Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates
Abstract
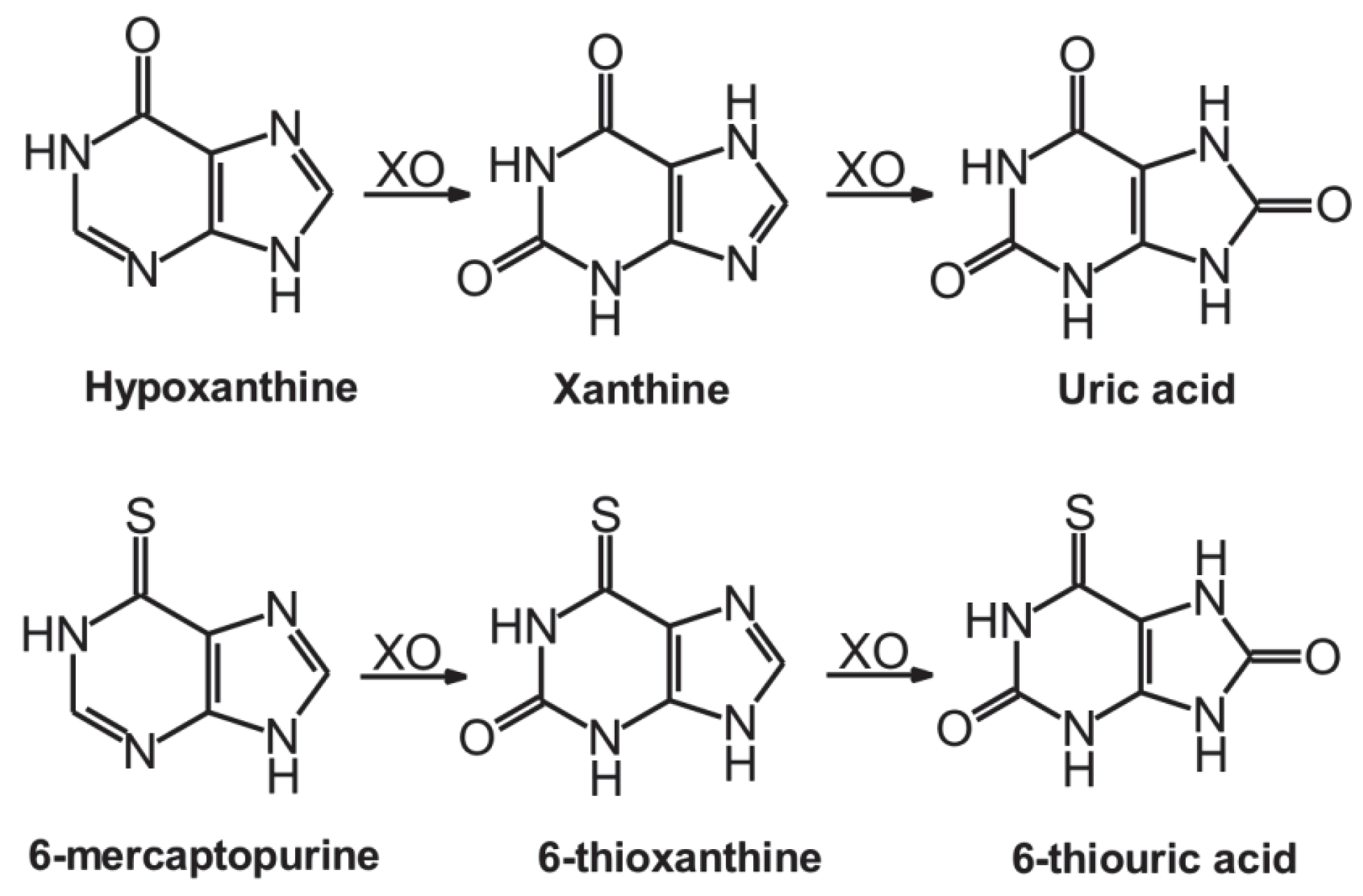
1. Introduction
2. Results
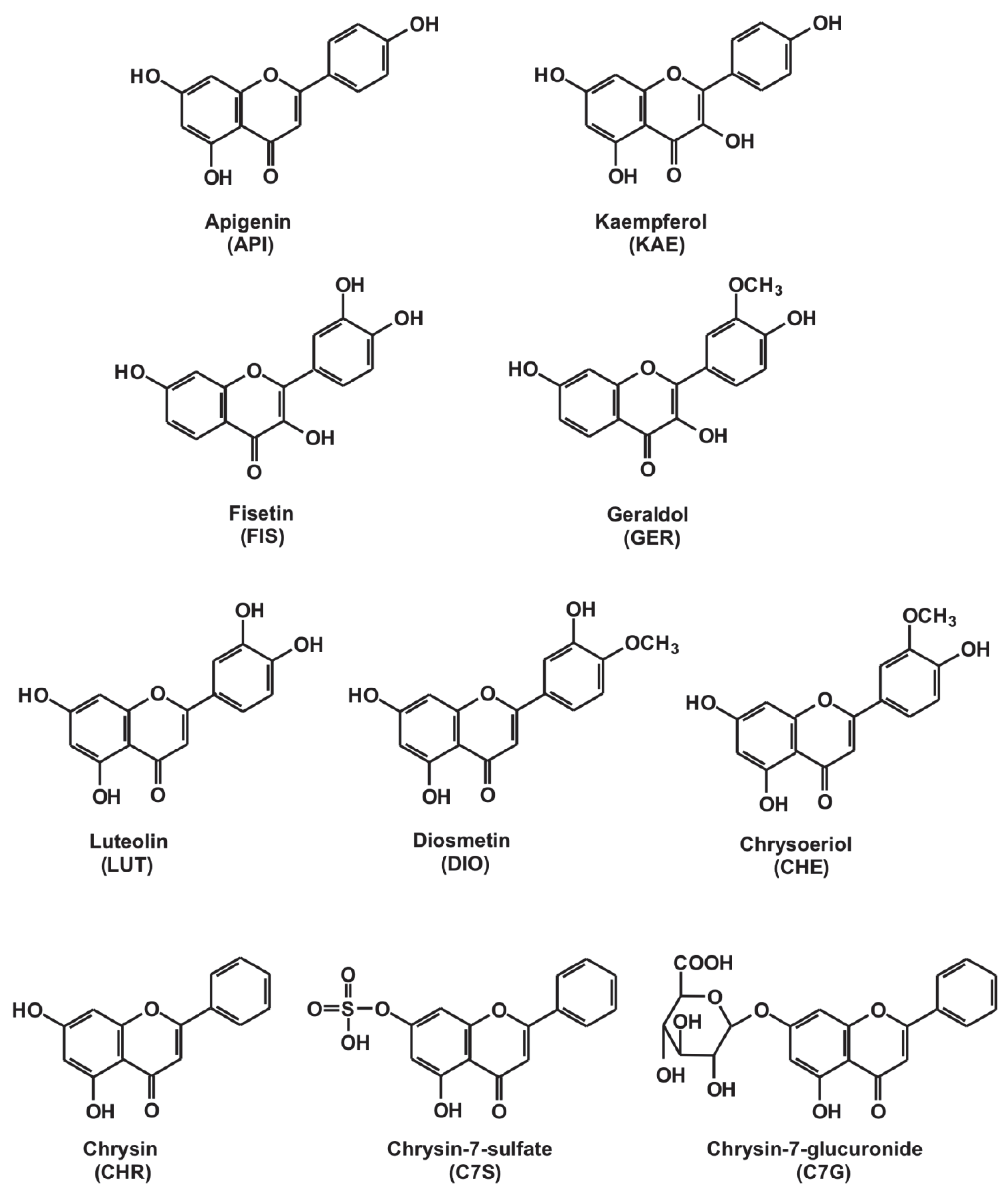
2.1. Inhibition of Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones
2.2. Inhibition of Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Conjugates
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. XO Assays
4.3. HPLC Analyses
4.4. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MP | 6-mercaptopurine |
| API | Apigenin |
| APU | Allopurinol |
| C7G | Chrysin-7-glucuronide |
| C7S | Chrysin-7-sulfate |
| CHE | Chrysoeriol |
| CHR | Chrysin |
| COMT | Catechol-O-methyltransferase |
| DIO | Diosmetin |
| DMSO | Dimethyl sulfoxide |
| FIS | Fisetin |
| GER | Geraldol |
| HPLC | High-performance liquid chromatography |
| KAE | Kaempferol |
| LUT | Luteolin |
| SEM | Standard error of the mean |
| SULT | Sulfotransferase |
| UGT | Uridine diphosphate glucuronosyltransferase |
| XO | Xanthine oxidase |
References
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high-performance liquid chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Donovan, J.L. Pharmacokinetics and Metabolism of Dietary Flavonoids in Humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Morris, M.E. Liquid chromatography–tandem mass spectroscopy assay for quercetin and conjugated quercetin metabolites in human plasma and urine. J. Chromatogr. 2005, 821, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, Excretion and Metabolite Profiling of Methyl-, Glucuronyl-, Glucosyl- and Sulpho-Conjugates of Quercetin in Human Plasma and Urine After Ingestion of Onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Brubaker, J.A.; Walle, U.K.; Halushka, P.V. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br. J. Clin. Pharmacol. 2001, 51, 143–146. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Touil, Y.S.; Auzeil, N.; Boulinguez, F.; Saighi, H.; Regazzetti, A.; Scherman, D.; Chabot, G.G. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem. Pharmacol. 2011, 82, 1731–1739. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Pan, H.; Sun, S.; Li, L.; Zeng, S.; Jiang, H. Role of Catechol-O-Methyltransferase in the Disposition of Luteolin in Rats. Drug Metab. Dispos. 2011, 39, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Dai, Y.Q.; Kong, S.S.; Song, F.F.; Li, L.P.; Ye, J.F.; Wang, R.W.; Zeng, S.; Zhou, H.; Jiang, H.D. Luteolin is a rare substrate of human catechol-O-methyltransferase favoring a para-methylation. Mol. Nutr. Food Res. 2013, 57, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.A.; Escolar, M.; Perez, G.; Garcia-Quetglas, E.; Sadaba, B.; Azanza, J.R. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Pharmac. Biomed. Anal. 2010, 51, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Gilani, A.H. Selective bronchodilatory effect of Rooibos tea (Aspalathus linearis) and its flavonoid, chrysoeriol. Eur. J. Nutr. 2006, 45, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Lee, J.Y.; Kim, M.R.; Woo, E.R.; Kim, Y.G.; Kang, K.W. Chrysoeriol potently inhibits the induction of nitric oxide synthase by blocking AP-1 activation. J. Biomed. Sci. 2005, 12, 949–959. [Google Scholar] [CrossRef]
- Wong, E.; Francis, C.M. Flavonoids in genotypes of Trifolium subterraneum—I: The normal flavonoid pattern of the Geraldton variety. Phytochemistry 1968, 7, 2123–2129. [Google Scholar] [CrossRef]
- Gupta, S.R.; Ravindranath, B.; Seshadri, T.R. Synthesis of some flavonoid glucosides of trifolium subterraneum. Phytochemistry 1971, 10, 877–882. [Google Scholar] [CrossRef]
- Day, R.O.; Graham, G.G.; Hicks, M.; McLachlan, A.J.; Stocker, S.L.; Williams, K.M. Clinical Pharmacokinetics and Pharmacodynamics of Allopurinol and Oxypurinol. Clin. Pharmacokinet. 2007, 46, 623–644. [Google Scholar] [CrossRef]
- Mohos, V.; Pánovics, A.; Fliszár-Nyúl, E.; Schilli, G.; Hetényi, C.; Mladěnka, P.; Needs, P.W.; Kroon, P.A.; Pethő, G.; Poór, M. Inhibitory Effects of Quercetin and Its Human and Microbial Metabolites on Xanthine Oxidase Enzyme. Int. J. Mol. Sci. 2019, 20, 2681. [Google Scholar] [CrossRef]
- Galbusera, C.; Orth, P.; Fedida, D.; Spector, T. Superoxide radical production by allopurinol and xanthine oxidase. Biochem. Pharmacol. 2006, 71, 1747–1752. [Google Scholar] [CrossRef]
- Leong, R.W.; Gearry, R.B.; Sparrow, M.P. Thiopurine hepatotoxicity in inflammatory bowel disease: The role for adding allopurinol. Expert Opin. Drug Saf. 2008, 7, 607–616. [Google Scholar] [CrossRef]
- McLeod, H.L. Clinically relevant drug–drug interactions in oncology. Br. J. Clin. Pharmacol. 1998, 45, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of Xanthine Oxidase by Flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J. Inhibition of chrysin on xanthine oxidase activity and its inhibition mechanism. Int. J. Biol. Macromol. 2015, 81, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure–Affinity and Structure–Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure−Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Van Hoorn, D.E.C.; Nijveldt, R.J.; Van Leeuwen, P.A.M.; Hofman, Z.; M’Rabet, L.; De Bont, D.B.A.; Van Norren, K. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharmacol. 2002, 451, 111–118. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.S.; Chen, C.T.; Liang, Y.C.; Lin, J.K. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002, 294, 167–172. [Google Scholar] [CrossRef]
- Iio, M.; Moriyama, A.; Matsumoto, Y.; Takaki, N.; Fukumoto, M. Inhibition of Xanthine Oxidase by Flavonoids. Agric. Biol. Chem. 1985, 49, 2173–2176. [Google Scholar] [CrossRef]
- Cimanga, K.; De Bruyne, T.; Hu, J.P.; Cos, P.; Apers, S.; Pieters, L.; Tona, L.; Kambu, K.; Vanden Berghe, D.; Vlietinck, A.J. Constituents from Morinda morindoides Leaves as Inhibitors of Xanthine Oxidase and Scavengers of Superoxide Anions. Pharm. Pharmacol. Commun. 1999, 5, 419–424. [Google Scholar] [CrossRef]
- Hayashi, T.; Sawa, K.; Kawasaki, M.; Arisawa, M.; Shimizu, M.; Morita, N. Inhibition of cow’s milk xanthine oxidase by flavonoids. J. Nat. Prod. 1988, 51, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Ueda, J.; Tran, Q.L.; Kadota, S. Xanthine Oxidase Inhibitors from the Flowers of Chrysanthemum sinense. Planta Med. 2006, 72, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Ruan, J.Y.; Jin, L.J.; Shi, W.Z.; Li, X.X.; Han, L.F.; Zhang, Y.; Wang, T. Xanthine oxidase inhibitory effects of the constituents of Chrysanthemum morifolium stems. Phytochem. Lett. 2017, 19, 39–45. [Google Scholar] [CrossRef]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Zhu, M.; Chen, J.; Zhu, X.; Chen, J.; Zhu, X. Effects of Genistein, Apigenin, Quercetin, Rutin and Astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem. Toxicol. 2011, 49, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Sarawek, S.; Feistel, B.; Pischel, I.; Butterweck, V. Flavonoids of Cynara scolymus Possess Potent Xanthinoxidase Inhibitory Activity in vitro but are Devoid of Hypouricemic Effects in Rats after Oral Application. Planta Med. 2008, 74, 221–227. [Google Scholar] [CrossRef]
- Mo, S.F.; Zhou, F.; Lv, Y.Z.; Hu, Q.H.; Zhang, D.M.; Kong, L.D. Hypouricemic Action of Selected Flavonoids in Mice: Structure–Activity Relationships. Biol. Pharm. Bull. 2007, 30, 1551–1556. [Google Scholar] [CrossRef]
- Abbey, E.L.; Rankin, J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 91–96. [Google Scholar] [CrossRef]
- Boots, A.W.; Drent, M.; De Boer, V.C.; Bast, A.; Haenen, G.R. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Chen, W.; Zhao, X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br. J. Nutr. 2010, 103, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with Quercetin Markedly Increases Plasma Quercetin Concentration without Effect on Selected Risk Factors for Heart Disease in Healthy Subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Turnheim, K.; Krivanek, P.; Oberbauer, R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br. J. Clin. Pharmacol. 1999, 48, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Mohos, V.; Fliszár-Nyúl, E.; Schilli, G.; Hetényi, C.; Lemli, B.; Kunsági-Máté, S.; Bognár, B.; Poór, M. Interaction of Chrysin and Its Main Conjugated Metabolites Chrysin-7-Sulfate and Chrysin-7-Glucuronide with Serum Albumin. Int. J. Mol. Sci. 2018, 19, 4073. [Google Scholar] [CrossRef]
- Huang, W.H.; Lee, A.R.; Yang, C.H. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Biosci. Biotechnol. Biochem. 2006, 70, 2371–2380. [Google Scholar] [CrossRef]
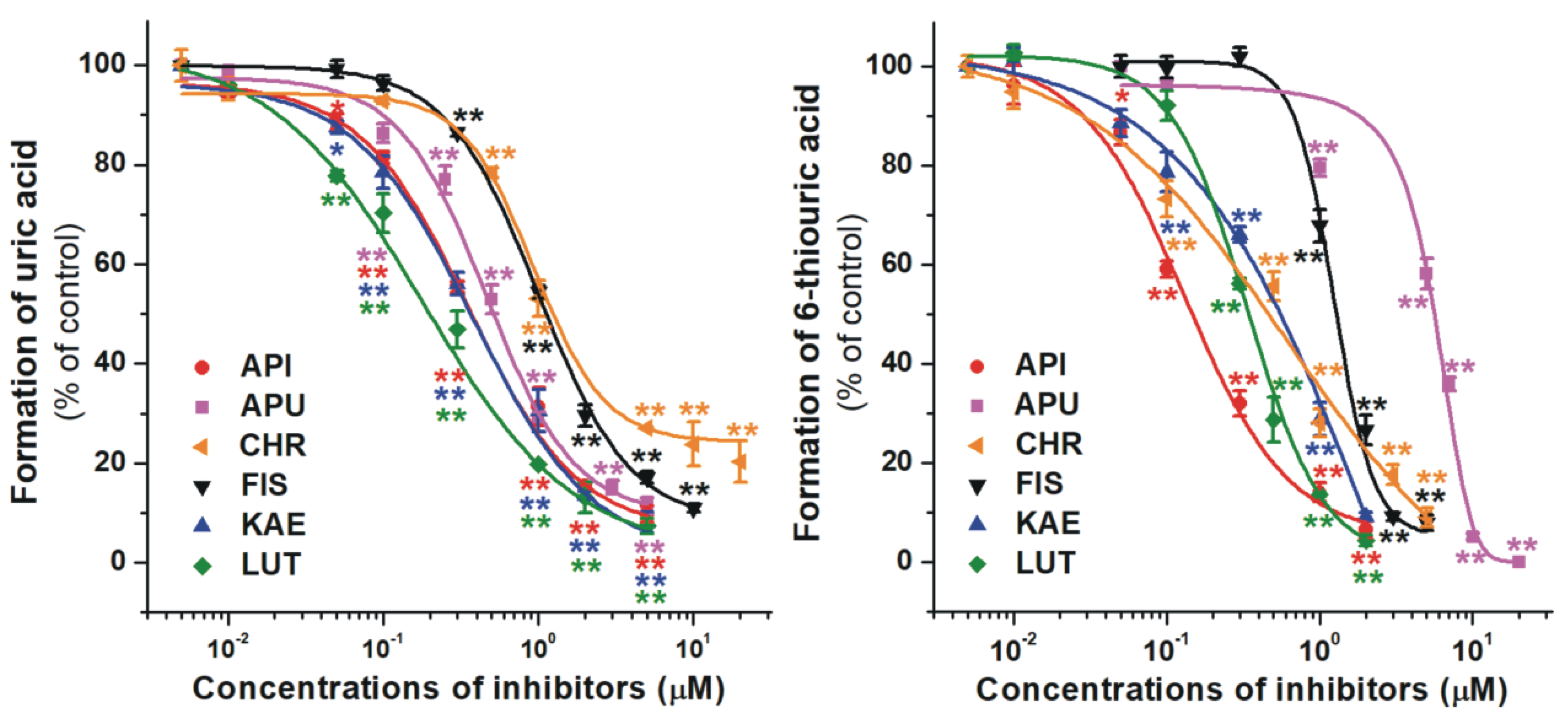
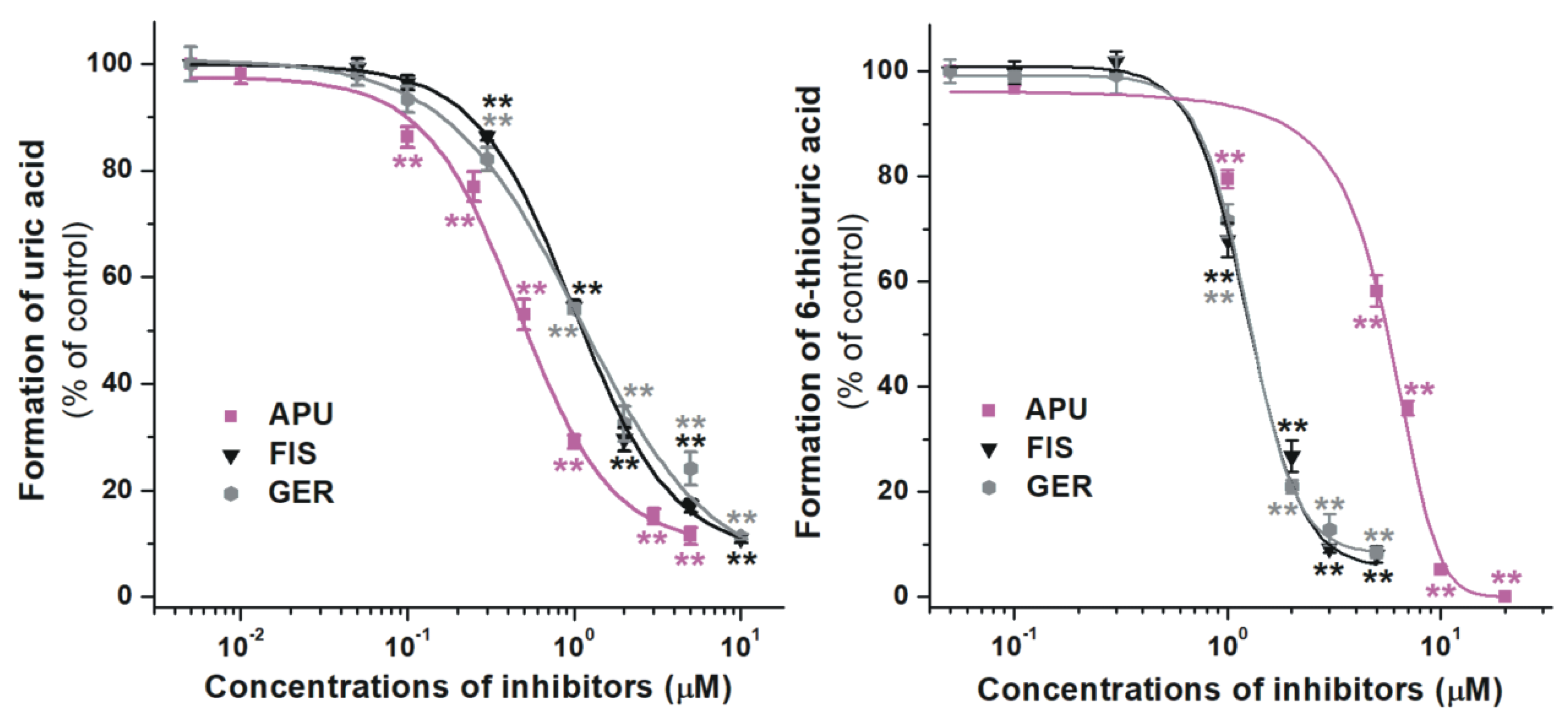
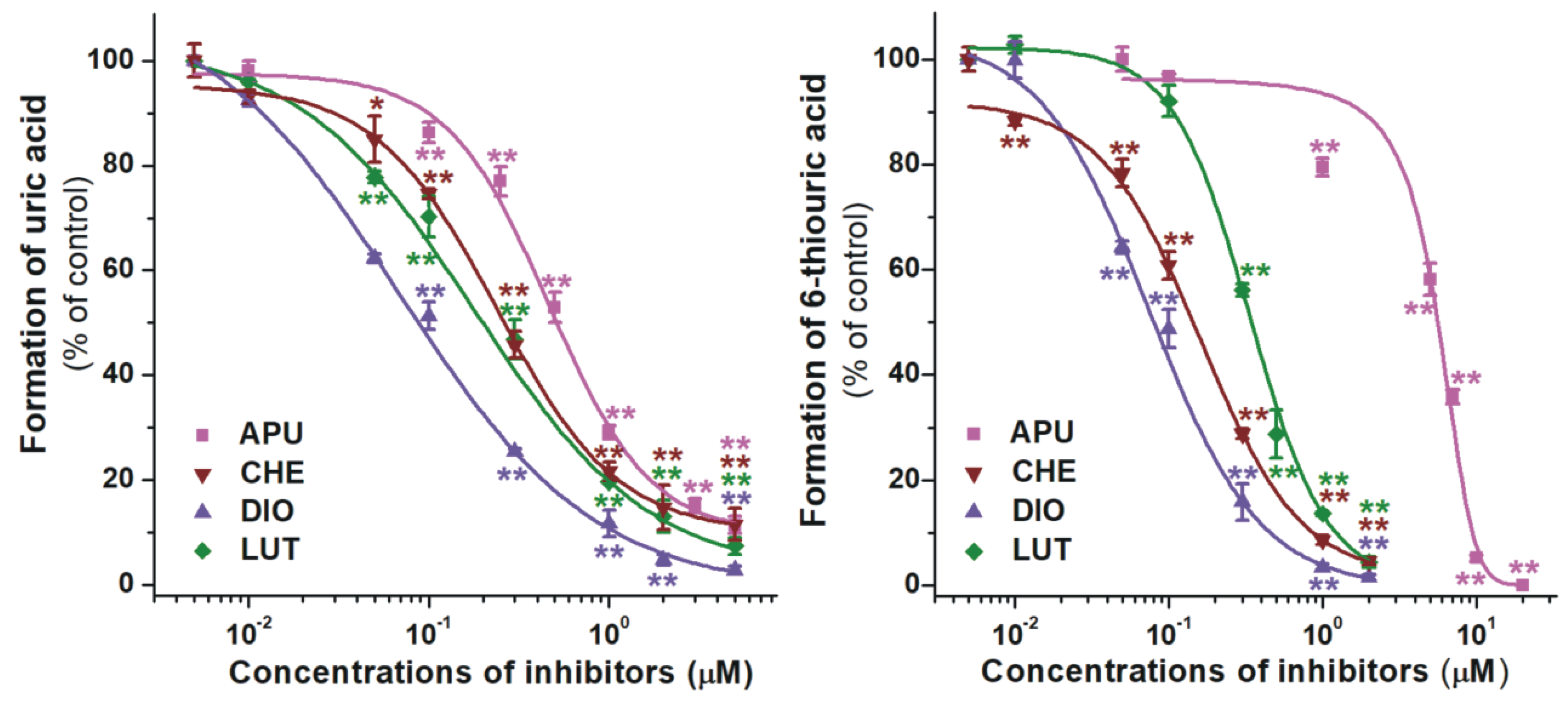
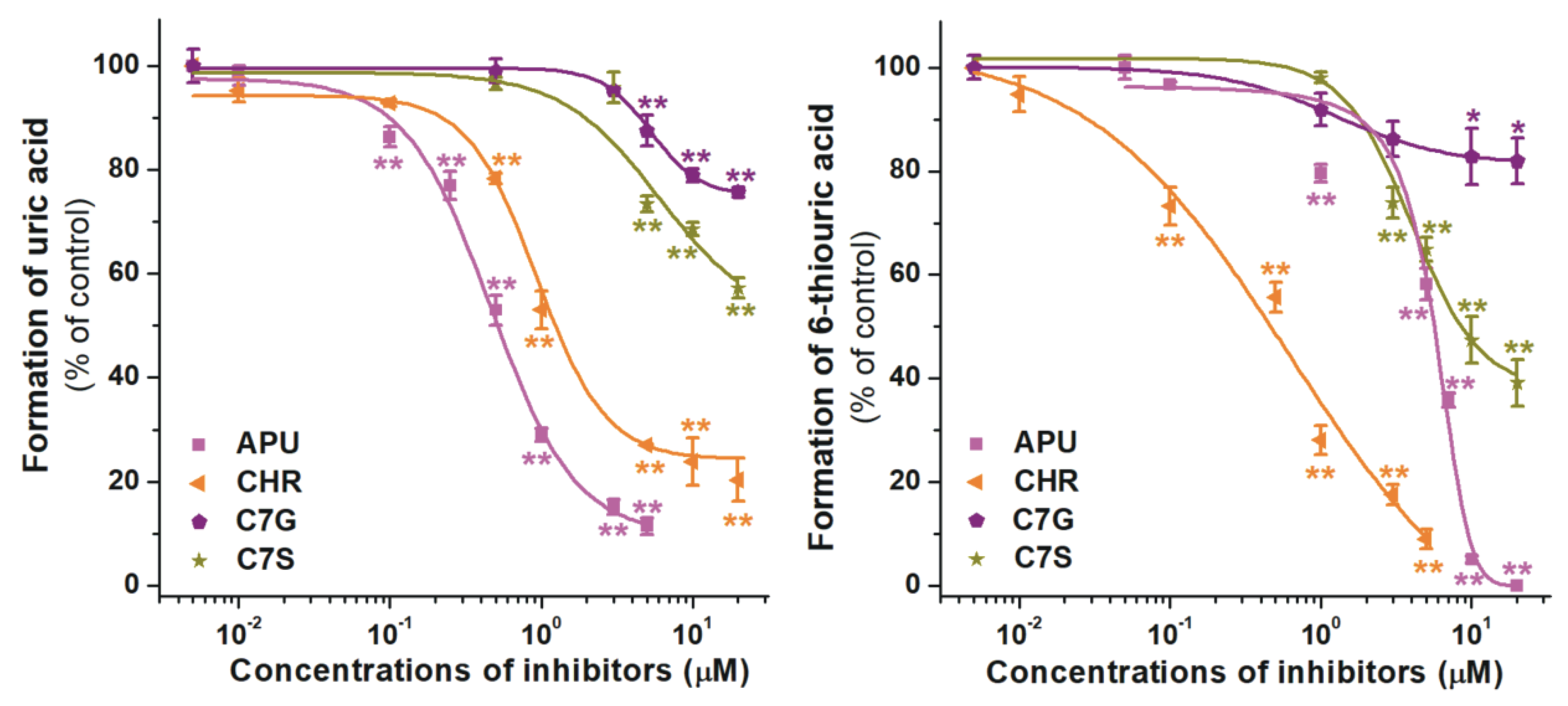
| Test Compound | IC50(xanthine) (μM) | IC50(6MP) (μM) | IC50 (xanthine)/IC50 (6MP) |
|---|---|---|---|
| Allopurinol | 0.38 | 1.97 | 0.19 |
| Apigenin | 0.27 | 0.09 | 3.00 |
| Kaempferol | 0.29 | 0.38 | 0.76 |
| Fisetin | 0.86 | 1.10 | 0.78 |
| Geraldol | 0.68 | 1.19 | 0.57 |
| Luteolin | 0.16 | 0.27 | 0.59 |
| Diosmetin | 0.04 | 0.05 | 0.80 |
| Chrysoeriol | 0.17 | 0.09 | 1.88 |
| Chrysin | 0.70 | 0.22 | 3.18 |
| Chrysin-7-sulfate | > 20.0 | 2.23 | - |
| Chrysin-7-glucuronide | > 20.0 | > 20.0 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohos, V.; Fliszár-Nyúl, E.; Poór, M. Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates. Int. J. Mol. Sci. 2020, 21, 3256. https://doi.org/10.3390/ijms21093256
Mohos V, Fliszár-Nyúl E, Poór M. Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates. International Journal of Molecular Sciences. 2020; 21(9):3256. https://doi.org/10.3390/ijms21093256
Chicago/Turabian StyleMohos, Violetta, Eszter Fliszár-Nyúl, and Miklós Poór. 2020. "Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates" International Journal of Molecular Sciences 21, no. 9: 3256. https://doi.org/10.3390/ijms21093256
APA StyleMohos, V., Fliszár-Nyúl, E., & Poór, M. (2020). Inhibition of Xanthine Oxidase-Catalyzed Xanthine and 6-Mercaptopurine Oxidation by Flavonoid Aglycones and Some of Their Conjugates. International Journal of Molecular Sciences, 21(9), 3256. https://doi.org/10.3390/ijms21093256






