Degradation Mechanism of 2,4-Dichlorophenol by Fungi Isolated from Marine Invertebrates
Abstract
1. Introduction
2. Results and Discussion
2.1. Biotransformation Potential of 2,4-DCP by Marine-Derived Fungi
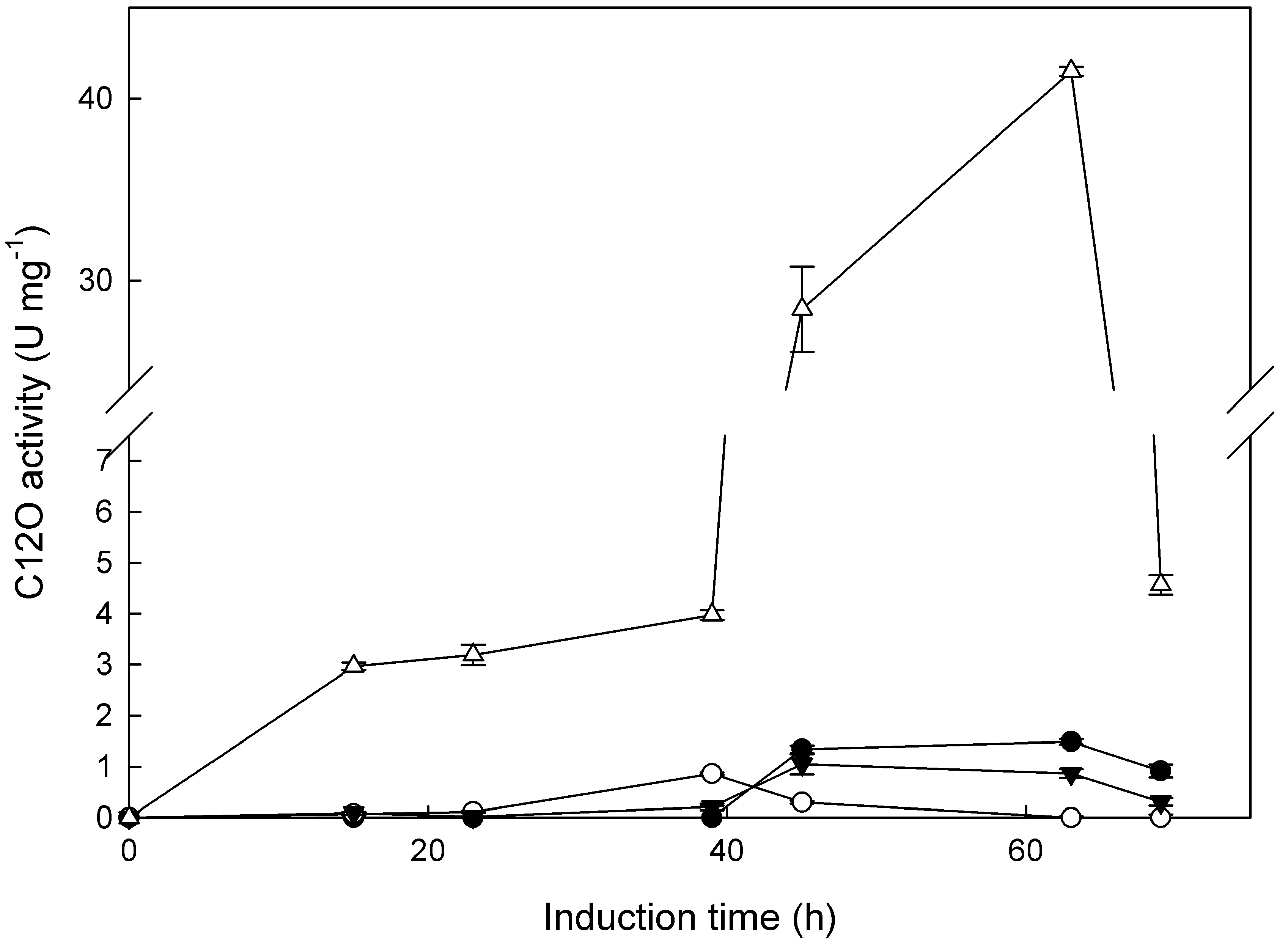
2.2. Expression of Catechol Dioxygenase Activities
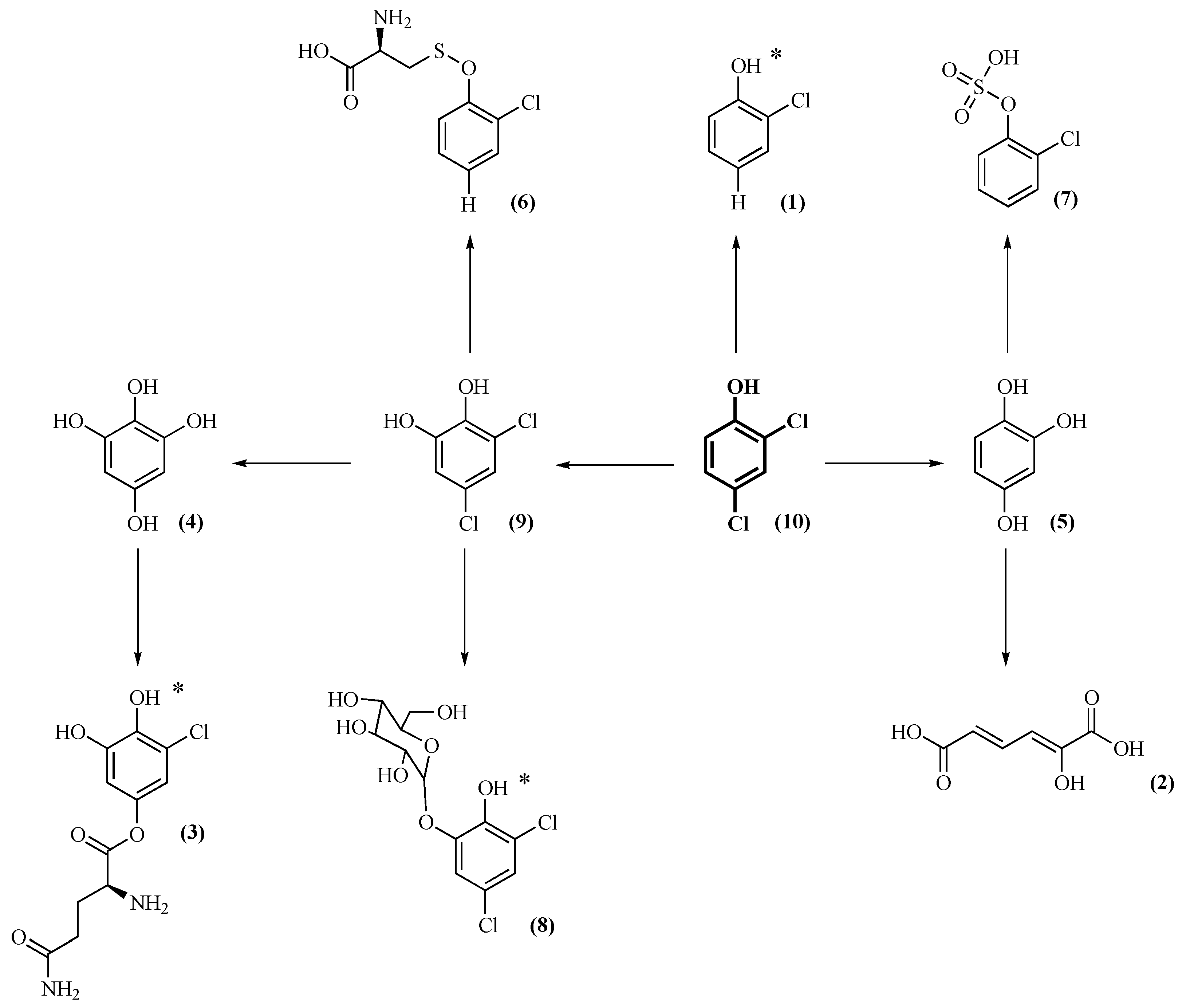
2.3. Identification of 2,4-DCP Metabolites
2.4. Metabolic Pathways of 2,4-DCP
3. Materials and Methods
3.1. Chemicals
3.2. Isolation and Identification of Invertebrate Symbionts
3.3. Culture Conditions and Resting-Cell Reactions
3.4. Detection and Quantification of 2,4-DCP
3.5. Identification of 2,4-DCP Metabolites by LC–MS
3.6. Measurement of Enzymatic Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhir, B. Bioremediation technologies for the removal of pollutants. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer: Singapore, 2017; pp. 69–91. ISBN 978-981-10-4041-2. [Google Scholar]
- Kalogerakis, N.; Arff, J.; Banat, I.M.; Broch, O.J.; Daffonchio, D.; Edvardsen, T.; Eguiraun, H.; Giuliano, L.; Handå, A.; López-de-Ipiña, K.; et al. The role of environmental biotechnology in exploring, exploiting, monitoring, preserving, protecting and decontaminating the marine environment. N. Biotechnol. 2015, 32, 157–167. [Google Scholar] [CrossRef]
- Gul, R.; Kumar, R. Introduction to Environmental Biotechnology. In Advances in Environmental Biotechnology; Kumar, R., Anil Kumar, S., Sarabjeet Singh, A., Eds.; Springer: Singapore, 2017; pp. 1–11. [Google Scholar]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Application of monooxygenases in dehalogenation, desulphurization, denitrification and hydroxylation of aromatic compounds. J. Bioremediat. Biodegrad. 2010. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszysk, D. Intradiol dioxygenases—The key enzymes in xenobiotics degradation. In Biodegradation of Hazardous and Special Products; Chamy, R., Rosenkranz, F., Eds.; InTech: Vienna, Austria, 2013; pp. 129–153. ISBN 978-953-51-1155-9. [Google Scholar]
- Siegbahn, P.E.M.; Haeffner, F. Mechanism for catechol ring-cleavage by non-heme iron extradiol dioxygenases. J. Am. Chem. Soc. 2004, 126, 8919–8932. [Google Scholar] [CrossRef]
- Fetzner, S. Ring-cleaving dioxygenases with a cupin fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Nikolaivits, E.; Dimarogona, M.; Fokialakis, N.; Topakas, E. Marine-derived biocatalysts: Importance, accessing and application in aromatic pollutant bioremediation. Front. Microbiol. 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Häggblom, M.M.; Smidt, H. Organohalide respiration in pristine environments: Implications for the natural halogen cycle. Environ. Microbiol. 2018, 20, 934–948. [Google Scholar] [CrossRef]
- Kumari, M.; Ghosh, P.; Thakur, I.S. Application of microbes in remediation of hazardous wastes: A review. In Bioremediation: Applications for Environmental Protection and Management. Energy, Environment, and Sustainability; Varjani, S., Agarwal, A., Gnansounou, E.G.B., Eds.; Springer: Singapore, 2018; pp. 223–241. ISBN 978-981-10-7485-1. [Google Scholar]
- Nikolaivits, E.; Agrafiotis, A.; Termentzi, A.; Machera, K.; Le Goff, G.; Álvarez, P.; Chavanich, S.; Benayahu, Y.; Ouazzani, J.; Fokialakis, N.; et al. Unraveling the detoxification mechanism of 2,4-dichlorophenol by marine-derived mesophotic symbiotic fungi isolated from marine invertebrates. Mar. Drugs 2019, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Vroumsia, T.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.-L. Groupe pour l’Étude du Devenir des Xénobiotiques dans l’Environnement (GEDEXE) Fungal bioconversion of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4-dichlorophenol (2,4-DCP). Chemosphere 2005, 60, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Shirapova, G.; Zimmer, C.; Giffhorn, F.; Batoev, V.; Kohring, G.-W. Degradation of 2,4-dichlorophenol by Bacillus sp. isolated from an aeration pond in the Baikalsk pulp and paper mill (Russia). Int. Biodeterior. Biodegrad. 2006, 58, 209–212. [Google Scholar] [CrossRef]
- Kargi, F.; Eker, S. Kinetics of 2,4-dichlorophenol degradation by Pseudomonas putida CP1 in batch culture. Int. Biodeterior. Biodegrad. 2005, 55, 25–28. [Google Scholar] [CrossRef]
- Chen, A.; Zeng, G.; Chen, G.; Fan, J.; Zou, Z.; Li, H.; Hu, X.; Long, F. Simultaneous cadmium removal and 2,4-dichlorophenol degradation from aqueous solutions by Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2011, 91, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Krastanov, A.; Stanchev, V.; Daniel, D.; Gerginova, M.; Alexieva, Z. Biodegradation of high amounts of phenol, catechol, 2,4-dichlorophenol and 2,6-dimethoxyphenol by Aspergillus awamori cells. Enzyme Microb. Technol. 2006, 39, 1036–1041. [Google Scholar] [CrossRef]
- Subbotina, N.M.; Kolomytseva, M.P.; Baskunov, B.P.; Golovlev, L.A. Catechol 1,2-dioxygenase induced in Rhodococcus opacus strain 1CP cultured in the presence of 3-hydroxybenzoate. Microbiology 2016, 85, 638–641. [Google Scholar] [CrossRef]
- Lin, J.; Milase, R.N. Purification and characterization of catechol 1,2-dioxygenase from Acinetobacter sp. Y64 strain and Escherichia coli transformants. Protein J. 2015, 34, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Sitnik, M.; Wojcieszyńska, D. High activity catechol 1,2-dioxygenase from Stenotrophomonas maltophilia strain KB2 as a useful tool in cis,cis-muconic acid production. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 103, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Giedraityte, G.; Kalėdienė, L. Catechol 1,2-dioxygenase from α-naphthol degrading thermophilic Geobacillus sp. strain: Purification and properties. Open Life Sci. 2009, 4, 68–73. [Google Scholar] [CrossRef]
- Santos, V.L.; Linardi, V.R. Biodegradation of phenol by a filamentous fungi isolated from industrial effluents-identification and degradation potential. Process Biochem. 2004, 39, 1001–1006. [Google Scholar] [CrossRef]
- Cai, W.; Li, J.; Zhang, Z. The characteristics and mechanisms of phenol biodegradation by Fusarium sp. J. Hazard. Mater. 2007, 148, 38–42. [Google Scholar] [CrossRef]
- Tsai, S.-C.; Li, Y.-K. Purification and characterization of a catechol 1,2-dioxygenase from a phenol degrading Candida albicans TL3. Arch. Microbiol. 2007, 187, 199–206. [Google Scholar] [CrossRef]
- Long, Y.; Yang, S.; Xie, Z.; Cheng, L. Cloning, expression, and characterization of catechol 1,2-dioxygenase from a phenol-degrading Candida tropicalis JH8 strain. Prep. Biochem. Biotechnol. 2016, 46, 673–678. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Mousa, H.A.; Gamal, M. El Microbial degradation of chlorophenols. In Microbe-Induced Degradation of Pesticides; Singh, S.N., Ed.; Springer: New York, NY, USA, 2017; pp. 23–58. [Google Scholar]
- Marco-Urrea, E.; Reddy, C.A. Degradation of chloro-organic pollutants by white rot fungi. In Microbial Degradation of Xenobiotics. Environmental Science and Engineering; Singh, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 31–66. ISBN 978-3-642-23789-8. [Google Scholar]
- Aranda, E. Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Osawa, S.; Hirata, T.; Yamagishi, Y.; Hosoda, J.; Horikoshi, T. 2,4-Dichlorophenol degradation by the soil fungus Mortierella sp. Biosci. Biotechnol. Biochem. 2006, 70, 525–527. [Google Scholar] [CrossRef]
- Arora, P.; Bae, H. Bacterial degradation of chlorophenols and their derivatives. Microb. Cell Fact. 2014, 13, 31. [Google Scholar] [CrossRef]
- Rubilar, O.; Diez, M.C.; Gianfreda, L. Transformation of chlorinated phenolic compounds by white rot fungi. Crit. Rev. Environ. Sci. Technol. 2008, 38, 227–268. [Google Scholar] [CrossRef]
- Hupert-Kocurek, K.; Wojcieszyſska, D.; Guzik, U.; Borowski, T.; Wojcieszyńska, D.; Guzik, U.; Borowski, T. A single amino acid substitution within catalytically non-active N-terminal domain of catechol 2,3-dioxygenase (C23O) increases enzyme activity towards 4-chlorocatechol. J. Mol. Catal. B Enzym. 2015, 122, 64–71. [Google Scholar] [CrossRef]
- Gioti, A.; Siaperas, R.; Nikolaivits, E.; Le Goff, G.; Ouazzani, J.; Kotoulas, G.; Topakas, E. Draft genome sequence of a Cladosporium species isolated from the mesophotic ascidian Didemnum maculosum. Microbiol. Resour. Announc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
| Isolate | Invertebrate | Location | Depth (m) | Isolate Identification | % DCP Removal |
|---|---|---|---|---|---|
| ML119-S1 | Bivalve (Pteria aegyptiaca) | Red | 35 | Aphanoascus fulvescens | 25.4 |
| ML123-S2 | Sponge (on top of bivalve) | Red | 35 | Cladosporium halotolerans | 21.1 |
| ML132-S1 | Amphimedon ochracea | Red | 35 | Aspergillus fumigatus | 27.3 |
| ML133-S2 | Soft coral (Sarcophyton glaucum) | Red | 35 | Cladosporium halotolerans | 9.3 |
| ML136-S2 | Bivalve (Pteria aegyptiaca) | Red | 35 | Aspergillus sp. (fumigatus) | 41.4 |
| ML147-S1 | Axinella verrucosa | Med E | 47 | Aspergillus terreus | 26.9 |
| ML147-S2 | Axinella verrucosa | Med E | 47 | Aspergillus sp. (terreus) | 55.1 |
| ML149-S1 | Sarcotragus muscarum | Med E | 49 | Aphanoascus fulvescens | 41.8 |
| ML150-S1 | Sarcotragus muscarum | Med E | 45 | Aphanoascus fulvescens | 35.8 |
| ML153-S1 | Sarcotragus muscarum | Med E | 45 | Penicillium fellutanum | 13.9 |
| ML153-S2 | Sarcotragus muscarum | Med E | 45 | Penicillium fellutanum | 24.7 |
| ML155-S1 | Sarcotragus muscarum | Med E | 35 | Lecanicillium sp. | 19.0 |
| ML-156-S8 | Ascidean | Med E | 35 | Penicillium chrysogenum | 59.5 |
| ML197-S3 | Protula intestinum | Med E | 6 | Tritirachium sp. | 66.3 |
| ML6-S1 | Sponge (Chondrilla australiensis) | Andaman | 10–15 | Cladosporium sp. | 64.0 |
| ML10-S1 | Sponge (Clathria (Thalysias) reinwardti) | Andaman | 10–15 | Penicillium coffeae | 37.1 |
| ML14-S1 | Sponge (Phobas arborescens) | Andaman | 10–15 | Aspergillus niger | 22.9 |
| ML15-S1 | Sponge (Phobas arborescens) | Andaman | 10–15 | Aspergillus terreus | 27.3 |
| ML16-S2 | Sponge (Iotrochota baculifera) | Andaman | 10–15 | Penicillium chrysogenum | 37.9 |
| ML45-S3 | Hydroid (Macrorhynchia philippina) | Andaman | 10–15 | Penicillium chrysogenum | 27.1 |
| ML45-S5 | Hydroid (Macrorhynchia philippina) | Andaman | 10–15 | Penicillium steckii | 36.3 |
| ML45-S6 | Hydroid (Macrorhynchia philippina) | Andaman | 10–15 | Purpureocillium lilacinum | 32.8 |
| ML52-S1 | Unknown hydroid | Andaman | 10–15 | Penicillium citrinum | 26.7 |
| ML52-S5 | Unknown hydroid | Andaman | 10–15 | Penicillium coffeae | 26.8 |
| ML52-S6 | Unknown hydroid | Andaman | 10–15 | Aspergillus niger | 29.3 |
| ML52-S7 | Unknown hydroid | Andaman | 10–15 | Penicillium steckii | 31.7 |
| ML52-S8 | Unknown hydroid | Andaman | 10–15 | Aspergillus fumigatus | 32.8 |
| A/A | Rt (min) | (M – H)− | EC | Found In |
|---|---|---|---|---|
| (1) | 0.89 | 126.9987 | C6H5ClO | Aspergillus sp. MLm147-S2 |
| (2) | 1.11 | 157.0146 | C6H6O5 | Tritirachium sp. MLm197-S3 |
| (3) | 1.45 | 287.0449 | C11H13O5N2Cl | Tritirachium sp. MLm197-S3 |
| (3) | 1.47 | 287.0452 | C11H13O5N2Cl | Tritirachium sp. MLm197-S3, Aspergillus sp. MLm147-S2 |
| (3) | 1.50 | 287.0452 | C11H13O5N2Cl | P. chrysogenum MLm156-S8 |
| (4) | 2.33 | 141.0197 | C6H6O4 | Tritirachium sp. MLm197-S3 |
| (4) | 2.44 | 141.0196 | C6H6O4 | P. chrysogenum MLm156-S8 |
| (4) | 2.45 | 141.0196 | C6H6O4 | Cladosporium sp. MLm6-S1 |
| (4) | 2.46 | 141.0196 | C6H6O4 | Cladosporium sp. MLm6-S1 |
| (4) | 2.50 | 141.0196 | C6H6O4 | Cladosporium sp. MLm6-S1 |
| (5) | 3.15 | 125.0248 | C6H6O3 | Aspergillus sp. MLm147-S2 |
| (5) | 3.18 | 125.0249 | C6H6O3 | Aspergillus sp. MLm147-S2 |
| (5) | 3.19 | 125.0248 | C6H6O3 | Tritirachium sp. MLm197-S3 |
| (5) | 3.19 | 125.0248 | C6H6O3 | Cladosporium sp. MLm6-S1 |
| (5) | 3.20 | 125.0247 | C6H6O3 | Cladosporium sp. MLm6-S1 |
| (5) | 3.20 | 125.0248 | C6H6O3 | P. chrysogenum MLm156-S8 |
| (5) | 3.22 | 125.0248 | C6H6O3 | Tritirachium sp. MLm197-S3 |
| (5) | 3.22 | 125.0248 | C6H6O3 | Cladosporium sp. MLm6-S1 |
| (5) | 3.23 | 125.0250 | C6H6O3 | P. chrysogenum MLm156-S8 |
| (5) | 3.25 | 125.0249 | C6H6O3 | Aspergillus sp. MLm147-S2 |
| (5) | 3.27 | 125.0250 | C6H6O3 | Tritirachium sp. MLm197-S3 |
| (5) | 3.27 | 125.0251 | C6H6O3 | P. chrysogenum MLm156-S8 |
| (6) | 6.93 | 245.9994 | C9H10NClO3S | Cladosporium sp. MLm6-S1 |
| (7) | 7.34 | 206.9523 | C6H5O4ClS | Aspergillus sp. MLm147-S2 |
| (8) | 10.62 | 339.0041 | C12H14Cl2O7 | Tritirachium sp. MLm197-S3 |
| (8) | 10.62 | 339.0042 | C12H14Cl2O7 | Aspergillus sp. MLm147-S2 |
| (8) | 10.64 | 339.0040 | C12H14Cl2O7 | Tritirachium sp. MLm197-S3 |
| (8) | 10.73 | 339.0041 | C12H14Cl2O7 | Aspergillus sp. MLm147-S2 |
| (9) | 11.91 | 176.9517 | Cl2C6H2(OH)2 | Cladosporium sp. MLm6-S1 |
| (9) | 11.92 | 176.9517 | Cl2C6H2(OH)2 | Aspergillus sp. MLm147-S2 |
| (9) | 11.93 | 176.9518 | C6H4Cl2O2 | P. chrysogenum MLm156-S8 |
| (9) | 11.94 | 176.9517 | C6H4Cl2O2 | Tritirachium sp. MLm197-S3 |
| (9) | 11.94 | 176.9517 | Cl2C6H2(OH)2 | Cladosporium sp. MLm6-S1 |
| (9) | 11.96 | 176.9521 | C6H4Cl2O2 | P. chrysogenum MLm156-S8 |
| (9) | 11.96 | 176.9521 | C6H4Cl2O2 | Tritirachium sp. MLm197-S3 |
| (9) | 12 | 176.9521 | C6H4Cl2O2 | P. chrysogenum MLm156-S8 |
| (10) | 13.46 | 160.9572 | C6H4Cl2O | Aspergillus sp. MLm147-S2 |
| (10) | 13.46 | 160.9571 | C6H4Cl2O | Control day 10 |
| (10) | 13.47 | 160.9571 | C6H4Cl2O | Tritirachium sp. MLm197-S3 |
| (10) | 13.48 | 160.9572 | C6H4Cl2O | Aspergillus sp. MLm147-S2 |
| (10) | 13.48 | 160.9572 | C6H4Cl2O | Cladosporium sp. MLm6-S1 |
| (10) | 13.48 | 160.9572 | C6H4Cl2O | P. chrysogenum MLm156-S8 |
| (10) | 13.49 | 160.9572 | C6H4Cl2O | Cladosporium sp. MLm6-S1 |
| (10) | 13.50 | 160.9573 | C6H4Cl2O | P. chrysogenum MLm156-S8 |
| (10) | 13.50 | 160.9571 | C6H4Cl2O | P. chrysogenum MLm156-S8 |
| (10) | 13.51 | 160.9571 | C6H4Cl2O | Tritirachium sp. MLm197-S3 |
| (10) | 13.51 | 160.9572 | C6H4Cl2O | Tritirachium sp. MLm197-S3 |
| (10) | 13.52 | 160.9572 | C6H4Cl2O | Control day 6 |
| (10) | 13.52 | 160.9573 | C6H4Cl2O | Aspergillus sp. MLm147-S2 |
| (10) | 13.53 | 160.9572 | C6H4Cl2O | Cladosporium sp. MLm6-S1 |
| (10) | 13.55 | 160.9572 | C6H4Cl2O | Control day 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaivits, E.; Agrafiotis, A.; Baira, E.; Le Goff, G.; Tsafantakis, N.; Chavanich, S.A.; Benayahu, Y.; Ouazzani, J.; Fokialakis, N.; Topakas, E. Degradation Mechanism of 2,4-Dichlorophenol by Fungi Isolated from Marine Invertebrates. Int. J. Mol. Sci. 2020, 21, 3317. https://doi.org/10.3390/ijms21093317
Nikolaivits E, Agrafiotis A, Baira E, Le Goff G, Tsafantakis N, Chavanich SA, Benayahu Y, Ouazzani J, Fokialakis N, Topakas E. Degradation Mechanism of 2,4-Dichlorophenol by Fungi Isolated from Marine Invertebrates. International Journal of Molecular Sciences. 2020; 21(9):3317. https://doi.org/10.3390/ijms21093317
Chicago/Turabian StyleNikolaivits, Efstratios, Andreas Agrafiotis, Eirini Baira, Géraldine Le Goff, Nikolaos Tsafantakis, Suchana A. Chavanich, Yehuda Benayahu, Jamal Ouazzani, Nikolas Fokialakis, and Evangelos Topakas. 2020. "Degradation Mechanism of 2,4-Dichlorophenol by Fungi Isolated from Marine Invertebrates" International Journal of Molecular Sciences 21, no. 9: 3317. https://doi.org/10.3390/ijms21093317
APA StyleNikolaivits, E., Agrafiotis, A., Baira, E., Le Goff, G., Tsafantakis, N., Chavanich, S. A., Benayahu, Y., Ouazzani, J., Fokialakis, N., & Topakas, E. (2020). Degradation Mechanism of 2,4-Dichlorophenol by Fungi Isolated from Marine Invertebrates. International Journal of Molecular Sciences, 21(9), 3317. https://doi.org/10.3390/ijms21093317









