Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression
Abstract
1. Introduction
2. Melatonin Synthesis and Signaling Ways of Embryogenesis
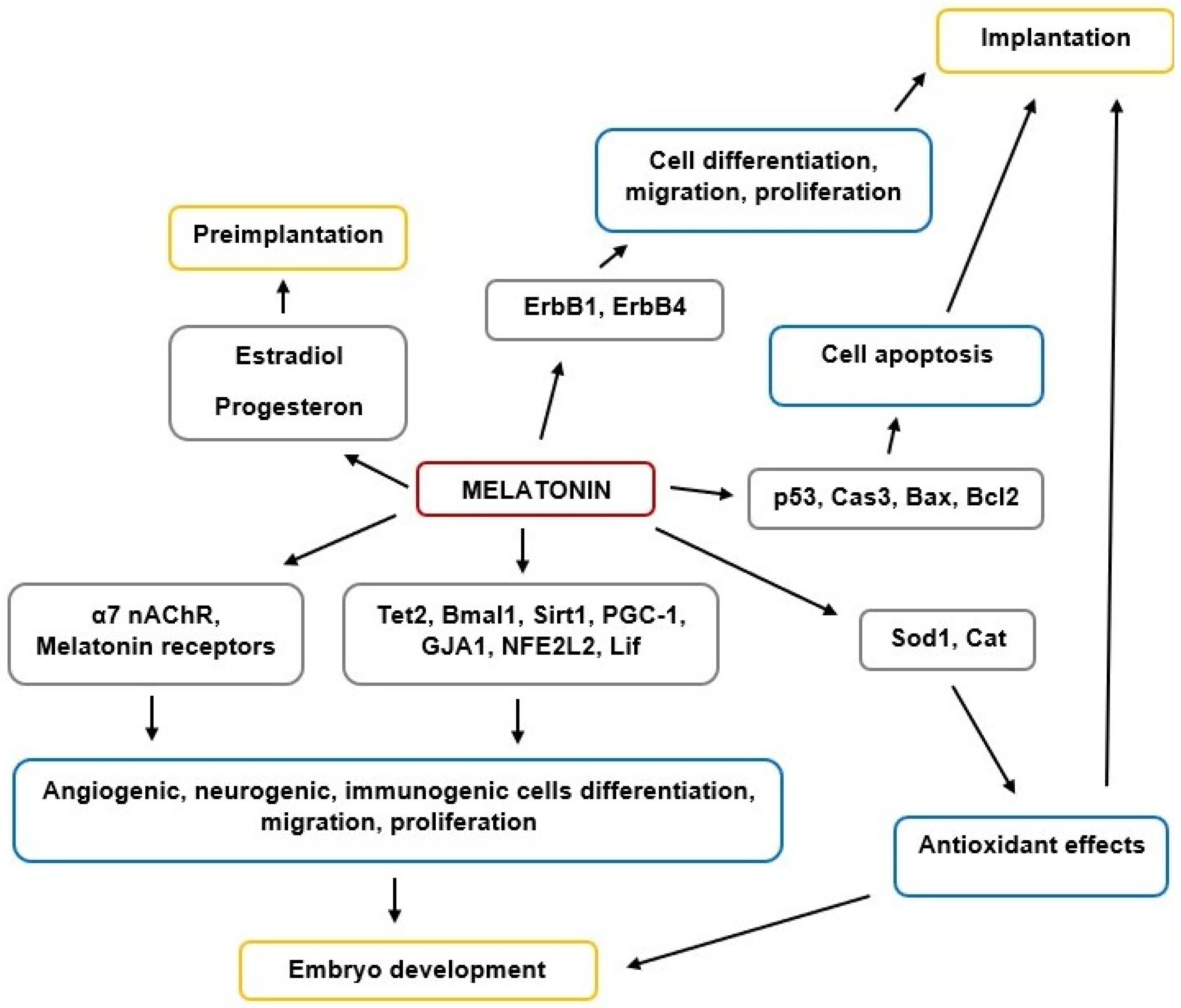
3. Melatonin’s Role in Preimplantation Embryo Development and Implantation
4. Melatonin’s Effects on Histogenesis, Morphogenesis, and Organogenesis in Different Organs and Tissues
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vaillancourt, C.; Lafond, J. Human Embryogenesis: Overview. Adv. Struct. Saf. Stud. 2009, 550, 3–7. [Google Scholar] [CrossRef]
- Bissiere, S.; Gasnier, M.; Alvarez, Y.D.; Plachta, N. Cell Fate Decisions during Preimplantation Mammalian Development. Curr. Top. Dev. Biol. 2018, 128, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J.; Amaral, F.G.D.; Cipolla-Neto, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharm. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Updat. 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Pandey, N.; Giri, S. Melatonin attenuates radiofrequency radiation (900 MHz)-induced oxidative stress, DNA damage and cell cycle arrest in germ cells of male Swiss albino mice. Toxicol. Ind. Health 2018, 34, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin′s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N.; Raikhlin, I.M.K.N.T. Melatonin may be synthesised in enterochromaffin cells. Nat. Cell Biol. 1975, 255, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Kvetnoy, I.M. Extrapineal Melatonin: Location and Role within Diffuse Neuroendocrine System. J. Mol. Histol. 1999, 31, 1–12. [Google Scholar] [CrossRef]
- Kvetnoy, I. Extrapineal melatonin in pathology: New perspectives for diagnosis, prognosis and treatment of illness. Neuroendocrinol. Lett. 2002, 23, 92–96. [Google Scholar] [PubMed]
- Maes, M.; Anderson, G.; Medina, S.R.B.; Seo, M.; Ojala, J.O. Integrating Autism Spectrum Disorder Pathophysiology: Mitochondria, Vitamin A, CD38, Oxytocin, Serotonin and Melatonergic Alterations in the Placenta and Gut. Curr. Pharm. Des. 2020, 25, 4405–4420. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.-M. Role of melatonin in embryo fetal development. J. Med. Life 2015, 7, 488–492. [Google Scholar]
- Yang, M.; Tao, J.; Wu, H.; Guan, S.; Liu, L.; Zhang, L.; Deng, S.-L.; He, C.; Ji, P.; Liu, J.; et al. Aanat Knockdown and Melatonin Supplementation in Embryo Development: Involvement of Mitochondrial Function and DNA Methylation. Antioxid. Redox Signal. 2019, 30, 2050–2065. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arto, M.; Hamilton, T.R.D.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.; Pérez-Pé, R.; Muiño-Blanco, T.; Pérez, J.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Itoh, M.T.; Takahashi, N.; Tarumi, W.; Ishizuka, B. The rat oocyte synthesises melatonin. Reprod. Fertil. Dev. 2013, 25, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, Y.; Xu, Y.; Zhou, C. Loss of Bmal1 decreases oocyte fertilization, early embryo development and implantation potential in female mice. Zygote 2016, 24, 760–767. [Google Scholar] [CrossRef]
- Anderson, G. Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation. Biomol. Concepts 2019, 10, 133–149. [Google Scholar] [CrossRef]
- Fu, L.; Yu, Z.; Chen, Y.-H.; Xia, M.-Z.; Wang, H.; Zhang, C.; Tao, F.-B.; Xu, D.-X. Orally Administered Melatonin Prevents Lipopolysaccharide-Induced Neural Tube Defects in Mice. PLoS ONE 2014, 9, e113763. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Sampaio, L.D.F.S. Eye and heart morphogenesis are dependent on the melatonin signaling in chick embryos. J. Exp. Biol. 2017, 220, 3826–3835. [Google Scholar] [CrossRef]
- Van Der Pol, C.W.; Van Roovert-Reijrink, I.A.M.; Gussekloo, S.W.S.; Kranenbarg, S.; Leon-Kloosterziel, K.M.; Van Eijk-Priester, M.H.; Zeman, M.; Kemp, B.; Brand, H.V.D. Effects of lighting schedule during incubation of broiler chicken embryos on leg bone development at hatch and related physiological characteristics. PLoS ONE 2019, 14, e0221083. [Google Scholar] [CrossRef]
- Yılmaz, H.; Ertekin, T.; Atay, E.; Nisari, M.; Güler, H.S.; Al, Ö.; Payas, A.; Yılmaz, S. Antioxidant role of melatonin against nicotine’s teratogenic effects on embryonic bone development. Iran. J. Basic Med. Sci. 2018, 21, 787–793. [Google Scholar] [PubMed]
- Kandemir, Y.B.; Konuk, E.; Katirci, E.; Behram, M. Is the efect of melatonin on vascular endothelial growth factor receptor-2 associated with angiogenesis in the rat ovary? Clinics 2019, 74, e658. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Ciccimarra, R.; Grasselli, F. Melatonin potentially acts directly on swine ovary by modulating granulosa cell function and angiogenesis. Reprod. Fertil. Dev. 2017, 29, 2305–2312. [Google Scholar] [CrossRef]
- Ullah, K.; Rahman, T.U.; Pan, H.-T.; Guo, M.-X.; Dong, X.-Y.; Liu, J.; Jin, L.-Y.; Cheng, Y.; Ke, Z.-H.; Ren, J.; et al. Serum estradiol levels in controlled ovarian stimulation directly affect the endometrium. J. Mol. Endocrinol. 2017, 59, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Ashary, N.; Tiwari, A.; Modi, D. Embryo Implantation: War in Times of Love. Endocrinology 2018, 159, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Favetta, L.A.; John, E.J.S.; King, W.A.; Betts, D.H. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic. Biol. Med. 2007, 42, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, X.; Sho, T.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. ROS-induced autophagy regulates porcine trophectoderm cell apoptosis, proliferation, and differentiation. Am. J. Physiol. Cell Physiol. 2019, 316, C198–C209. [Google Scholar] [CrossRef] [PubMed]
- McGowen, M.R.; Erez, O.; Romero, R.; Wildman, D.E. The evolution of embryo implantation. Int. J. Dev. Biol. 2014, 58, 155–161. [Google Scholar] [CrossRef]
- Moshkdanian, G.; Moghani-Ghoroghi, F.; Pasbakhsh, P.; Nematollahi-Mahani, S.N.; Najafi, A.; Kashani, S.R. Melatonin upregulates ErbB1 and ErbB4, two primary implantation receptors, in pre-implantation mouse embryos. Iran. J. Basic Med. Sci. 2017, 20, 655–661. [Google Scholar]
- Niu, Y.; Zhou, W.; Nie, Z.; Shin, K.; Cui, X. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2019, 68, e12627. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhang, L.; Ji, P.; Wang, J.; Lv, D.; Li, G.; Chai, M.; Lian, Z.; Liu, G. Melatonin Promotes the In Vitro Development of Microinjected Pronuclear Mouse Embryos via Its Anti-Oxidative and Anti-Apoptotic Effects. Int. J. Mol. Sci. 2017, 18, 988. [Google Scholar] [CrossRef]
- Mehaisen, G.M.K.; Saeed, A.M.; Gad, A.; Abass, A.O.; Arafa, M.; El-Sayed, A. Antioxidant Capacity of Melatonin on Preimplantation Development of Fresh and Vitrified Rabbit Embryos: Morphological and Molecular Aspects. PLoS ONE 2015, 10, e0139814. [Google Scholar] [CrossRef] [PubMed]
- Stauch, B.; Johansson, L.; McCorvy, J.D.; Patel, N.; Han, G.W.; Huang, X.-P.; Gati, C.; Batyuk, A.; Slocum, S.T.; Ishchenko, A.; et al. Structural basis of ligand recognition at the human MT1 melatonin receptor. Nat. Cell Biol. 2019, 569, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Zhou, J.-N.; Balesar, R.; Unmehopa, U.; Bao, A.; Jockers, R.; Van Heerikhuize, J.; Swaab, D.F. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 2006, 499, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Sharan, K.; Lewis, K.; Furukawa, T.; Yadav, V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal Res. 2017, 63, e12423. [Google Scholar] [CrossRef]
- Jumnongprakhon, P.; Sivasinprasasn, S.; Govitrapong, P.; Tocharus, C.; Tocharus, J. Activation of melatonin receptor (MT1/2) promotes P-gp transporter in methamphetamine-induced toxicity on primary rat brain microvascular endothelial cells. Toxicol. Vitr. 2017, 41, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.-P.; Savych, O.; Moroz, Y.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nat. Cell Biol. 2020, 579, 609–614. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Tomasini, R.; McKeon, F.D.; Mak, T.W.; Melino, G. The p53 family: Guardians of maternal reproduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 259–265. [Google Scholar] [CrossRef]
- Koifman, G.; Aloni-Grinstein, R.; Rotter, V. p53 balances between tissue hierarchy and anarchy. J. Mol. Cell Biol. 2019, 11, 553–563. [Google Scholar] [CrossRef]
- Guan, S.; Xie, L.; Ma, T.; Lv, D.; Jing, W.; Tian, X.; Song, Y.; Liu, Z.; Xiao, X.; Liu, G. Effects of Melatonin on Early Pregnancy in Mouse: Involving the Regulation of StAR, Cyp11a1, and Ihh Expression. Int. J. Mol. Sci. 2017, 18, 1637. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Ji, D.-M.; Liu, Z.-B.; Wang, T.-J.; Xie, F.-F.; Zhang, Z.-G.; Wei, Z.-L.; Zhou, P.; Cao, Y.-X. Melatonin maintains mitochondrial membrane potential and decreases excessive intracellular Ca2+ levels in immature human oocytes. Life Sci. 2019, 235, 116810. [Google Scholar] [CrossRef]
- Tanabe, M.; Tamura, H.; Taketani, T.; Okada, M.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; Sugino, N. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J. Reprod. Dev. 2015, 61, 35–41. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.-X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2016, 62, e12381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheung, H.H.; Zhang, C.; Wu, J.; Chan, W.-Y. Melatonin as Potential Targets for Delaying Ovarian Aging. Curr. Drug Targets 2018, 20, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar] [PubMed]
- Unfer, V.; Raffone, E.; Rizzo, P.; Buffo, S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011, 27, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improvingin vitrofertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2015, 32, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Poloni, J.D.F.; Feltes, B.C.; Bonatto, D. Melatonin as a central molecule connecting neural development and calcium signaling. Funct. Integr. Genom. 2011, 11, 383–388. [Google Scholar] [CrossRef]
- López, A.; García, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; Lopez, L.C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46, 188–198. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Soliman, A.; Vaillancourt, C. Maternal and placental melatonin: Actions and implication for successful pregnancies. Minerva Ginecol. 2014, 66, 251–266. [Google Scholar]
- Bates, K.; Herzog, E.D. Maternal-Fetal Circadian Communication during Pregnancy. Front. Endocrinol. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Purvis, C.C.; Drew, J.E.; Abramovich, D.R.; Williams, L.M. Melatonin receptors in human fetal brain: 2-[125I]iodomelatonin binding and MT1 gene expression. J. Pineal Res. 2002, 33, 218–224. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J. Epigenetic regulation: A new research area for melatonin? J. Pineal Res. 2007, 44, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, Y.; Yuan, Q.; Pan, Y.; Wang, L.; Liu, Q.; Wang, F.; Wang, J.; Hao, A. Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J. Pineal Res. 2015, 59, 508–517. [Google Scholar] [CrossRef]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Olszańska, B.; Majewski, P.; Lewczuk, B.; Stepińska, U. Melatonin and its synthesizing enzymes (arylalkylamine N-acetyltransferase-like and hydroxyindole-O-methyltransferase) in avian eggs and early embryos. J. Pineal Res. 2007, 42, 310–318. [Google Scholar]
- Anderson, G.; Rodriguez, M.; Reiter, R.J. Multiple Sclerosis: Melatonin, Orexin, and Ceramide Interact with Platelet Activation Coagulation Factors and Gut-Microbiome-Derived Butyrate in the Circadian Dysregulation of Mitochondria in Glia and Immune Cells. Int. J. Mol. Sci. 2019, 20, 5500. [Google Scholar] [CrossRef]
- Jang, S.-W.; Liu, X.; Pradoldej, S.; Tosini, G.; Chang, Q.; Iuvone, P.M.; Ye, K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876–3881. [Google Scholar] [CrossRef]
- Gahring, L.C.; Myers, E.; Palumbos, S.; Rogers, S.W. Nicotinic Receptor Alpha7 Expression during Mouse Adrenal Gland Development. PLoS ONE 2014, 9, e103861. [Google Scholar] [CrossRef]
- Gergalova, G.; Lykhmus, O.; Kalashnyk, O.; Koval, L.; Chernyshov, V.; Kryukova, E.; Tsetlin, V.; Komisarenko, S.; Skok, M. Mitochondria Express α7 Nicotinic Acetylcholine Receptors to Regulate Ca2+ Accumulation and Cytochrome c Release: Study on Isolated Mitochondria. PLoS ONE 2012, 7, e31361. [Google Scholar] [CrossRef]
- Markus, R.P.; Silva, C.L.; Gil Franco, D.; Barbosa, E.M.; Ferreira, Z.S. Is modulation of nicotinic acetylcholine receptors by melatonin relevant for therapy with cholinergic drugs? Pharmacol. Ther. 2010, 126, 251–262. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, D.; Mazzoccoli, G.; Anderson, G.; Linkova, N.; Dyatlova, A.; Mironova, E.; Polyakova, V.; Kvetnoy, I.; Evsyukova, I.; Carbone, A.; et al. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. Int. J. Mol. Sci. 2021, 22, 5885. https://doi.org/10.3390/ijms22115885
Ivanov D, Mazzoccoli G, Anderson G, Linkova N, Dyatlova A, Mironova E, Polyakova V, Kvetnoy I, Evsyukova I, Carbone A, et al. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. International Journal of Molecular Sciences. 2021; 22(11):5885. https://doi.org/10.3390/ijms22115885
Chicago/Turabian StyleIvanov, Dmitry, Gianluigi Mazzoccoli, George Anderson, Natalia Linkova, Anastasiia Dyatlova, Ekaterina Mironova, Victoria Polyakova, Igor Kvetnoy, Inna Evsyukova, Annalucia Carbone, and et al. 2021. "Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression" International Journal of Molecular Sciences 22, no. 11: 5885. https://doi.org/10.3390/ijms22115885
APA StyleIvanov, D., Mazzoccoli, G., Anderson, G., Linkova, N., Dyatlova, A., Mironova, E., Polyakova, V., Kvetnoy, I., Evsyukova, I., Carbone, A., & Nasyrov, R. (2021). Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. International Journal of Molecular Sciences, 22(11), 5885. https://doi.org/10.3390/ijms22115885









