Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth
Abstract
1. Introduction
1.1. Background
1.2. Melatonin
1.3. Myo-Inositol
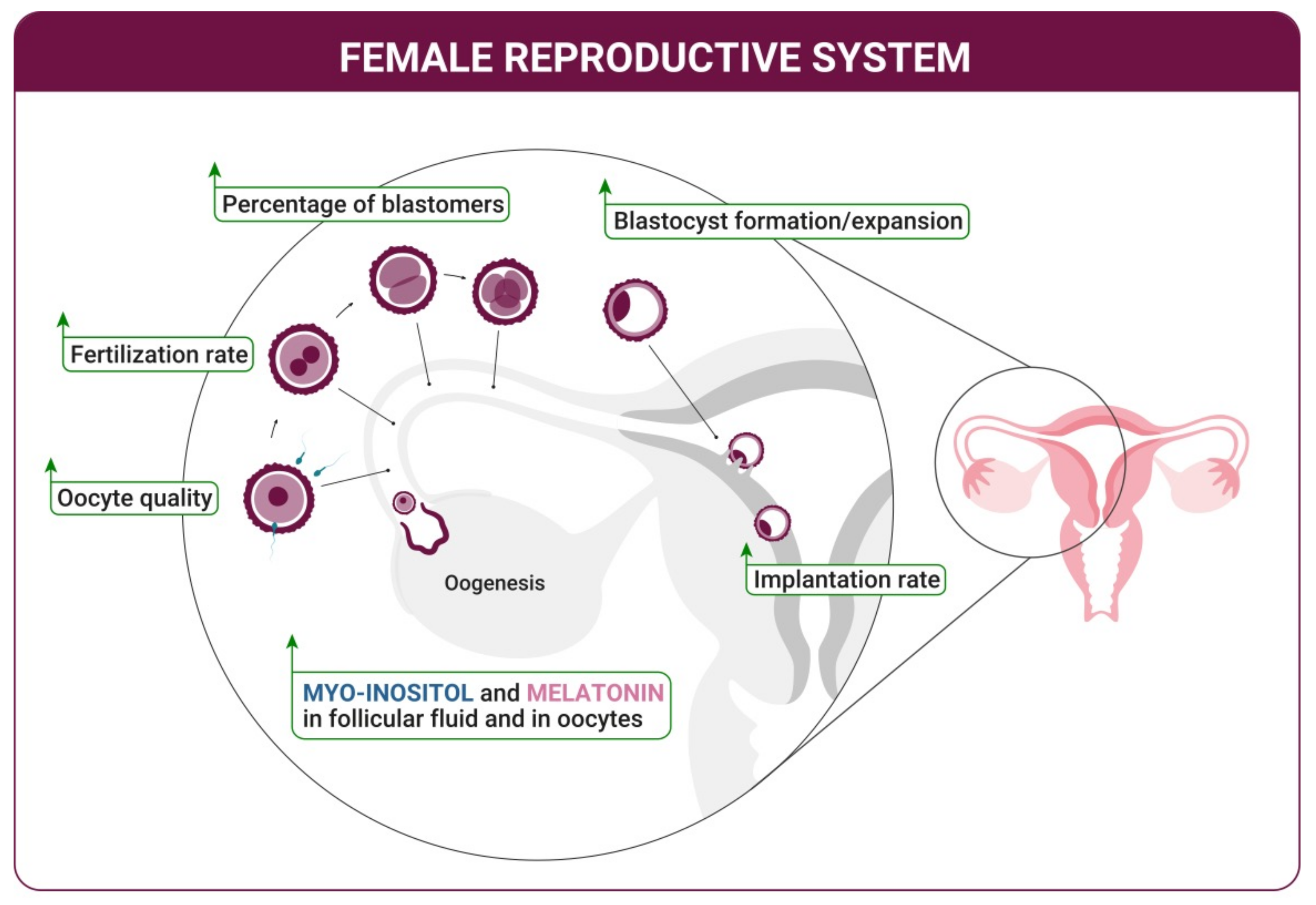
2. Improvement of the Ovulation Quality—Oogenesis
3. Oocyte Maturation and Fertilization
4. Blastocyst Development and Implantation
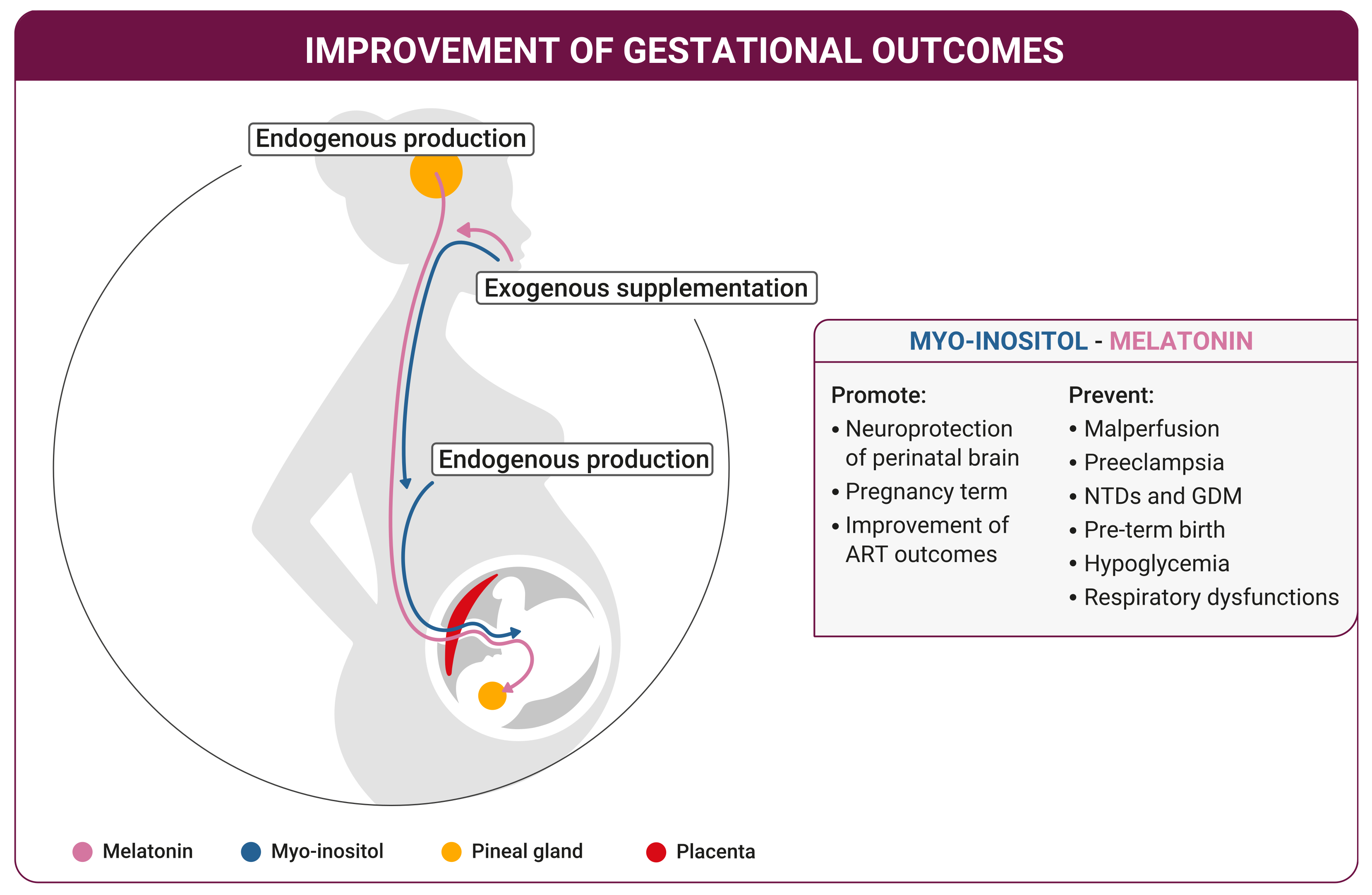
5. Fetus Growth
6. Childbirth
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiarello, D.I.; Abad, C.L.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Wdowiak, A.; Filip, M. The effect of myo-inositol, vitamin D3 and melatonin on the oocyte quality and pregnancy in in vitro fertilization: A randomized prospective controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Carlomagno, G.; Antonini, G.; Pacchiarotti, A. Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol. Endocrinol. 2016, 32, 69–73. [Google Scholar] [CrossRef]
- Iervolino, M.; Lepore, E.; Forte, G.; Laganà, A.S.; Buzzaccarini, G.; Unfer, V. Natural Molecules in the Management of Polycystic Ovary Syndrome (PCOS): An Analytical Review. Nutrients 2021, 13, 1677. [Google Scholar] [CrossRef]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Cell. Mol. Life Sci. 1993, 49, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Deurveilher, S.; Burns, J.; Semba, K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: A dual tract-tracing study in rat. Eur. J. Neurosci. 2002, 16, 1195–1213. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Baumgartner, J.E.; Edwards, M.S.B. Pineal Tumors. Neurosurg. Clin. N. Am. 1992, 3, 853–862. [Google Scholar] [CrossRef]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; de Amorim, J.P.; Teixeira, G.R.; Mendes, L.O.; Fioruci, B.A.; Pinheiro, P.F.; Seiva, F.R.; Novelli, E.L.; Mello Júnior, W.; Martinez, M.; et al. Long-term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Braz. J. Med. Biol. Res. 2011, 44, 217–223. [Google Scholar] [CrossRef]
- Minguini, I.P.; Luquetti, C.M.; Baracat, M.C.P.; Maganhin, C.C.; Nunes, C.O.; Simões, R.S.; Veiga, E.C.A.; Cipolla Neto, J.; Baracat, E.C.; Soares Junior, J.M. Melatonin effects on ovarian follicular cells: A systematic review. Rev. Assoc. Med. Bras. 2019, 65, 1122–1127. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef]
- Charollais, E.; Posternak, T. Research on the biochemistry of cyclitols. Ix. Contribution to the study of the metabolism of ms-inositol in the rat. Ii. Helv. Chim. Acta 1965, 48, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Bizzarri, M. Inositols in Insulin Signaling and Glucose Metabolism. Int. J. Endocrinol. 2018, 2018, 1968450. [Google Scholar] [CrossRef]
- Harland, B.F.; Smikle-Williams, S.; Oberleas, D. High performance liquid chromatography analysis of phytate (IP6) in selected foods. J. Food Compos. Anal. 2004, 17, 227–233. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Verón, G.L. Myo-inositol in health and disease: Its impact on semen parameters and male fertility. Andrology 2020, 8, 277–298. [Google Scholar] [CrossRef]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef]
- Milewska, E.M.; Czyzyk, A.; Meczekalski, B.; Genazzani, A.D. Inositol and human reproduction. From cellular metabolism to clinical use. Gynecol. Endocrinol. 2016, 32, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Gambioli, R.; Forte, G.; Buzzaccarini, G.; Unfer, V.; Laganà, A.S. Myo-Inositol as a Key Supporter of Fertility and Physiological Gestation. Pharmaceuticals 2021, 14, 504. [Google Scholar] [CrossRef]
- Valenzuela, F.J.; Torres-Farfan, C.; Richter, H.G.; Mendez, N.; Campino, C.; Torrealba, F.; Valenzuela, G.J.; Serón-Ferré, M. Clock gene expression in adult primate suprachiasmatic nuclei and adrenal: Is the adrenal a peripheral clock responsive to melatonin? Endocrinology 2008, 149, 1454–1461. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Gitto, E.; Sainz, R.M.; Mayo, J.C.; Leon, J.; Manchester, L.C.; Vijayalaxmi; Kilic, E.; Kilic, U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol. J. Pharmacol. 2004, 56, 159–170. [Google Scholar] [PubMed]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef]
- Vitale, S.G.; Rossetti, P.; Corrado, F.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Valenti, G.; Sapia, F.; Laganà, A.S.; Buscema, M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int. J. Endocrinol. 2016, 2016, 4987436. [Google Scholar] [CrossRef] [PubMed]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; JiaJia, L.; Yang, L. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.H.; Leal, C.L.; Cruz, J.F.; Tan, D.X.; Reiter, R.J. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 2014, 82, 925–932. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef]
- Unfer, V.; Forte, G. Does inositol ratio orchestrate the fate of ovarian follicles? Med. Hypotheses 2020, 144, 109983. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.-P.; Fusi, F.; Occhi, F.; De Santis, L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil. Steril. 2009, 91, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.T.; Rogers, M.S.; Briton-Jones, C.; Haines, C. Effects of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Hum. Reprod. 2003, 18, 408–416. [Google Scholar] [CrossRef]
- Vartanyan, E.V.; Tsaturova, K.A.; Devyatova, E.A.; Mikhaylyukova, A.S.; Levin, V.A.; Petuhova, N.L.; Markin, A.V.; Steptsova, E.M. Improvement in quality of oocytes in polycystic ovarian syndrome in programs of in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 8–11. [Google Scholar] [CrossRef][Green Version]
- Unfer, V.; Dinicola, S.; Laganà, A.S.; Bizzarri, M. Altered Ovarian Inositol Ratios May Account for Pathological Steroidogenesis in PCOS. Int. J. Mol. Sci. 2020, 21, 7157. [Google Scholar] [CrossRef]
- Kamenov, Z.; Gateva, A. Inositols in PCOS. Molecules 2020, 25, 5566. [Google Scholar] [CrossRef]
- Akbari Sene, A.; Tabatabaie, A.; Nikniaz, H.; Alizadeh, A.; Sheibani, K.; Mortezapour Alisaraie, M.; Tabatabaie, M.; Ashrafi, M.; Amjadi, F. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: A randomized clinical trial. Arch. Gynecol. Obstet. 2019, 299, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Lisi, F.; Carfagna, P.; Oliva, M.M.; Rago, R.; Lisi, R.; Poverini, R.; Manna, C.; Vaquero, E.; Caserta, D.; Raparelli, V.; et al. Pretreatment with myo-inositol in non polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: A pilot study. Reprod. Biol. Endocrinol. 2012, 10, 52. [Google Scholar] [CrossRef]
- Caprio, F.; D’Eufemia, M.D.; Trotta, C.; Campitiello, M.R.; Ianniello, R.; Mele, D.; Colacurci, N. Myo-inositol therapy for poor-responders during IVF: A prospective controlled observational trial. J. Ovarian. Res. 2015, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Raffone, E.; Rizzo, P.; Buffo, S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: A prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011, 27, 857–861. [Google Scholar] [CrossRef]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar]
- Khan, H.L.; Bhatti, S.; Abbas, S.; Kaloglu, C.; Qurat-Ul-Ain Zahra, S.; Khan, Y.L.; Hassan, Z.; Turhan, N.; Aydin, H.H. Melatonin levels and microRNA (miRNA) relative expression profile in the follicular ambient microenvironment in patients undergoing in vitro fertilization process. J. Assist. Reprod. Genet. 2021, 38, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Wu, K.; Liu, H.; Huang, T.; Chen, Z.J.; Zhao, S.; Ma, J.; Zhao, H. Melatonin promotes human oocyte maturation and early embryo development by enhancing clathrin-mediated endocytosis. J. Pineal Res. 2019, 67, e12601. [Google Scholar] [CrossRef]
- Facchinetti, F.; Espinola, M.S.B.; Dewailly, D.; Ozay, A.C.; Prapas, N.; Vazquez-Levin, M.; Wdowiak, A.; Unfer, V.; Appetecchia, M.; Aragona, C.; et al. Breakthroughs in the Use of Inositols for Assisted Reproductive Treatment (ART). Trends Endocrinol. Metab. 2020, 31, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Colazingari, S.; Fiorenza, M.T.; Carlomagno, G.; Najjar, R.; Bevilacqua, A. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J. Assist. Reprod. Genet. 2014, 31, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, M.T.; Torcia, S.; Canterini, S.; Bevilacqua, A.; Narducci, M.G.; Ragone, G.; Croce, C.M.; Russo, G.; Mangia, F. TCL1 promotes blastomere proliferation through nuclear transfer, but not direct phosphorylation, of AKT/PKB in early mouse embryos. Cell Death Differ. 2008, 15, 420–422. [Google Scholar] [CrossRef]
- Garg, D.; Tal, R. Inositol Treatment and ART Outcomes in Women with PCOS. Int. J. Endocrinol. 2016, 2016, 1979654. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, D.; Zhang, Y.; Lin, Y.; Song, J.; Li, S.; Sun, Y. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine 2017, 96, e8842. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Rizzo, P.; Raffone, E.; Roseff, S. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 452–457. [Google Scholar]
- Paria, B.C.; Reese, J.; Das, S.K.; Dey, S.K. Deciphering the cross-talk of implantation: Advances and challenges. Science 2002, 296, 2185–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, F.; Tian, X.; Ji, P.; Liu, G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod. Biol. Endocrinol. 2017, 15, 78. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, B.; Peng, W.; Mao, T.; Wu, H.; Zhang, Y. Melatonin protect the development of preimplantation mouse embryos from sodium fluoride-induced oxidative injury. Env. Toxicol. Pharm. 2017, 54, 133–141. [Google Scholar] [CrossRef]
- Asgari, Z.; Ghasemian, F.; Ramezani, M.; Bahadori, M.H. The effect of melatonin on the developmental potential and implantation rate of mouse embryos. Cell J. 2012, 14, 203–208. [Google Scholar] [PubMed]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef]
- Gray, C.A.; Taylor, K.M.; Ramsey, W.S.; Hill, J.R.; Bazer, F.W.; Bartol, F.F.; Spencer, T.E. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol. Reprod. 2001, 64, 1608–1613. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Martin, D.; Carrocera, S.; Alonso-Guervos, M.; Mora, M.I.; Corrales, F.J.; Peynot, N.; Giraud-Delville, C.; Duranthon, V.; Sandra, O.; et al. Localisation of stem cell factor, stanniocalcin-1, connective tissue growth factor and heparin-binding epidermal growth factor in the bovine uterus at the time of blastocyst formation. Reprod. Fertil. Dev. 2017, 29, 2127–2139. [Google Scholar] [CrossRef]
- Zou, H.; Chen, B.; Ding, D.; Gao, M.; Chen, D.; Liu, Y.; Hao, Y.; Zou, W.; Ji, D.; Zhou, P.; et al. Melatonin promotes the development of immature oocytes from the COH cycle into healthy offspring by protecting mitochondrial function. J. Pineal Res. 2020, 68, e12621. [Google Scholar] [CrossRef] [PubMed]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The regulations and role of circadian clock and melatonin in uterine receptivity and pregnancy-An immunological perspective. Am. J. Reprod. Immunol. 2017, 78, e12715. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yao, G.; Wang, Y.; Xu, H.; Ji, X.; He, Y.; Zhu, Q.; Chen, Z.; Sun, Y. Transcriptomic changes during the pre-receptive to receptive transition in human endometrium detected by RNA-Seq. J. Clin. Endocrinol. Metab. 2014, 99, E2744–E2753. [Google Scholar] [CrossRef]
- Muter, J.; Lucas, E.S.; Chan, Y.W.; Brighton, P.J.; Moore, J.D.; Lacey, L.; Quenby, S.; Lam, E.W.; Brosens, J.J. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J. 2015, 29, 1603–1614. [Google Scholar] [CrossRef]
- Warner, S.M.; Conlon, F.V.; Kane, M.T. Inositol transport in preimplantation rabbit embryos: Effects of embryo stage, sodium, osmolality and metabolic inhibitors. Reproduction 2003, 125, 479–493. [Google Scholar] [CrossRef]
- Kuşcu, N.; Bizzarri, M.; Bevilacqua, A. Myo-Inositol Safety in Pregnancy: From Preimplantation Development to Newborn Animals. Int. J. Endocrinol. 2016, 2016, 2413857. [Google Scholar] [CrossRef] [PubMed]
- Hynes, A.C.; Sreenan, J.M.; Kane, M.T. Uptake and incorporation of myo-inositol by bovine preimplantation embryos from two-cell to early blastocyst stages. Mol. Reprod. Dev. 2000, 55, 265–269. [Google Scholar] [CrossRef]
- Hynes, A.C.; Sreenan, J.M.; Kane, M.T. The phosphatidylinositol signalling system in elongating bovine blastocysts; Formation of phosphoinositides, inositol phosphates and stimulation by growth factors. Reprod. Fertil. Dev. 2002, 14, 515–523. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Dante, G.; Facchinetti, F. Effects of myo-inositol in women with PCOS: A systematic review of randomized controlled trials. Gynecol. Endocrinol. 2012, 28, 509–515. [Google Scholar] [CrossRef]
- Unfer, V.; Nestler, J.E.; Kamenov, Z.A.; Prapas, N.; Facchinetti, F. Effects of Inositol(s) in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Int. J. Endocrinol. 2016, 2016, 1849162. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Saharkhiz, N.; Azizi, E.; Hashemi, T.; Ghodssi-Ghassemabadi, R. Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: A randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101698. [Google Scholar] [CrossRef] [PubMed]
- West, R.C.; Ming, H.; Logsdon, D.M.; Sun, J.; Rajput, S.K.; Kile, R.A.; Schoolcraft, W.B.; Roberts, R.M.; Krisher, R.L.; Jiang, Z.; et al. Dynamics of trophoblast differentiation in peri-implantation-stage human embryos. Proc. Natl. Acad. Sci. USA 2019, 116, 22635–22644. [Google Scholar] [CrossRef]
- Landgraf, D.; Achten, C.; Dallmann, F.; Oster, H. Embryonic development and maternal regulation of murine circadian clock function. Chronobiol. Int. 2015, 32, 416–427. [Google Scholar] [CrossRef]
- Akiyama, S.; Ohta, H.; Watanabe, S.; Moriya, T.; Hariu, A.; Nakahata, N.; Chisaka, H.; Matsuda, T.; Kimura, Y.; Tsuchiya, S.; et al. The uterus sustains stable biological clock during pregnancy. Tohoku J. Exp. Med. 2010, 221, 287–298. [Google Scholar] [CrossRef]
- Chuffa, L.G.A.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300. [Google Scholar] [CrossRef]
- Waddell, B.J.; Wharfe, M.D.; Crew, R.C.; Mark, P.J. A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta 2012, 33, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, S.; Shin, N.E.; Na, Q.; Dong, J.; Jia, B.; Jones-Beatty, K.; McLane, M.W.; Ozen, M.; Lei, J.; et al. Melatonin for prevention of placental malperfusion and fetal compromise associated with intrauterine inflammation-induced oxidative stress in a mouse model. J. Pineal Res. 2019, 67, e12591. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, H.; Dash, O.; Park, M.; Shin, N.E.; McLane, M.W.; Lei, J.; Hwang, J.Y.; Burd, I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am. J. Reprod. Immunol. 2019, 82, e13151. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Gao, Y.; Wan, J.; Tong, M.; Lee, A.C.; Zhao, M.; Chen, Q. The reduction in circulating levels of melatonin may be associated with the development of preeclampsia. J. Hum. Hypertens. 2016, 30, 666–671. [Google Scholar] [CrossRef]
- Lanoix, D.; Guérin, P.; Vaillancourt, C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: New insights into the role of this hormone in pregnancy. J. Pineal Res. 2012, 53, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Loren, P.; Sánchez, R.; Arias, M.E.; Felmer, R.; Risopatrón, J.; Cheuquemán, C. Melatonin Scavenger Properties against Oxidative and Nitrosative Stress: Impact on Gamete Handling and In Vitro Embryo Production in Humans and Other Mammals. Int. J. Mol. Sci. 2017, 18, 1119. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.W.; Copp, A.J.; Greene, N.D.E.; Glazier, J.D. Maternal Inositol Status and Neural Tube Defects: A Role for the Human Yolk Sac in Embryonic Inositol Delivery? Adv. Nutr. 2021, 12, 212–222. [Google Scholar] [CrossRef]
- Jauniaux, E.; Hempstock, J.; Teng, C.; Battaglia, F.C.; Burton, G.J. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: Maintenance of redox potential in a low oxygen environment. J. Clin. Endocrinol. Metab. 2005, 90, 1171–1175. [Google Scholar] [CrossRef]
- Yang, F.; Huang, L.; Tso, A.; Wang, H.; Cui, L.; Lin, L.; Wang, X.; Ren, M.; Fang, X.; Liu, J.; et al. Inositol 1,4,5-trisphosphate receptors are essential for fetal-maternal connection and embryo viability. PLoS Genet. 2020, 16, e1008739. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Development of the Human Placenta and Fetal Heart: Synergic or Independent? Front. Physiol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.Y.; Copp, A.J. Inositol, neural tube closure and the prevention of neural tube defects. Birth. Defects Res. 2017, 109, 68–80. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Palomba, S.; Laganà, A.S.; Orio, F. Inositols in the Treatment of Insulin-Mediated Diseases. Int. J. Endocrinol. 2016, 2016, 3058393. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Marin, I.; Picconi, O.; Laganà, A.S.; Costabile, L.; Unfer, V. A multicenter clinical study with myo-inositol and alpha-lactalbumin in Mexican and Italian PCOS patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F. Editorial—Update on Inositol(s). Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1–3. [Google Scholar] [PubMed]
- Noventa, M.; Vitagliano, A.; Quaranta, M.; Borgato, S.; Abdulrahim, B.; Gizzo, S. Preventive and Therapeutic Role of Dietary Inositol Supplementation in Periconceptional Period and During Pregnancy: A Summary of Evidences and Future Applications. Reprod. Sci. 2016, 23, 278–288. [Google Scholar] [CrossRef]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef]
- Facchinetti, F.; Cavalli, P.; Copp, A.J.; D’Anna, R.; Kandaraki, E.; Greene, N.D.E.; Unfer, V. An update on the use of inositols in preventing gestational diabetes mellitus (GDM) and neural tube defects (NTDs). Expert Opin. Drug Metab. Toxicol. 2020, 16, 1187–1198. [Google Scholar] [CrossRef]
- Cavalli, P.; Ronda, E. Myoinositol: The Bridge (PONTI) to Reach a Healthy Pregnancy. Int. J. Endocrinol. 2017, 2017, 5846286. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.Y.; Gay, V.; Burren, K.; Mills, K.; Chitty, L.S.; Copp, A.J. Inositol for the prevention of neural tube defects: A pilot randomised controlled trial. Br. J. Nutr. 2016, 115, 974–983. [Google Scholar] [CrossRef]
- “How Many People Are Affected by or at Risk for Spina Bifida?”. Available online: https://www.nichd.nih.gov/health/topics/spinabifida/conditioninfo/affected-risk (accessed on 23 March 2021).
- Özturan, A.; Arslan, S.; Kocaadam, B.; Elibol, E.; İmamoğlu, İ.; Karadağ, M.G. Effect of inositol and its derivatives on diabetes: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1124–1136. [Google Scholar] [CrossRef]
- Oskovi-Kaplan, Z.A.; Ozgu-Erdinc, A.S. Management of Gestational Diabetes Mellitus. Adv. Exp. Med. Biol. 2021, 1307, 257–272. [Google Scholar] [CrossRef]
- Pillai, R.A.; Islam, M.O.; Selvam, P.; Sharma, N.; Chu, A.H.Y.; Watkins, O.C.; Godfrey, K.M.; Lewis, R.M.; Chan, S.Y. Placental Inositol Reduced in Gestational Diabetes as Glucose Alters Inositol Transporters and IMPA1 Enzyme Expression. J. Clin. Endocrinol. Metab. 2021, 106, e875–e890. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Bizzarri, M.; Benvenga, S.; D’Anna, R.; Lanzone, A.; Soulage, C.; Di Renzo, G.C.; Hod, M.; Cavalli, P.; Chiu, T.T.; et al. Results from the International Consensus Conference on Myo-inositol and d-chiro-inositol in Obstetrics and Gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 72–76. [Google Scholar] [CrossRef]
- Gateva, A.; Unfer, V.; Kamenov, Z. The use of inositol(s) isomers in the management of polycystic ovary syndrome: A comprehensive review. Gynecol. Endocrinol. 2018, 34, 545–550. [Google Scholar] [CrossRef]
- D’Anna, R.; Di Benedetto, V.; Rizzo, P.; Raffone, E.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. Myo-inositol may prevent gestational diabetes in PCOS women. Gynecol. Endocrinol. 2012, 28, 440–442. [Google Scholar] [CrossRef]
- D’Anna, R.; Santamaria, A.; Alibrandi, A.; Corrado, F.; Di Benedetto, A.; Facchinetti, F. Myo-Inositol for the Prevention of Gestational Diabetes Mellitus. A Brief Review. J. Nutr. Sci. Vitam. 2019, 65, S59–S61. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.; Lee, J.; Olcese, J. Circadian clock regulation of melatonin MTNR1B receptor expression in human myometrial smooth muscle cells. Mol. Hum. Reprod. 2015, 21, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef]
- Van Dalum, J.; Melum, V.J.; Wood, S.H.; Hazlerigg, D.G. Maternal Photoperiodic Programming: Melatonin and Seasonal Synchronization Before Birth. Front. Endocrinol. 2019, 10, 901. [Google Scholar] [CrossRef]
- Domínguez Rubio, A.P.; Correa, F.; Aisemberg, J.; Dorfman, D.; Bariani, M.V.; Rosenstein, R.E.; Zorrilla Zubilete, M.; Franchi, A.M. Maternal administration of melatonin exerts short- and long-term neuroprotective effects on the offspring from lipopolysaccharide-treated mice. J. Pineal Res. 2017, 63, e12439. [Google Scholar] [CrossRef]
- Carloni, S.; Favrais, G.; Saliba, E.; Albertini, M.C.; Chalon, S.; Longini, M.; Gressens, P.; Buonocore, G.; Balduini, W. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal Res. 2016, 61, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Proietti, F.; Rocchi, M.; Longini, M.; Marseglia, L.; D’Angelo, G.; Balduini, W.; Gitto, E.; Buonocore, G. Melatonin Pharmacokinetics Following Oral Administration in Preterm Neonates. Molecules 2017, 22, 2115. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the prevention of gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Matarrelli, B.; Vitacolonna, E.; D’Angelo, M.; Pavone, G.; Mattei, P.A.; Liberati, M.; Celentano, C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: A randomized controlled trial. J. Matern. Fetal. Neonatal. Med. 2013, 26, 967–972. [Google Scholar] [CrossRef]
- Celentano, C.; Matarrelli, B.; Pavone, G.; Vitacolonna, E.; Mattei, P.A.; Berghella, V.; Liberati, M. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: A randomized controlled trial. J. Matern. Fetal. Neonatal. Med. 2020, 33, 743–751. [Google Scholar] [CrossRef]
- Chu, A.H.Y.; Tint, M.T.; Chang, H.F.; Wong, G.; Yuan, W.L.; Tull, D.; Nijagal, B.; Narayana, V.K.; Meikle, P.J.; Chang, K.T.E.; et al. High placental inositol content associated with suppressed pro-adipogenic effects of maternal glycaemia in offspring: The GUSTO cohort. Int. J. Obes. 2021, 45, 247–257. [Google Scholar] [CrossRef]
- Catalano, P.M.; Thomas, A.; Huston-Presley, L.; Amini, S.B. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am. J. Obstet. Gynecol. 2003, 189, 1698–1704. [Google Scholar] [CrossRef]
- Longo, M.; Alrais, M.; Tamayo, E.H.; Ferrari, F.; Facchinetti, F.; Refuerzo, J.S.; Blackwell, S.C.; Sibai, B.M. Vascular and metabolic profiles in offspring born to pregnant mice with metabolic syndrome treated with inositols. Am. J. Obstet. Gynecol. 2019, 220, 279.e1–279.e9. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Laganà, A.S.; Aragona, D.; Unfer, V. Inositol and pulmonary function. Could myo-inositol treatment downregulate inflammation and cytokine release syndrome in SARS-CoV-2? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3426–3432. [Google Scholar] [CrossRef]
- Buccafusca, R.; Venditti, C.P.; Kenyon, L.C.; Johanson, R.A.; Van Bockstaele, E.; Ren, J.; Pagliardini, S.; Minarcik, J.; Golden, J.A.; Coady, M.J.; et al. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: Myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol. Genet. Metab. 2008, 95, 81–95. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, P.M.; Di Fiore, J.M. Myo-inositol Effects on the Developing Respiratory Neural Control System. Adv. Exp. Med. Biol. 2018, 1071, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Menichini, D.; Facchinetti, F. Myo-inositol in the treatment of airways diseases: A minireview. Int. J. Med. Device Adjuv. Treat. 2021, 4, e296. [Google Scholar] [CrossRef]
- Hallman, M.; Bry, K.; Hoppu, K.; Lappi, M.; Pohjavuori, M. Inositol supplementation in premature infants with respiratory distress syndrome. N. Engl. J. Med. 1992, 326, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.; Ohlsson, A.; Plakkal, N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst. Rev. 2015, 2, CD000366. [Google Scholar] [CrossRef] [PubMed]
| Melatonin | Myo-Inositol | |
|---|---|---|
| Reproductive Apparatus |
|
|
| Oogenesis |
|
|
| Oocyte Maturationand and Fertilization |
|
|
| Blastocyst Development and Implantation |
|
|
| Fetus Growth |
|
|
| Childbirth |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Forte, G.; Montanino Oliva, M.; Laganà, A.S.; Unfer, V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. Int. J. Mol. Sci. 2021, 22, 8433. https://doi.org/10.3390/ijms22168433
Russo M, Forte G, Montanino Oliva M, Laganà AS, Unfer V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. International Journal of Molecular Sciences. 2021; 22(16):8433. https://doi.org/10.3390/ijms22168433
Chicago/Turabian StyleRusso, Michele, Gianpiero Forte, Mario Montanino Oliva, Antonio Simone Laganà, and Vittorio Unfer. 2021. "Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth" International Journal of Molecular Sciences 22, no. 16: 8433. https://doi.org/10.3390/ijms22168433
APA StyleRusso, M., Forte, G., Montanino Oliva, M., Laganà, A. S., & Unfer, V. (2021). Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. International Journal of Molecular Sciences, 22(16), 8433. https://doi.org/10.3390/ijms22168433








