Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking
Abstract
:1. Introduction
2. Results and Discussion
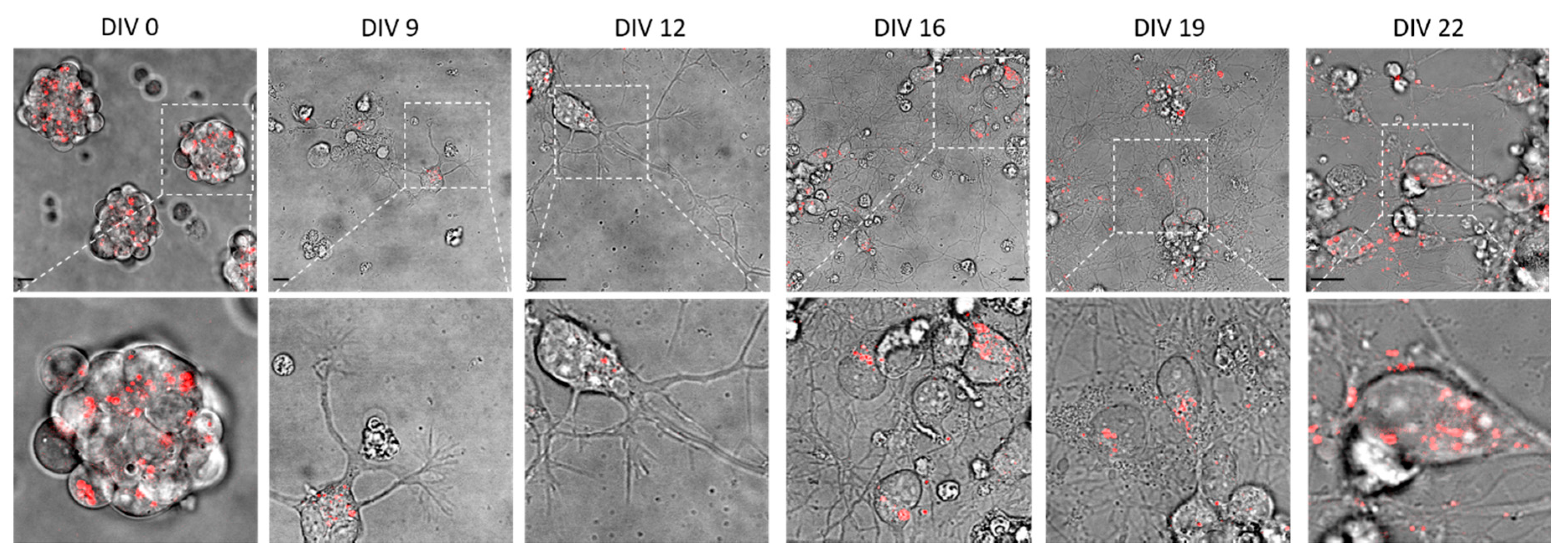
2.1. The Biological System
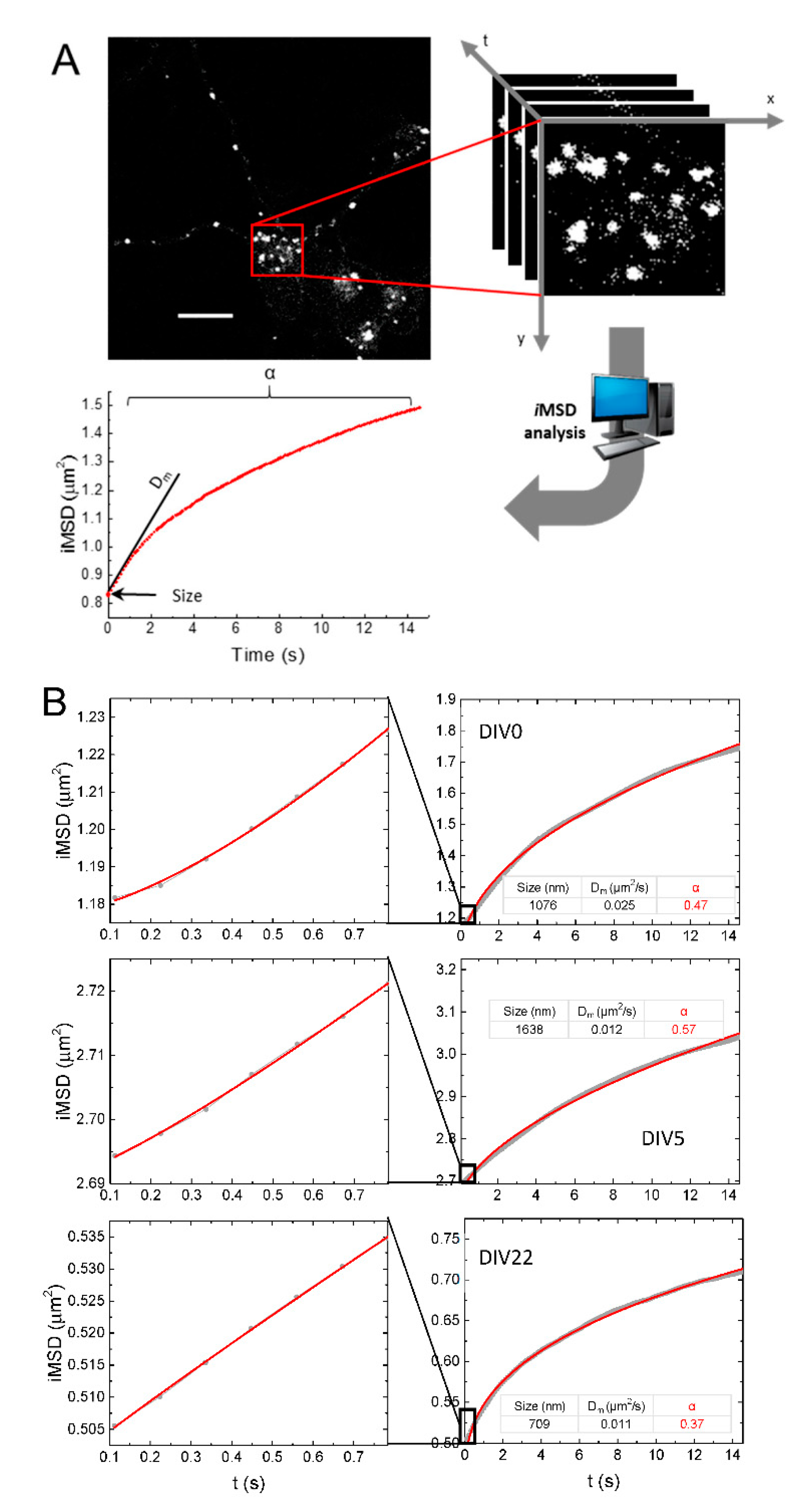
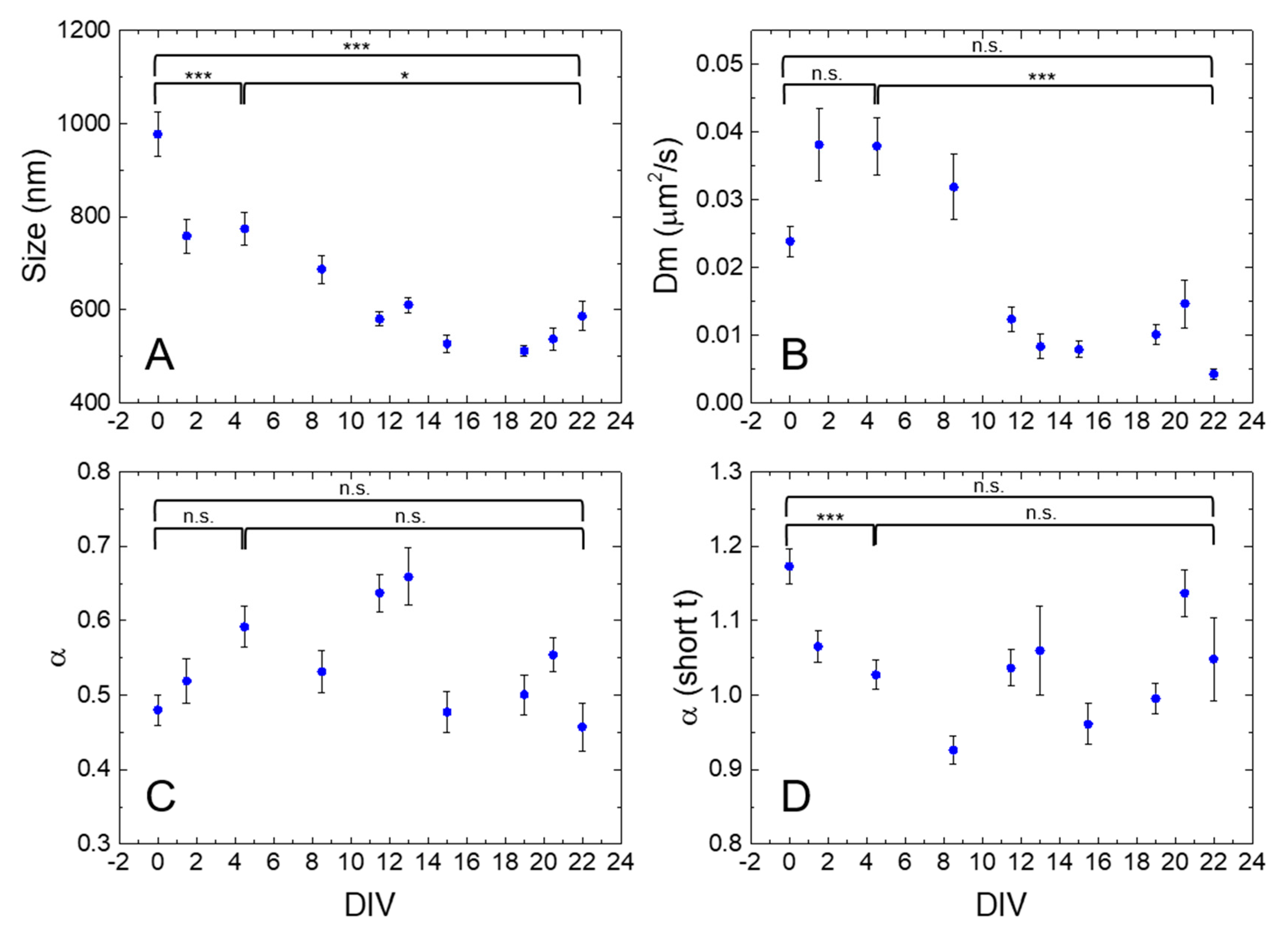
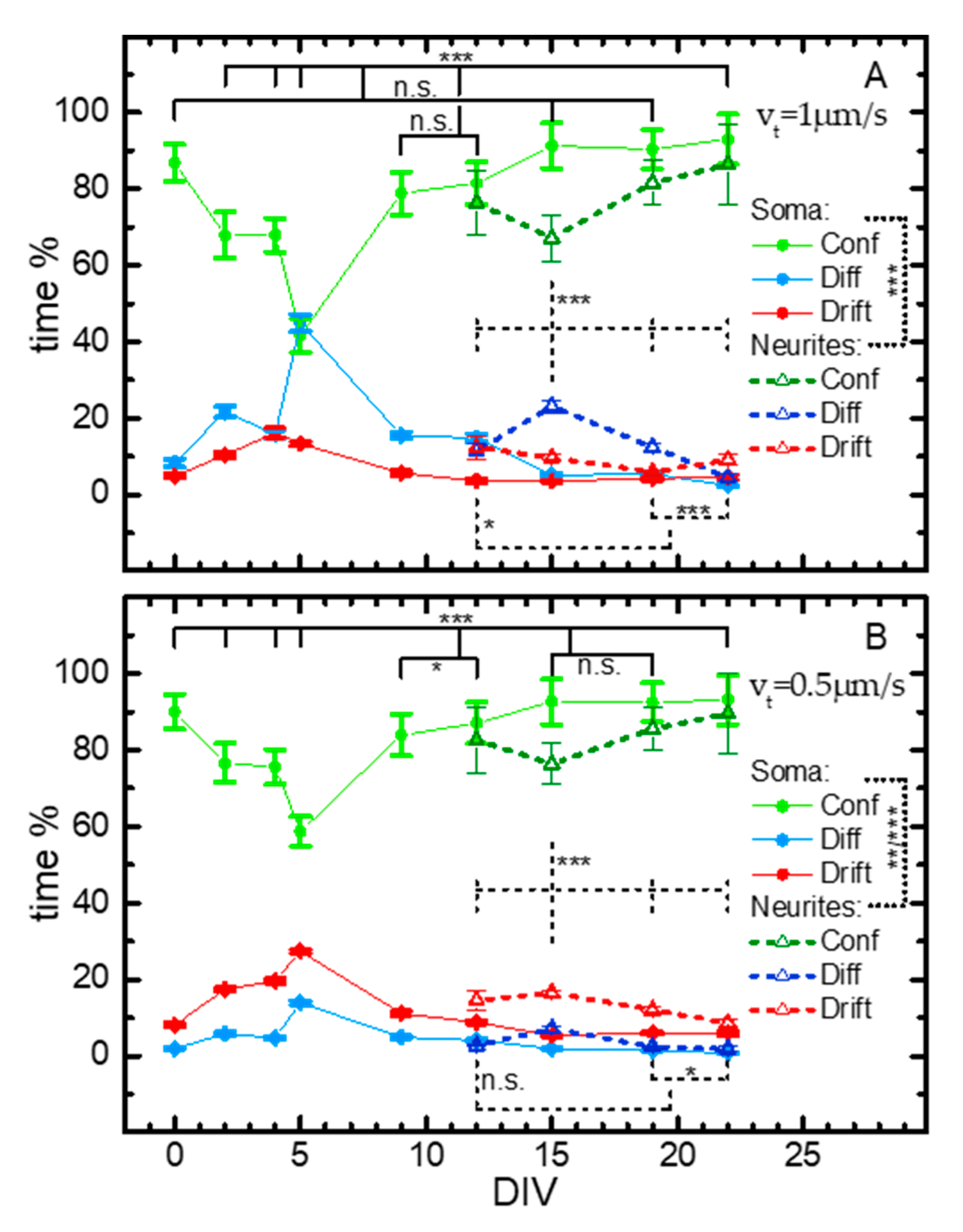
2.2. iMSD Analysis of Lysosome Dynamics during NSC Differentiation
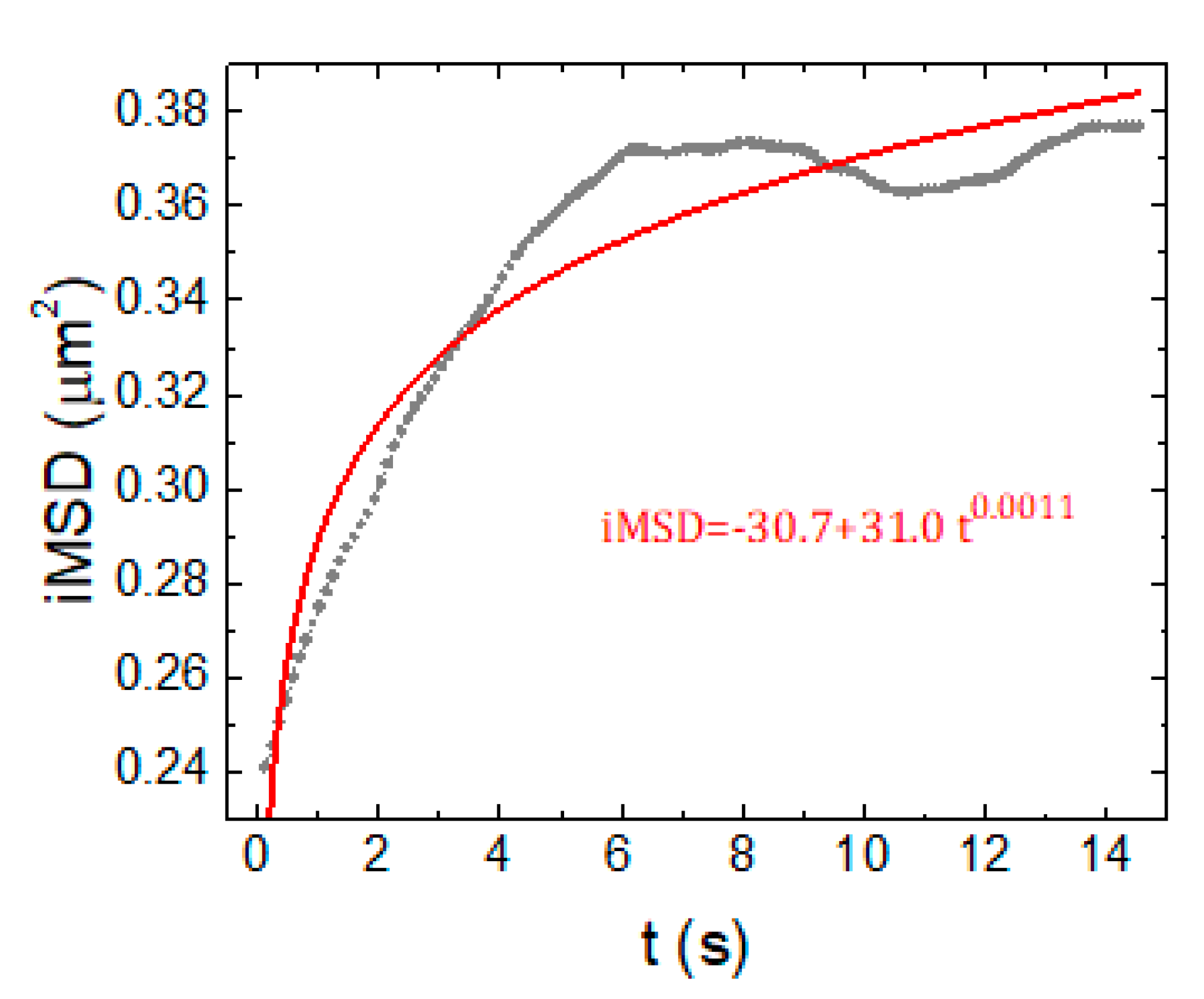
2.3. SPT Analysis of Lysosome Motion during NSC Differentiation
3. Conclusions
4. Materials and Methods
4.1. Mouse ES Cell-Derived Neural Cell Culture
4.2. Cell Fluorescence Staining
4.3. Live-Cell Imaging
4.4. Image Processing and Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pryor, P.R. Analyzing lysosomes in Live Cells, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 505, ISBN 9780123884480. [Google Scholar]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeman, D.S.; Hebestreit, K.; Ruetz, T.; Webb, A.E.; McKay, A.; Pollina, E.A.; Dulken, B.W.; Zhao, X.; Yeo, R.W.; Ho, T.T.; et al. Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 2018, 359, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes-Dias, P.; Holzbaur, E.L.F. Axonal transport: Driving synaptic function. Science 2019, 366, 6462. [Google Scholar] [CrossRef] [PubMed]
- Hangen, E.; Cordelières, F.P.; Petersen, J.D.; Choquet, D.; Coussen, F. Neuronal Activity and Intracellular Calcium Levels Regulate Intracellular Transport of Newly Synthesized AMPAR. Cell Rep. 2018, 24, 1001–1012. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, N.; Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nat. Rev. Neurosci. 2005, 6, 201–214. [Google Scholar] [CrossRef]
- Ferguson, S.M. Axonal transport and maturation of lysosomes. Curr. Opin. Neurobiol. 2018, 51, 45–51. [Google Scholar] [CrossRef]
- Digiacomo, L.; D’Autilia, F.; Durso, W.; Tentori, P.M.; Caracciolo, G.; Cardarelli, F. Dynamic fingerprinting of sub-cellular nanostructures by image mean square displacement analysis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Durso, W.; D’Autilia, F.; Amodeo, R.; Marchetti, L.; Cardarelli, F. Probing labeling-induced lysosome alterations in living cells by imaging-derived mean squared displacement analysis. Biochem. Biophys. Res. Commun. 2018, 503, 2704–2709. [Google Scholar] [CrossRef]
- De Nadai, T.; Marchetti, L.; Di Rienzo, C.; Calvello, M.; Signore, G.; Di Matteo, P.; Gobbo, F.; Turturro, S.; Meucci, S.; Viegi, A.; et al. Precursor and mature NGF live tracking: One versus many at a time in the axons. Sci. Rep. 2016, 6, 20272. [Google Scholar] [CrossRef] [Green Version]
- Convertino, D.; Fabbri, F.; Mishra, N.; Mainardi, M.; Cappello, V.; Testa, G.; Capsoni, S.; Albertazzi, L.; Luin, S.; Marchetti, L.; et al. Graphene promotes axon elongation through local stall of Nerve Growth Factor signaling endosomes. Nano Lett. 2020. [CrossRef]
- Di Rienzo, C.; Gratton, E.; Beltram, F.; Cardarelli, F. Fast spatiotemporal correlation spectroscopy to determine protein lateral diffusion laws in live cell membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 12307–12312. [Google Scholar] [CrossRef] [Green Version]
- Ferri, G.; Digiacomo, L.; D’autilia, F.; Durso, W.; Caracciolo, G.; Cardarelli, F. Data descriptor: Time-lapse confocal imaging datasets to assess structural and dynamic properties of subcellular nanostructures. Sci. Data 2018, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, G.; Digiacomo, L.; Lavagnino, Z.; Occhipinti, M.; Bugliani, M.; Cappello, V.; Caracciolo, G.; Marchetti, P.; Piston, D.W.; Cardarelli, F. Insulin secretory granules labelled with phogrin-fluorescent proteins show alterations in size, mobility and responsiveness to glucose stimulation in living β-cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Luin, S.; Bonsignore, F.; de Nadai, T.; Beltram, F.; Cattaneo, A. Ligand-induced dynamics of neurotrophin receptors investigated by single-molecule imaging approaches. Int. J. Mol. Sci. 2015, 16, 1949–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, L.; Bonsignore, F.; Gobbo, F.; Amodeo, R.; Calvello, M.; Jacob, A.; Signore, G.; Spagnolo, C.S.; Porciani, D.; Mainardi, M.; et al. Fast-diffusing p75NTR monomers support apoptosis and growth cone collapse by neurotrophin ligands. Proc. Natl. Acad. Sci. USA 2019, 116, 21563–21572. [Google Scholar] [CrossRef] [Green Version]
- Amodeo, R.; Nifosì, R.; Giacomelli, C.; Ravelli, C.; La Rosa, L.; Callegari, A.; Trincavelli, M.L.; Mitola, S.; Luin, S.; Marchetti, L. Molecular insight on the altered membrane trafficking of TrkA kinase dead mutants. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118614. [Google Scholar] [CrossRef]
- Ahmed, W.W.; Saif, T.A. Active transport of vesicles in neurons is modulated by mechanical tension. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bertacchi, M.; Pandolfini, L.; D’Onofrio, M.; Brandi, R.; Cremisi, F. The double inhibition of endogenously produced bmp and wnt factors synergistically triggers dorsal telencephalic differentiation of mouse es cells. Dev. Neurobiol. 2015, 75, 66–79. [Google Scholar] [CrossRef]
- Afroze, S.H.; Jensen, K.; Rahal, K.; Meng, F.; Alpini, G.; Glaser, S.S. Liver Regeneration. The Stem Cell Approach; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780123985231. [Google Scholar]
- Tropepe, V.; Hitoshi, S.; Sirard, C.; Mak, T.W.; Rossant, J.; Van Der Kooy, D. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 2001, 30, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Smukler, S.R.; Runciman, S.B.; Xu, S.; Van Der Kooy, D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J. Cell Biol. 2006, 172, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Shimogori, T.; Banuchi, V.; Ng, H.Y.; Strauss, J.B.; Grove, E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 2004, 131, 5639–5647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupo, G.; Bertacchi, M.; Carucci, N.; Augusti-Tocco, G.; Biagioni, S.; Cremisi, F. From pluripotency to forebrain patterning: An in vitro journey astride embryonic stem cells. Cell. Mol. Life Sci. 2014, 71, 2917–2930. [Google Scholar] [CrossRef] [Green Version]
- Boergermann, J.H.; Kopf, J.; Yu, P.B.; Knaus, P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 2010, 42, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Hebert, B.; Costantino, S.; Wiseman, P.W. Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Biophys. J. 2005, 88, 3601–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukoja, A.; Rey, U.; Petzoldt, A.G.; Ott, C.; Vollweiter, D.; Quentin, C.; Puchkov, D.; Reynolds, E.; Lehmann, M.; Hohensee, S.; et al. Presynaptic Biogenesis Requires Axonal Transport of Lysosome-Related Vesicles. Neuron 2018, 99, 1216–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozumi, M.; Nakatsu, F.; Katoh, K.; Igarashi, M. Coordinated Movement of Vesicles and Actin Bundles during Nerve Growth Revealed by Superresolution Microscopy. Cell Rep. 2017, 18, 2203–2216. [Google Scholar] [CrossRef] [Green Version]
- Callegari, A.; Duci, A.; Cattaneo, A.; Beltram, F.; Marchetti, L.; Luin, S. Single particle tracking of acyl carrier protein (ACP)-tagged TrkA receptors in PC12nnr5 cells. J. Neurosci. Methods 2012, 204, 82–86. [Google Scholar] [CrossRef]
- Marchetti, L.; Callegari, A.; Luin, S.; Signore, G.; Viegi, A.; Beltram, F.; Cattaneo, A. Ligand signature in the membrane dynamics of single TrkA receptor molecules. J. Cell Sci. 2013, 126, 4445–4456. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Dou, X.; Liu, C.; Wang, L.; Xing, C.; Peng, G.; Chen, J.; Yu, F.; Qiao, Y.; Song, L.; et al. Histone deacetylation promotes mouse neural induction by restricting Nodal-dependent mesendoderm fate. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ying, Q.L.; Stavridis, M.; Griffiths, D.; Li, M.; Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003, 21, 183–186. [Google Scholar] [CrossRef]
- Abranches, E.; Silva, M.; Pradier, L.; Schulz, H.; Hummel, O.; Henrique, D.; Bekman, E. Neural differentiation of embryonic stem cells in vitro: A road map to neurogenesis in the embryo. PLoS ONE 2009, 4, e6286. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A. The awesome lysosome. EMBO Mol. Med. 2016, 8, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, F.; Marchetti, L.; Jacob, A.; Pinto, B.; Binini, N.; Pecoraro Bisogni, F.; Alia, C.; Luin, S.; Caleo, M.; Fellin, T.; et al. Activity-dependent expression of Channelrhodopsin at neuronal synapses. Nat. Commun. 2017, 8, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.-C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA Granules Hitchhike on Lysosomes for Long-Distance Transport, Using Annexin A11 as a Molecular Tether. Cell 2019, 179, 147–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durso, W.; Martins, M.; Marchetti, L.; Cremisi, F.; Luin, S.; Cardarelli, F. Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking. Int. J. Mol. Sci. 2020, 21, 3397. https://doi.org/10.3390/ijms21093397
Durso W, Martins M, Marchetti L, Cremisi F, Luin S, Cardarelli F. Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking. International Journal of Molecular Sciences. 2020; 21(9):3397. https://doi.org/10.3390/ijms21093397
Chicago/Turabian StyleDurso, William, Manuella Martins, Laura Marchetti, Federico Cremisi, Stefano Luin, and Francesco Cardarelli. 2020. "Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking" International Journal of Molecular Sciences 21, no. 9: 3397. https://doi.org/10.3390/ijms21093397
APA StyleDurso, W., Martins, M., Marchetti, L., Cremisi, F., Luin, S., & Cardarelli, F. (2020). Lysosome Dynamic Properties during Neuronal Stem Cell Differentiation Studied by Spatiotemporal Fluctuation Spectroscopy and Organelle Tracking. International Journal of Molecular Sciences, 21(9), 3397. https://doi.org/10.3390/ijms21093397





