Free Radic. Biol. Med. 2009, 47, 92–102. [Google Scholar] [CrossRef]
- Stöckigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet–Spengler Reaction in Nature and in Organic Chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef]
- Nemet, I.; Varga-Defterdarović, L. Methylglyoxal-derived β-carbolines formed from tryptophan and its derivates in the Maillard reaction. Amino Acids 2007, 32, 291–293. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Snytnikova, O.A.; Forbes, M.D.E.; Chernyak, E.I.; Morozov, S.V. Photochemical and thermal reactivity of kynurenine. Exp. Eye Res. 2006, 83, 1439–1445. [Google Scholar] [CrossRef]
- Chu, N.T.; Clydesdale, F.M. Reactions between amino acids and organic acids: Reaction of tryptophan and pyruvic acid. J. Food Sci. 1976, 41, 891–894. [Google Scholar] [CrossRef]
- White, J.D.; Li, Y.; Ihle, D.C. Tandem Intramolecular Photocycloaddition−Retro-Mannich Fragmentation as a Route to Spiro[pyrrolidine-3,3′-oxindoles]. Total Synthesis of (±)-Coerulescine, (±)-Horsfiline, (±)-Elacomine, and (±)-6-Deoxyelacomine. J. Org. Chem. 2010, 75, 3569–3577. [Google Scholar] [CrossRef]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Biorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef]
- Creed, D. The photophysics and photochemistry of the near-UV absorbing amino acids—I. Tryptophan and its simple derivatives. Photochem. Photobiol. 1984, 39, 537–562. [Google Scholar] [CrossRef]
- Domingues, M.R.M.; Domingues, P.; Reis, A.; Fonseca, C.; Amado, F.M.L.; Ferrer-Correia, A.J.V. Identification of oxidation products and free radicals of tryptophan by mass spectrometry. J. Am. Soc. Mass. Spectrom. 2003, 14, 406–416. [Google Scholar] [CrossRef]
- Mayser, P.; Schäfer, U.; Krämer, H.-J.; Irlinger, B.; Steglich, W. Pityriacitrin—An ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch. Dermatol. Res. 2002, 294, 131–134. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, X.; Xu, B.; Bijian, K.; Wan, S.; Li, G.; Alaoui-Jamali, M.; Jiang, T. Total synthesis and bioactivity of the marine alkaloid pityriacitrin and some of its derivatives. Eur. J. Med. Chem. 2011, 46, 6089–6097. [Google Scholar] [CrossRef]
- Roggero, C.M.; Giulietti, J.M.; Mulcahy, S.P. Efficient synthesis of eudistomin U and evaluation of its cytotoxicity. Bioorg. Med. Chem. Lett. 2014, 24, 3549–3551. [Google Scholar] [CrossRef]
- Liang, J.L.; Park, S.-E.; Kwon, Y.; Jahng, Y. Synthesis of benzo-annulated tryptanthrins and their biological properties. Bioorg. Med. Chem. 2012, 20, 4962–4967. [Google Scholar] [CrossRef]
- Kirpotina, L.N.; Schepetkin, I.A.; Hammaker, D.; Kuhs, A.; Khlebnikov, A.I.; Quinn, M.T. Therapeutic Effects of Tryptanthrin and Tryptanthrin-6-Oxime in Models of Rheumatoid Arthritis. Front. Pharmacol. 2020, 11, 1145. [Google Scholar] [CrossRef]
- Kuschelewski, J.; Schnellbaecher, A.; Pering, S.; Wehsling, M.; Zimmer, A. Antioxidant effect of thiazolidine molecules in cell culture media improves stability and performance. Biotechnol. Prog. 2017, 33, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Marison, I.W.; von Stockar, U. The importance of ammonia in mammalian cell culture. J. Biotechnol. 1996, 46, 161–185. [Google Scholar] [CrossRef]
- Kim, D.Y.; Chaudhry, M.A.; Kennard, M.L.; Jardon, M.A.; Braasch, K.; Dionne, B.; Butler, M.; Piret, J.M. Fed-batch CHO cell t-PA production and feed glutamine replacement to reduce ammonia production. Biotechnol. Prog. 2013, 29, 165–175. [Google Scholar] [CrossRef]
- Yang, M.; Butler, M. Effect of Ammonia on the Glycosylation of Human Recombinant Erythropoietin in Culture. Biotechnol. Prog. 2000, 16, 751–759. [Google Scholar] [CrossRef]
- Sohn, M.; Ho, C.-T. Ammonia Generation during Thermal Degradation of Amino Acids. J. Agric. Food Chem. 1995, 43, 3001–3003. [Google Scholar] [CrossRef]
- Rice, G.B.; Yerabolu, J.R.; Krishnamurthy, R.; Springsteen, G. The Abiotic Oxidation of Organic Acids to Malonate. Synlett 2017, 28, 98–102. [Google Scholar] [CrossRef]
- Ling, J.; Reynolds, N.; Ibba, M. Aminoacyl-tRNA Synthesis and Translational Quality Control. Annu. Rev. Microbiol. 2009, 63, 61–78. [Google Scholar] [CrossRef]
- Graham, R.J.; Bhatia, H.; Yoon, S. Consequences of trace metal variability and supplementation on Chinese hamster ovary (CHO) cell culture performance: A review of key mechanisms and considerations. Biotechnol. Bioeng. 2019, 116, 3446–3456. [Google Scholar] [CrossRef]
- Mohammad, A.; Agarabi, C.; Rogstad, S.; DiCioccio, E.; Brorson, K.; Ashraf, M.; Faustino, P.J.; Madhavarao, C.N. An ICP-MS platform for metal content assessment of cell culture media and evaluation of spikes in metal concentration on the quality of an IgG3:κ monoclonal antibody during production. J. Pharm. Biomed. Anal. 2019, 162, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, C.; Bareford, L.; Matthews, T.E.; Brantley, T.; Moore, B.; Kolwyck, D. Elemental metal variance in cell culture raw materials for process risk profiling. Biotechnol. Prog. 2020, 36, e3004. [Google Scholar] [CrossRef] [PubMed]
- Argentova, V.; Aliev, T.; Dolgikh, D.; Kirpichnikov, M. Effects of Succinic Acid Supplementation on Stable Cell Line Growth, Aggregation, and IgG and IgA Production. Curr. Pharm. Biotechnol. 2020, 21, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, R.; Lin, H.; Xu, S. Feeding tricarboxylic acid cycle intermediates improves lactate consumption and antibody production in Chinese hamster ovary cell cultures. Biotechnol. Prog. 2020, 36, e2975. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnellbaecher, A.; Lindig, A.; Le Mignon, M.; Hofmann, T.; Pardon, B.; Bellmaine, S.; Zimmer, A. Degradation Products of Tryptophan in Cell Culture Media: Contribution to Color and Toxicity. Int. J. Mol. Sci. 2021, 22, 6221. https://doi.org/10.3390/ijms22126221
Schnellbaecher A, Lindig A, Le Mignon M, Hofmann T, Pardon B, Bellmaine S, Zimmer A. Degradation Products of Tryptophan in Cell Culture Media: Contribution to Color and Toxicity. International Journal of Molecular Sciences. 2021; 22(12):6221. https://doi.org/10.3390/ijms22126221
Chicago/Turabian StyleSchnellbaecher, Alisa, Anton Lindig, Maxime Le Mignon, Tim Hofmann, Brit Pardon, Stephanie Bellmaine, and Aline Zimmer. 2021. "Degradation Products of Tryptophan in Cell Culture Media: Contribution to Color and Toxicity" International Journal of Molecular Sciences 22, no. 12: 6221. https://doi.org/10.3390/ijms22126221
APA StyleSchnellbaecher, A., Lindig, A., Le Mignon, M., Hofmann, T., Pardon, B., Bellmaine, S., & Zimmer, A. (2021). Degradation Products of Tryptophan in Cell Culture Media: Contribution to Color and Toxicity. International Journal of Molecular Sciences, 22(12), 6221. https://doi.org/10.3390/ijms22126221





