Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice
Abstract
1. Introduction
2. Results
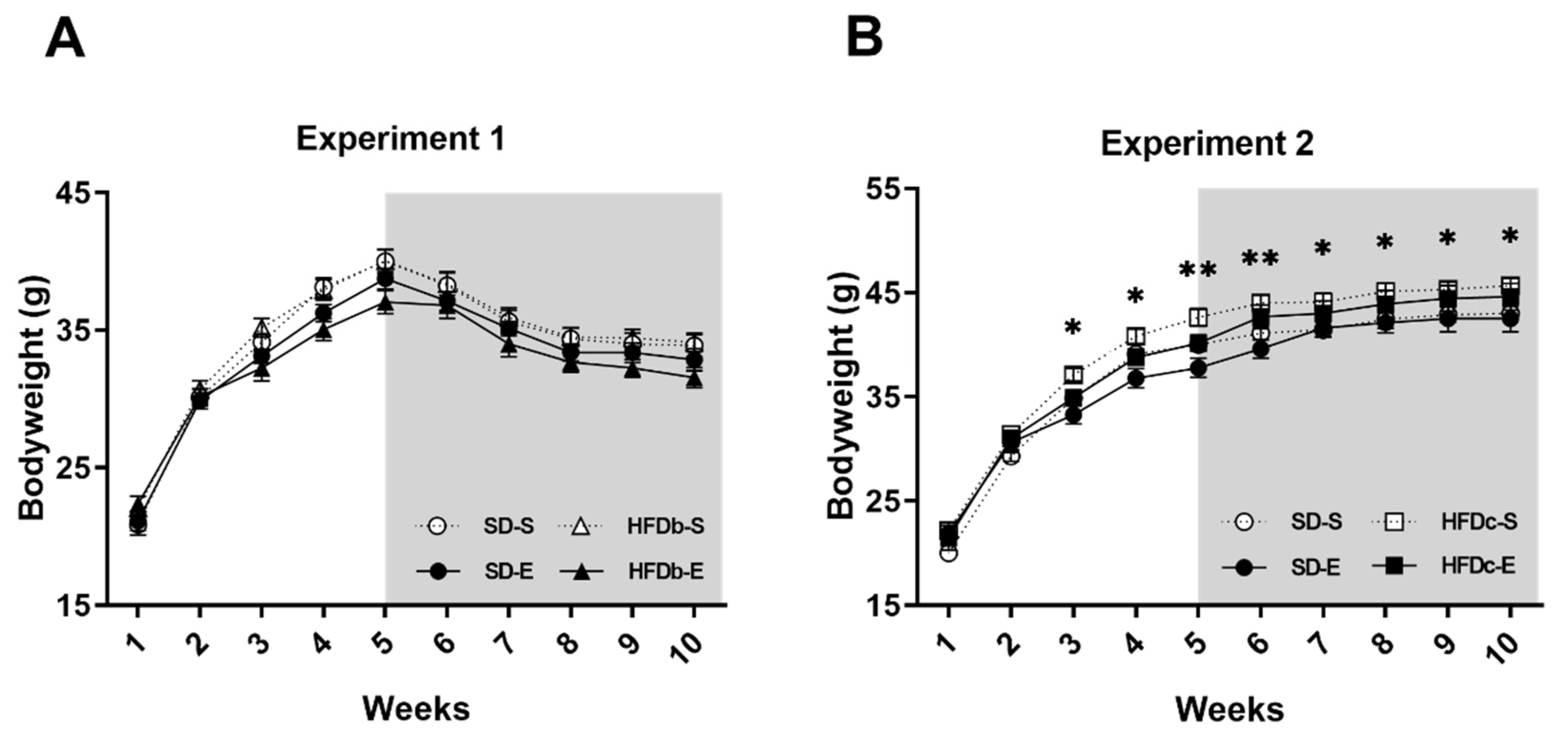
2.1. Bodyweight Is Increased by Continuous but Not Intermittent Access to HFD
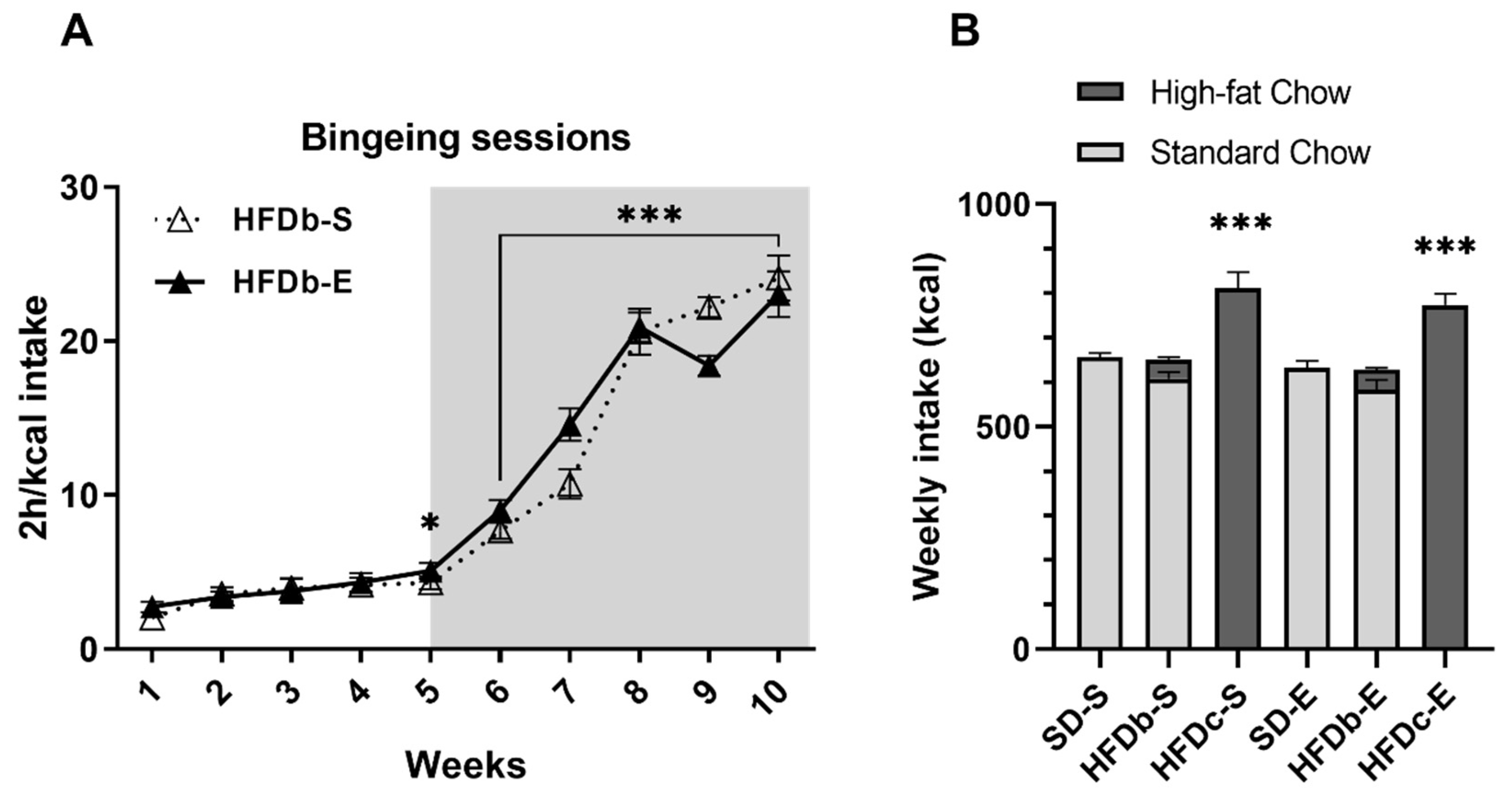
2.2. Intermittent Access to HFD Induces Bingeing Behavior and Continuous Access Increases Caloric Intake
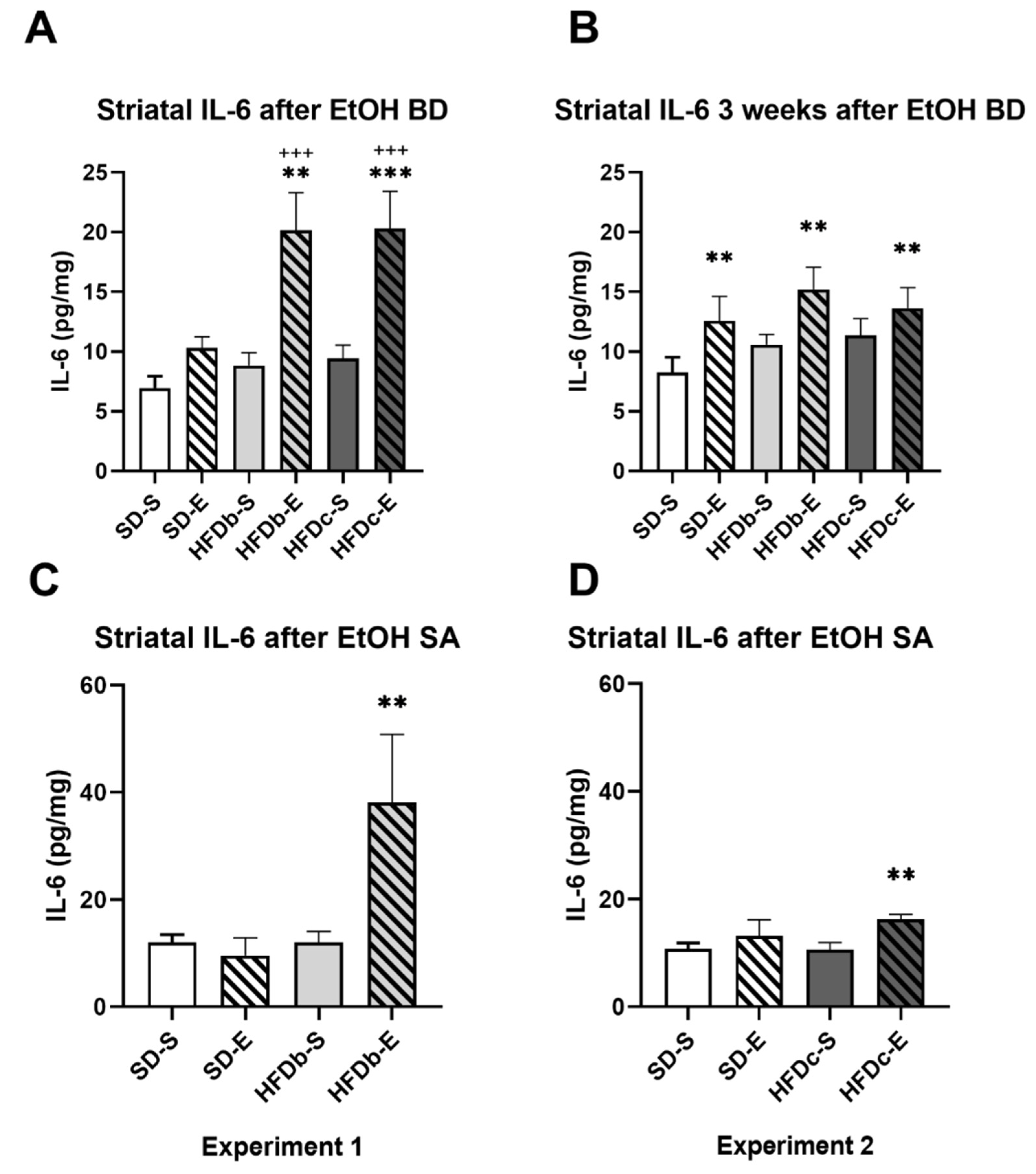
2.3. A High Fat Diet and Intermittent Ethanol Intake during Adolescence Increases the Neuroinflammatory Response
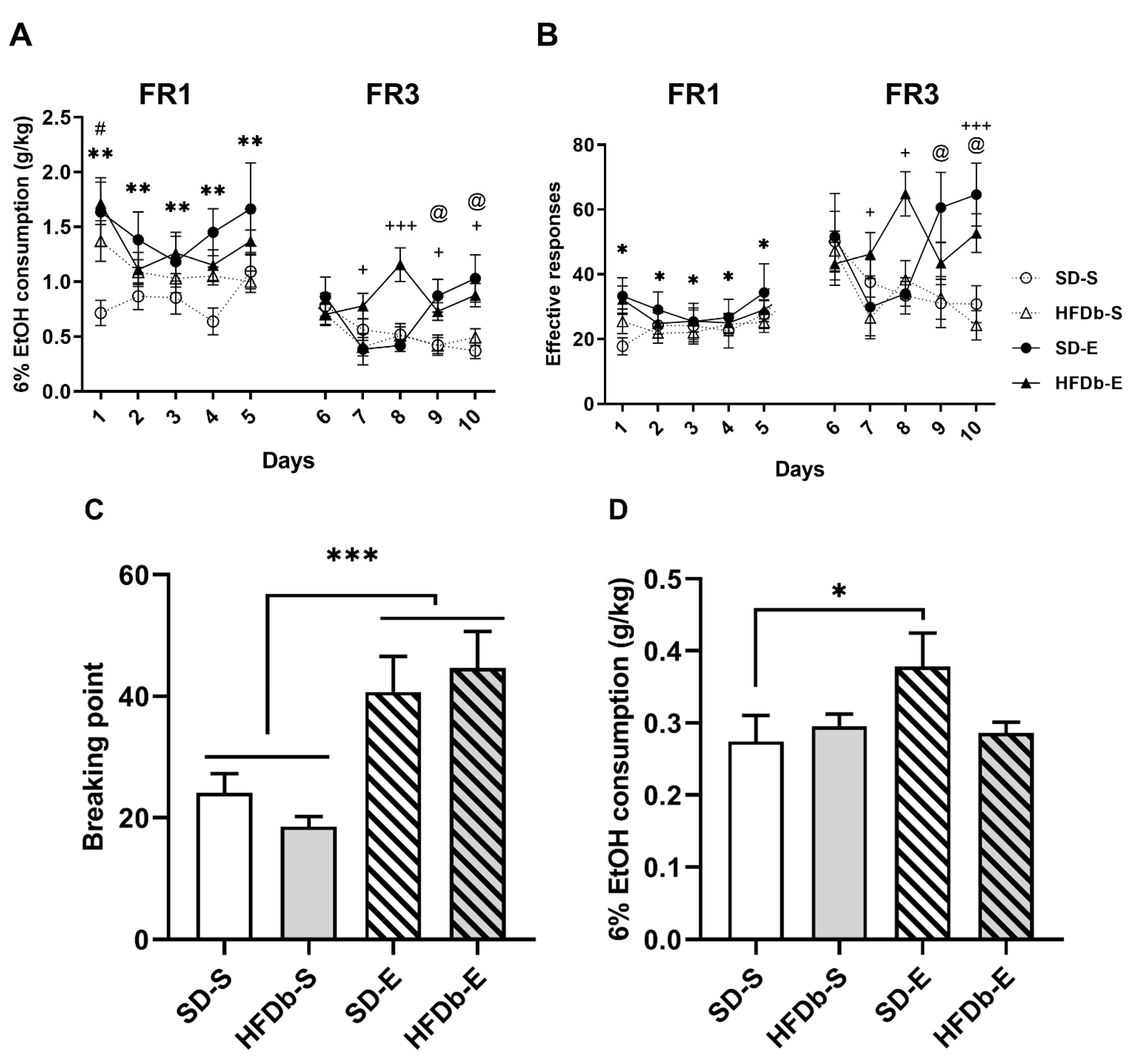
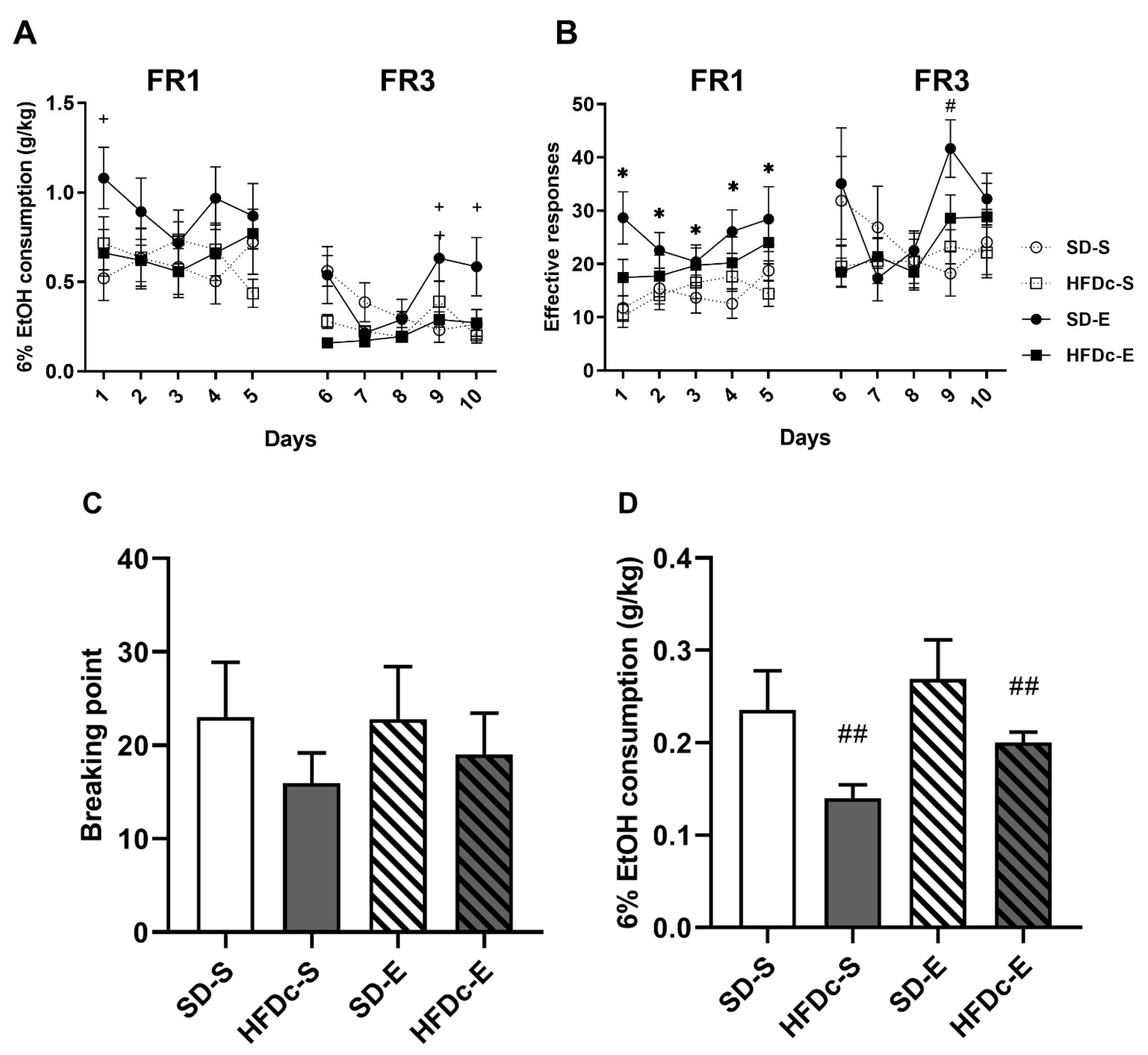
2.4. Oral Self-Administration of Ethanol
2.4.1. Experiment 1
2.4.2. Experiment 2
3. Discussion
3.1. Access Schedule to HFD Exposure Differentially Affects the Increase in Ethanol Intake Induced by BD Exposure during Adolescence
3.2. HFD Increases the Neuroinflammatory Response Induced by Exposure to Binge Drinking during Adolescence
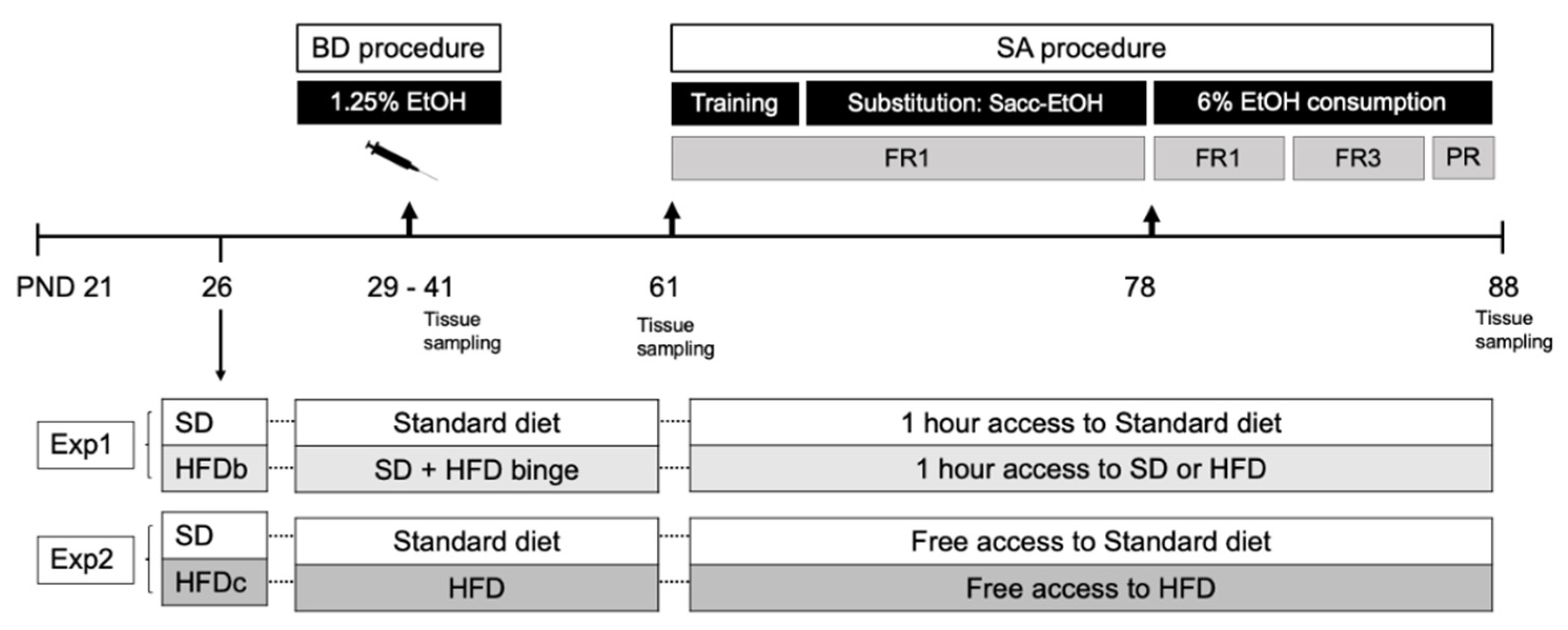
4. Materials and Methods
4.1. Animals and Experimental Procedure
4.2. Feeding Conditions
4.3. Drugs
4.4. Binge Drinking Protocol in Adolescent Mice
4.5. Oral Ethanol Self-Administration
4.5.1. Training Phase (8 Days)
4.5.2. Saccharin Fading (9 Days)
4.5.3. Ethanol Consumption 6% (11 Days)
4.5.4. Progressive Responding Ratio for Alcohol
4.6. Tissue Sampling
4.7. IL-6 and CX3CL1 Measurements
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization [WHO]. Available online: https://apps.who.int/iris/handle/10665/274603 (accessed on 18 January 2021).
- Zheng, Y.; Brendgen, M.; Dionne, G.; Boivin, M.; Vitaro, F. Genetic and environmental influences on developmental trajectories of adolescent alcohol use. Eur. Child Adolesc. Psychiatry 2019, 28, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse, and Alcoholism [NIAAA]. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/binge-drinking (accessed on 2 April 2021).
- Kuntsche, E.; Kuntsche, S.; Thrul, J.; Gmel, G. Binge drinking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 976–1017. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.E.; Polich, J. Binge drinking in young adults: Data, definitions, and determinants. Psychol. Bull. 2009, 135, 142–156. [Google Scholar] [CrossRef]
- Younis, R.M.; Wolstenholme, J.T.; Bagdas, D.; Bettinger, J.C.; Miles, M.F.; Damaj, M.I. Adolescent but not adult ethanol binge drinking modulates ethanol behavioral effects in mice later in life. Pharm. Biochem. Behav. 2019, 184, 172740. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Alcohol Related Disease Consortium; Vassallo, G.A.; Antonelli, G.; Antonelli, M.; Tarli, C.; Mirijello, A.; Agyei-Nkansah, A.; Mentella, M.C.; Ferrarese, D.; et al. Binge Drinking among adolescents is related to the development of Alcohol Use Disorders: Results from a Cross-Sectional Study. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- King, K.M.; Chassin, L. A prospective study of the effects of age of initiation of alcohol and drug use on young adult substance dependence. J. Stud. Alcohol. Drugs 2007, 68, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Effects of adolescent alcohol consumption on the brain and behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P.; Broadwater, M.A.; Robinson, D.L. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharm. Rev. 2016, 68, 1074–1109. [Google Scholar] [CrossRef]
- Marco, E.M.; Peñasco, S.; Hernández, M.-D.; Gil, A.; Borcel, E.; Moya, M.; Giné, E.; López-Moreno, J.A.; Guerri, C.; López-Gallardo, M.; et al. Long-Term effects of intermittent adolescent alcohol exposure in male and female rats. Front. Behav. Neurosci. 2017, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.; Crews, F. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience 2012, 226, 475–488. [Google Scholar] [CrossRef]
- Pascual, M.; Boix, J.; Felipo, V.; Guerri, C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009, 108, 920–931. [Google Scholar] [CrossRef]
- Waller, R.; Murray, L.; Shaw, D.S.; Forbes, E.E.; Hyde, L.W. Accelerated alcohol use across adolescence predicts early adult symptoms of alcohol use disorder via reward-related neural function. Psychol. Med. 2019, 49, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Molnar, S.M.; Beaton, L.E.; Happer, J.P.; Holcomb, L.A.; Huang, S.; Arienzo, D.; Marinkovic, K. Behavioral and brain activity indices of cognitive control deficits in binge drinkers. Brain Sci. 2018, 8, 9. [Google Scholar] [CrossRef]
- Carbia, C.; López-Caneda, E.; Corral, M.; Cadaveira, F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci. Biobehav. Rev. 2018, 90, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Ezra-Nevo, G.; Henriques, S.F.; Ribeiro, C. The diet-microbiome tango: How nutrients lead the gut brain axis. Curr. Opin. Neurobiol. 2020, 62, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Kao, C.-Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Nakandakari, S.C.B.R.; Muñoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Sant’Ana, M.R.; Pavan, I.C.B.; da Silva, L.G.S.; Morelli, A.P.; Simabuco, F.M.; da Silva, A.S.R.; et al. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav. Immun. 2019, 79, 284–293. [Google Scholar] [CrossRef]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Ota, K.T.; Wohleb, E.S.; Rasmussen, K.; Duman, R.S. High-Fat diet induced anxiety and anhedonia: Impact on brain homeostasis and inflammation. Neuropsychopharmacology 2016, 41, 1874–1887. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.-E. Impact of drinking alcohol on gut microbiota: Recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.-C.; Chen, S.-J.; Lee, C.-H.; Cheng, C.-M. Alcohol addiction, gut microbiota, and alcoholism treatment: A review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; López-Hidalgo, R.; Montagud-Romero, S.; Ureña-Peralta, J.R.; Rodríguez-Arias, M.; Guerri, C. Role of mTOR-regulated autophagy in spine pruning defects and memory impairments induced by binge-like ethanol treatment in adolescent mice. Brain Pathol. 2021, 31, 174–188. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Montagud-Romero, S.; Forteza, J.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J. Neuroinflammation 2017, 14, 145. [Google Scholar] [CrossRef]
- Guerri, C.; Pascual, M. Impact of neuroimmune activation induced by alcohol or drug abuse on adolescent brain development. Int. J. Dev. Neurosci. 2019, 77, 89–98. [Google Scholar] [CrossRef]
- Montesinos, J.; Alfonso-Loeches, S.; Guerri, C. Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol. Clin. Exp. Res. 2016, 40, 2260–2270. [Google Scholar] [CrossRef]
- Vetreno, R.; Crews, F. Current hypotheses on the mechanisms of alcoholism. Handb. Clin. Neurol. 2014, 125, 477–497. [Google Scholar] [CrossRef]
- Crews, F.T.; Bechara, R.; Brown, L.A.; Guidot, D.M.; Mandrekar, P.; Oak, S.; Qin, L.; Szabo, G.; Wheeler, M.; Zou, J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006, 30, 720–730. [Google Scholar] [CrossRef]
- Mayfield, J.; Ferguson, L.; Harris, R.A. Neuroimmune signaling: A key component of alcohol abuse. Curr. Opin. Neurobiol. 2013, 23, 513–520. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; Crews, F.T. Innate immune signaling and alcohol use disorders. Handb. Exp. Pharm. 2018, 248, 369–396. [Google Scholar]
- Montesinos, J.; Pascual, M.; Rodríguez-Arias, M.; Miñarro, J.; Guerri, C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav. Immun. 2016, 53, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alavez, M.; Nguyen, W.; Mori, S.; Wills, D.N.; Otero, D.; Aguirre, C.A.; Singh, M.; Ehlers, C.L.; Conti, B. Time course of blood and brain Cytokine/Chemokine levels following adolescent alcohol exposure and withdrawal in rats. Alcohol. Clin. Exp. Res. 2019, 43, 2547–2558. [Google Scholar] [CrossRef]
- Pascual, M.; Montesinos, J.; Guerri, C. Role of the innate immune system in the neuropathological consequences induced by adolescent binge drinking. J. Neurosci. Res. 2018, 96, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.J.M.; Phelan, K.D.; Douglas, J.C.; Wagoner, G.; Johnson, J.W.; Xu, J.; Phelan, P.S.; Drew, P.D. Effects of Ethanol on immune response in the brain: Region-specific changes in adolescent versus adult mice. Alcohol. Clin. Exp. Res. 2013, 38, 384–391. [Google Scholar] [CrossRef]
- Bogusz, K.; Kopera, M.; Jakubczyk, A.; Trucco, E.M.; Kucharska, K.; Walenda, A.; Wojnar, M. Prevalence of alcohol use disorder among individuals who binge eat: A systematic review and meta-analysis. Addiction 2021, 116, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Giel, K.E.; Teufel, M.; Junne, F.; Zipfel, S.; Schag, K. Food-Related impulsivity in obesity and binge eating disorder—A systematic update of the evidence. Nutrients 2017, 9, 1170. [Google Scholar] [CrossRef]
- Davis, C. Compulsive overeating as an addictive behavior: Overlap between food addiction and binge eating disorder. Curr. Obes. Rep. 2013, 2, 171–178. [Google Scholar] [CrossRef]
- American Psychiatric Association [APA]. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Herpertz-Dahlmann, B. Adolescent eating disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.M.; Yokum, S.; Potenza, M.N.; Gearhardt, A.N. Neural systems implicated in obesity as an addictive disorder. Neuroendocrinol. Pathol. Situat. Dis. 2016, 223, 329–346. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, I.C.; de Freitas, J.S.; Torres, I.L.D.S. The influence of palatable diets in reward system activation: A mini review. Adv. Pharm. Sci. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2011, 1–24. [Google Scholar] [CrossRef]
- Blanco-Gandía, M.C.; Cantacorps, L.; Aracil-Fernández, A.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Valverde, O.; Miñarro, J.; Rodríguez-Arias, M. Effects of bingeing on fat during adolescence on the reinforcing effects of cocaine in adult male mice. Neuropharmacology 2017, 113, 31–44. [Google Scholar] [CrossRef]
- Corwin, R.L.; Buda-Levin, A. Behavioral models of binge-type eating. Physiol. Behav. 2004, 82, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.D.; McCutcheon, J.E.; Cone, J.J.; Ragozzino, M.; Roitman, M.F. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur. J. Neurosci. 2011, 34, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Durst, M.; Könczöl, K.; Balázsa, T.; Eyre, M.D.; Tóth, Z.E. Reward-representing D1-type neurons in the medial shell of the accumbens nucleus regulate palatable food intake. Int. J. Obes. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- Spierling, S.; de Guglielmo, G.; Kirson, D.; Kreisler, A.; Roberto, M.; George, O.; Zorrilla, E.P. Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacology 2020, 45, 579–588. [Google Scholar] [CrossRef]
- O’Connor, R.M.; Kenny, P.J. Role of striatal dopamine signaling in compulsive eating associated with obesity. Curr. Opin. Behav. Sci. 2016, 9, 152–157. [Google Scholar] [CrossRef]
- Mazzone, C.M.; Liang-Guallpa, J.; Li, C.; Wolcott, N.S.; Boone, M.H.; Southern, M.; Kobzar, N.P.; Salgado, I.D.A.; Reddy, D.M.; Sun, F.; et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat. Neurosci. 2020, 23, 1253–1266. [Google Scholar] [CrossRef]
- Coker, C.R.; Aguilar, E.A.; Snyder, A.E.; Bingaman, S.S.; Graziane, N.M.; Browning, K.N.; Arnold, A.C.; Silberman, Y. Access schedules mediate the impact of high fat diet on ethanol intake and insulin and glucose function in mice. Alcohol 2020, 86, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, N.; Blanco-Gandía, M.C.; Mateos-García, A.; Del Rio, D.; Miñarro, J.; Ruiz-Gayo, M.; Rodríguez-Arias, M. Differential impact of ad libitum or intermittent high-fat diets on bingeing ethanol-mediated behaviors. Nutrients 2019, 11, 2253. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.A.; Leibowitz, S.F.; Karatayev, O.; Hoebel, B.G. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol 2004, 34, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gandía, M.C.; Ledesma, J.C.; Aracil-Fernández, A.; Navarrete, F.; Montagud-Romero, S.; Aguilar, M.A.; Manzanares, J.; Miñarro, J.; Rodríguez-Arias, M. The rewarding effects of ethanol are modulated by binge eating of a high-fat diet during adolescence. Neuropharmacology 2017, 121, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.; Van Cleef, A.; Davis, J.F. Intermittent access to a nutritionally complete high-fat diet attenuates alcohol drinking in rats. Pharm. Biochem. Behav. 2017, 153, 105–115. [Google Scholar] [CrossRef]
- Gelineau, R.R.; Arruda, N.L.; Hicks, J.A.; De Pina, I.M.; Hatzidis, A.; Seggio, J.A. The behavioral and physiological effects of high-fat diet and alcohol consumption: Sex differences in C57BL6/J mice. Brain Behav. 2017, 7, e00708. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, A.; Käser, M.; Lichtinghagen, R.; Rhein, M.; Lenz, B.; Kornhuber, J.; Bleich, S.; Hillemacher, T. TNF-α and IL-6 serum levels: Neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol 2014, 48, 671–676. [Google Scholar] [CrossRef]
- Gruol, D.L.; Melkonian, C.; Huitron-Resendiz, S.; Roberts, A.J. Alcohol alters IL-6 signal transduction in the CNS of transgenic mice with increased astrocyte expression of IL-6. Cell. Mol. Neurobiol. 2021, 41, 733–750. [Google Scholar] [CrossRef]
- Aurelian, L.; Balan, I. GABAAR α2-activated neuroimmune signal controls binge drinking and impulsivity through regulation of the CCL2/CX3CL1 balance. Psychopharmacology 2019, 236, 3023–3043. [Google Scholar] [CrossRef]
- Aiken, A.; Clare, P.J.; Wadolowski, M.; Hutchinson, D.; Najman, J.M.; Slade, T.; Bruno, R.; McBride, N.; Kypri, K.; Mattick, R.P. Age of alcohol initiation and progression to binge drinking in adolescence: A prospective cohort study. Alcohol. Clin. Exp. Res. 2017, 42, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Escrivá-Martínez, T.; Herrero, R.; Molinari, G.; Rodríguez-Arias, M.; Verdejo-García, A.; Baños, R.M. Binge eating and binge drinking: A two-way road? An integrative review. Curr. Pharm. Des. 2020, 26, 2402–2415. [Google Scholar] [CrossRef]
- Pascual, M.; Blanco, A.M.; Cauli, O.; Miñarro, J.; Guerri, C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007, 25, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Romero, S.; Daza-Losada, M.; Vidal-Infer, A.; Maldonado, C.; Aguilar, M.A.; Miñarro, J.; Rodríguez-Arias, M. The novelty-seeking phenotype modulates the long-lasting effects of intermittent ethanol administration during adolescence. PLoS ONE 2014, 9, e92576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, L.; Sonne, S.B.; Feng, Q.; Chen, N.; Xia, Z.; Li, X.; Fang, Z.; Zhang, D.; Fjære, E.; Midtbø, L.K.; et al. High-fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Gholami, A.M.; Berry, D.; Desmarchelier, C.; Hahne, H.; Loh, G.; Mondot, S.; Lepage, P.; Rothballer, M.; Walker, A.; et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014, 8, 295–308. [Google Scholar] [CrossRef]
- Klingbeil, E.A.; De La Serre, C.B. Microbiota modulation by eating patterns and diet composition: Impact on food intake. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R1254–R1260. [Google Scholar] [CrossRef]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut microbiota-induced changes in β-Hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef]
- Vena, A.A.; Zandy, S.L.; Cofresí, R.U.; Gonzales, R.A. Behavioral, neurobiological, and neurochemical mechanisms of ethanol self-administration: A translational review. Pharmacology 2020, 212, 107573. [Google Scholar] [CrossRef] [PubMed]
- McGinn, M.A.; Tunstall, B.J.; Schlosburg, J.E.; Gregory-Flores, A.; George, O.; de Guglielmo, G.; Mason, B.J.; Hunt, H.J.; Koob, G.F.; Vendruscolo, L.F. Glucocorticoid receptor modulators decrease alcohol self-administration in male rats. Neuropharmacology 2021, 188, 108510. [Google Scholar] [CrossRef]
- Montanari, C.; Secci, M.E.; Driskell, A.; McDonald, K.O.; Schratz, C.L.; Gilpin, N.W. Chronic nicotine increases alcohol self-administration in adult male Wistar rats. Psychopharmacology 2021, 238, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Augier, E.; Dulman, R.S.; Damadzic, R.; Pilling, A.; Hamilton, J.P.; Heilig, M. The GABAB positive allosteric modulator adx71441 attenuates alcohol self-administration and relapse to alcohol seeking in rats. Neuropsychopharmacology 2017, 42, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Randall, P.A.; Stewart, R.T.; Besheer, J. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharm. Biochem. Behav. 2017, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, J.T.; Younis, R.M.; Toma, W.; Damaj, M.I. Adolescent low-dose ethanol drinking in the dark increases ethanol intake later in life in C57BL/6J, but not DBA/2J mice. Alcohol 2020, 89, 85–91. [Google Scholar] [CrossRef]
- Vargas, W.M.; Bengston, L.; Gilpin, N.W.; Whitcomb, B.W.; Richardson, H.N. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. J. Neurosci. 2014, 34, 14777–14782. [Google Scholar] [CrossRef]
- Strong, M.N.; Yoneyama, N.; Fretwell, A.M.; Snelling, C.; Tanchuck, M.A.; Finn, D.A. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm. Behav. 2010, 58, 82–90. [Google Scholar] [CrossRef]
- Alaux-Cantin, S.; Warnault, V.; Legastelois, R.; Botia, B.; Pierrefiche, O.; Vilpoux, C.; Naassila, M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 2013, 67, 521–531. [Google Scholar] [CrossRef]
- Blanco-Gandía, M.C.; Miñarro, J.; Aguilar, M.A.; Rodríguez-Arias, M. Increased ethanol consumption after interruption of fat bingeing. PLoS ONE 2018, 13, e0194431. [Google Scholar] [CrossRef]
- Jones, S.R.; Fordahl, S.C. Bingeing on high-fat food enhances evoked dopamine release and reduces dopamine uptake in the nucleus accumbens. Obesity. 2021, 29, 721–730. [Google Scholar] [CrossRef]
- Corwin, R.L.; Wojnicki, F.H.; Zimmer, D.J.; Babbs, R.K.; McGrath, L.E.; Olivos, D.R.; Mietlicki-Baase, E.G.; Hayes, M.R. Binge-type eating disrupts dopaminergic and GABAergic signaling in the prefrontal cortex and ventral tegmental area. Obesity 2016, 24, 2118–2125. [Google Scholar] [CrossRef]
- Valdivia, S.; Cornejo, M.P.; Reynaldo, M.; De Francesco, P.N.; Perello, M. Escalation in high fat intake in a binge eating model differentially engages dopamine neurons of the ventral tegmental area and requires ghrelin signaling. Psychoneuroendocrinology 2015, 60, 206–216. [Google Scholar] [CrossRef]
- Fordahl, S.; Jones, S. Limited access to a palatable high fat diet promotes binge-like food intake and addiction-like dopamine terminal adaptations in the nucleus accumbens core of mice. FASEB J. 2017, 31, 636-33. [Google Scholar]
- Puhl, M.D.; Cason, A.M.; Wojnicki, F.H.E.; Corwin, R.L.; Grigson, P.S. A history of bingeing on fat enhances cocaine seeking and taking. Behav. Neurosci. 2011, 125, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Olney, J.J.; Burnham, N.W.; Mazzone, C.M.; Lowery-Gionta, E.G.; E Pleil, K.; Kash, T.L.; E Thiele, T. Lateral hypothalamus gabaergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology 2015, 41, 1505–1512. [Google Scholar] [CrossRef]
- Barson, J.R.; Leibowitz, S.F. Hypothalamic neuropeptide signaling in alcohol addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 321–329. [Google Scholar] [CrossRef] [PubMed]
- España, R.A.; Oleson, E.B.; Locke, J.L.; Brookshire, B.R.; Roberts, D.C.S.; Jones, S.R. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur. J. Neurosci. 2010, 31, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Wellman, P.J.; Nation, J.R.; Davis, K.W. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharm. Biochem. Behav. 2007, 88, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Avesson, L.; Liljequist, S.; Lindblom, J.; Schiöth, H.B. The role of hypothalamic peptide gene expression in alcohol self-administration behavior. Peptides 2007, 28, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Goldstein, N.; Park, O.; Klima, M.L.; Vargas, A.; Betley, J.N. Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron 2019, 103, 891–908.e6. [Google Scholar] [CrossRef]
- Kao, K.; Hisatsune, T. Differential effects of dopamine D1-like and D2-like receptor agonists on water drinking behaviour under thirsty conditions in mice with reduced dopamine secretion. Eur. J. Neurosci. 2020, 51, 584–597. [Google Scholar] [CrossRef]
- Kroemer, N.B.; Small, D.M. Fuel not fun: Reinterpreting attenuated brain responses to reward in obesity. Physiol. Behav. 2016, 162, 37–45. [Google Scholar] [CrossRef]
- Davis, J.F.; Tracy, A.L.; Schurdak, J.D.; Tschöp, M.H.; Lipton, J.W.; Clegg, D.J.; Benoit, S.C. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci. 2008, 122, 1257–1263. [Google Scholar] [CrossRef]
- Rospond, B.; Sadakierska-Chudy, A.; Kazek, G.; Krośniak, M.; Bystrowska, B.; Filip, M. Assessment of metabolic and hormonal profiles and striatal dopamine D2 receptor expression following continuous or scheduled high-fat or high-sucrose diet in rats. Pharm. Rep. 2019, 71, 1–12. [Google Scholar] [CrossRef]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged high fat diet reduces dopamine reuptake without altering dat gene expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Bocarsly, M.E.; Barson, J.R.; Hoebel, B.G.; Leibowitz, S.F. Reduced accumbens dopamine in Sprague–Dawley rats prone to overeating a fat-rich diet. Physiol. Behav. 2010, 101, 394–400. [Google Scholar] [CrossRef]
- Alsiö, J.; Olszewski, P.; Norbäck, A.; Gunnarsson, Z.; Levine, A.; Pickering, C.; Schiöth, H. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 2010, 171, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.N.; Wallace, C.W.; Jacobowitz, B.S.; Fordahl, S.C. Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat. Nutr. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Erickson, E.K.; Grantham, E.K.; Warden, A.S.; Harris, R. Neuroimmune signaling in alcohol use disorder. Pharm. Biochem. Behav. 2019, 177, 34–60. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Barak, S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat. Rev. Neurosci. 2016, 17, 576–591. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Guerri, C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit. Rev. Clin. Lab. Sci. 2011, 48, 19–47. [Google Scholar] [CrossRef]
- Adams, C.; Conigrave, J.H.; Lewohl, J.; Haber, P.; Morley, K.C. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 89, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lamont, M.G.; McCallum, P.; Head, N.; Blundell, J.; Weber, J.T. Binge drinking in male adolescent rats and its relationship to persistent behavioral impairments and elevated proinflammatory/proapoptotic proteins in the cerebellum. Psychopharmacology 2020, 237, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Lallemand, F.; De Witte, P. Influence of adolescent heavy session drinking on the systemic and brain innate immune system. Alcohol Alcohol. 2014, 49, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.V.; Puro, A.C.; Manos, J.C.; Huitron-Resendiz, S.; Reyes, K.C.; Liu, K.; Vo, K.; Roberts, A.J.; Gruol, D.L. Transgenic mice with increased astrocyte expression of IL-6 show altered effects of acute ethanol on synaptic function. Neuropharmacology 2016, 103, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Khom, S.; Bajo, M.; Vlkolinsky, R.; Polis, I.; Cates-Gatto, C.; Roberto, M.; Gruol, D.L. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav. Immun. 2019, 82, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Doremus-Fitzwater, T.L.; Buck, H.M.; Bordner, K.; Richey, L.; Jones, M.E.; Deak, T. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol. Clin. Exp. Res. 2014, 38, 2186–2198. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: Significance for metabolic inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.-J. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J. Gastroenterol. 2016, 22, 8905–8909. [Google Scholar] [CrossRef]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Kim, K.-A.; Gu, W.; Lee, I.-A.; Joh, E.-H.; Kim, D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Giralt, M.; Esposito, F.L.; Fernandez-Gayol, O.; Hidalgo, J. Astrocytic IL-6 mediates locomotor activity, exploration, anxiety, learning and social behavior. Horm. Behav. 2015, 73, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Aniszewska, A.; Chłodzińska, N.; Bartkowska, K.; Winnicka, M.; Turlejski, K.; Djavadian, R. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J. Neuroimmunol. 2015, 284, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chourbaji, S.; Urani, A.; Inta, I.; Sanchis-Segura, C.; Brandwein, C.; Zink, M.; Schwaninger, M.; Gass, P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 2006, 23, 587–594. [Google Scholar] [CrossRef]
- Leclercq, S.; Cani, P.D.; Neyrinck, A.M.; Stärkel, P.; Jamar, F.; Mikolajczak, M.; Delzenne, N.M.; De Timary, P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 2012, 26, 911–918. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 axis and inflammatory signaling pathways in tissue injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Tarozzo, G.; Bortolazzi, S.; Crochemore, C.; Chen, S.-C.; Lira, A.; Abrams, J.; Beltramo, M. Fractalkine protein localization and gene expression in mouse brain. J. Neurosci. Res. 2003, 73, 81–88. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Ebisht, K.; Tremblay, M.-E. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, M.D.; Thaler, J.P. Hypothalamic inflammation and gliosis in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 325–330. [Google Scholar] [CrossRef]
- Polyák, Á.; Ferenczi, S.; Dénes, Á.; Winkler, Z.; Kriszt, R.; Pintér-Kübler, B.; Kovács, K.J. The fractalkine/Cx3CR1 system is implicated in the development of metabolic visceral adipose tissue inflammation in obesity. Brain Behav. Immun. 2014, 38, 25–35. [Google Scholar] [CrossRef][Green Version]
- Morari, J.; Anhe, G.F.; Nascimento, L.F.; De Moura, R.F.; Razolli, D.; Solon, C.; Guadagnini, D.; Souza, G.; Mattos, A.H.; Tobar, N.; et al. Fractalkine (CX3CL1) Is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 2014, 63, 3770–3784. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Hinkle, C.C.; Ferguson, J.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Freire, D.; Reyes, R.E.; Baghram, A.; Davies, D.L.; Asatryan, L. P2X7 receptor antagonist A804598 inhibits inflammation in brain and liver in C57BL/6J mice exposed to chronic ethanol and high fat diet. J. Neuroimmune Pharm. 2018, 14, 263–277. [Google Scholar] [CrossRef]
- Asatryan, L.; Khoja, S.; Rodgers, K.E.; Alkana, R.L.; Tsukamoto, H.; Davies, D.L. Chronic ethanol exposure combined with high fat diet up-regulates P2X7 receptors that parallels neuroinflammation and neuronal loss in C57BL/6J mice. J. Neuroimmunol. 2015, 285, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Coppens, V.; Morrens, M.; Destoop, M.; Dom, G. The interplay of inflammatory processes and cognition in alcohol use disorders—A systematic review. Front. Psychiatry 2019, 10, 632. [Google Scholar] [CrossRef]
- Schneider, R.; Bandiera, S.; Souza, D.G.; Bellaver, B.; Caletti, G.; Quincozes-Santos, A.; Elisabetsky, E.; Gomez, R. N-acetylcysteine prevents alcohol related neuroinflammation in rats. Neurochem. Res. 2017, 42, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Corwin, R.L.; Babbs, R.K. Rodent models of binge eating: Are they models of addiction? Ilar J. 2012, 53, 23–34. [Google Scholar] [CrossRef]
- Navarrete, F.; Rubio, G.; Manzanares, J. Effects of naltrexone plus topiramate on ethanol self-administration and tyrosine hydroxylase gene expression changes. Addict. Biol. 2013, 19, 862–873. [Google Scholar] [CrossRef]
- Roberts, A.J.; Gold, L.H.; Polis, I.; McDonald, J.S.; Filliol, D.; Kieffer, B.L.; Koob, G.F. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol. Clin. Exp. Res. 2001, 25, 1249–1256. [Google Scholar] [CrossRef]
- Samson, H.H. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol. Clin. Exp. Res. 1986, 10, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Heffner, T.G.; Hartman, J.A.; Seiden, L.S. A rapid method for the regional dissection of the rat brain. Pharm. Biochem. Behav. 1980, 13, 453–456. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Portilla, M.; Montagud-Romero, S.; Navarrete, F.; Gasparyan, A.; Manzanares, J.; Miñarro, J.; Rodríguez-Arias, M. Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice. Int. J. Mol. Sci. 2021, 22, 5279. https://doi.org/10.3390/ijms22105279
González-Portilla M, Montagud-Romero S, Navarrete F, Gasparyan A, Manzanares J, Miñarro J, Rodríguez-Arias M. Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice. International Journal of Molecular Sciences. 2021; 22(10):5279. https://doi.org/10.3390/ijms22105279
Chicago/Turabian StyleGonzález-Portilla, Macarena, Sandra Montagud-Romero, Francisco Navarrete, Ani Gasparyan, Jorge Manzanares, José Miñarro, and Marta Rodríguez-Arias. 2021. "Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice" International Journal of Molecular Sciences 22, no. 10: 5279. https://doi.org/10.3390/ijms22105279
APA StyleGonzález-Portilla, M., Montagud-Romero, S., Navarrete, F., Gasparyan, A., Manzanares, J., Miñarro, J., & Rodríguez-Arias, M. (2021). Pairing Binge Drinking and a High-Fat Diet in Adolescence Modulates the Inflammatory Effects of Subsequent Alcohol Consumption in Mice. International Journal of Molecular Sciences, 22(10), 5279. https://doi.org/10.3390/ijms22105279








