Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing
Abstract
1. Introduction
2. Results
2.1. In Vitro
2.1.1. Quantification of ATP Contents Released from Activated Platelets
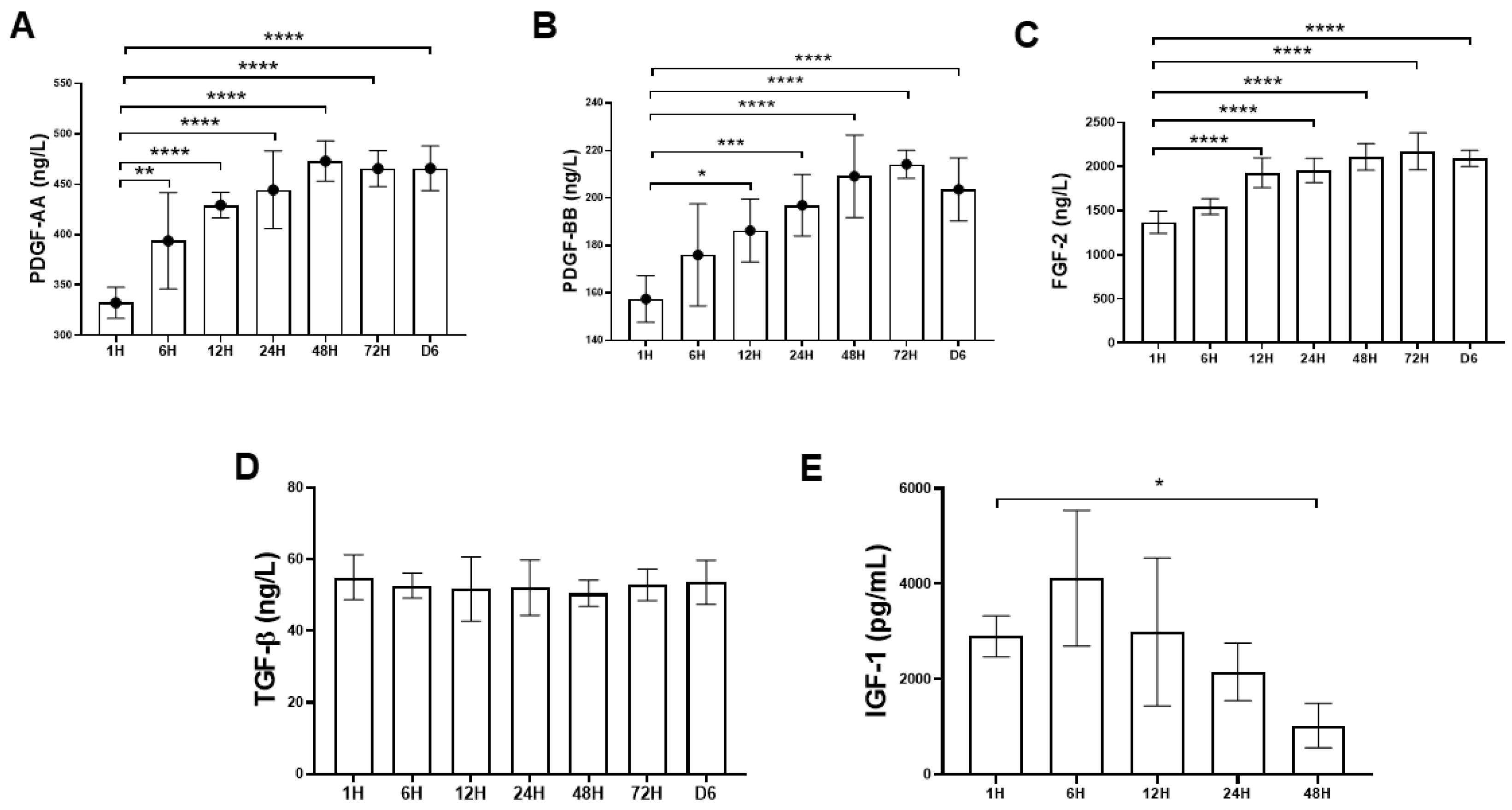
2.1.2. Time-Sequential Cytokine Release
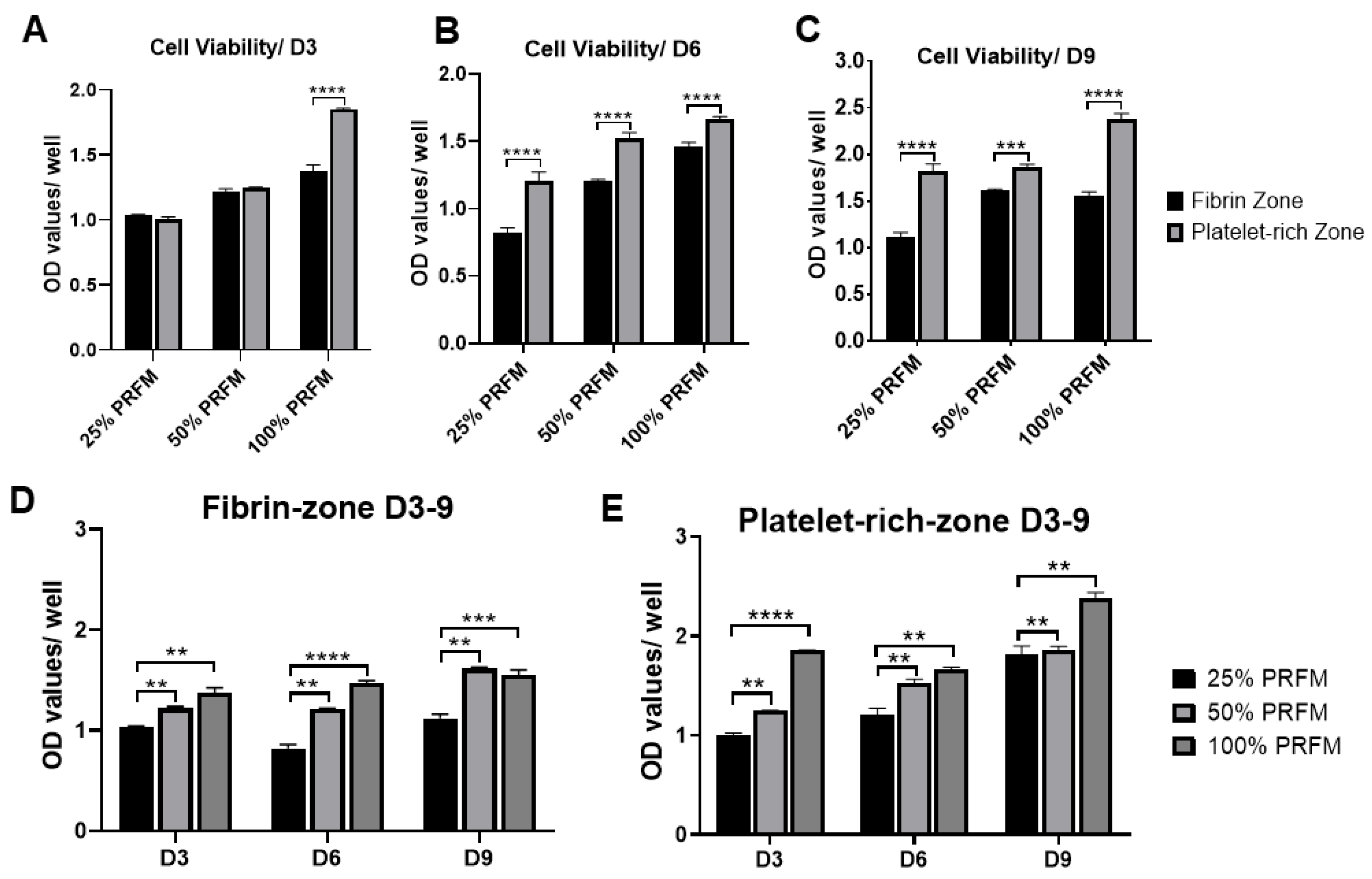
2.1.3. Dose-Dependent Effects of PRF on Tenocytes Viability and Proliferation
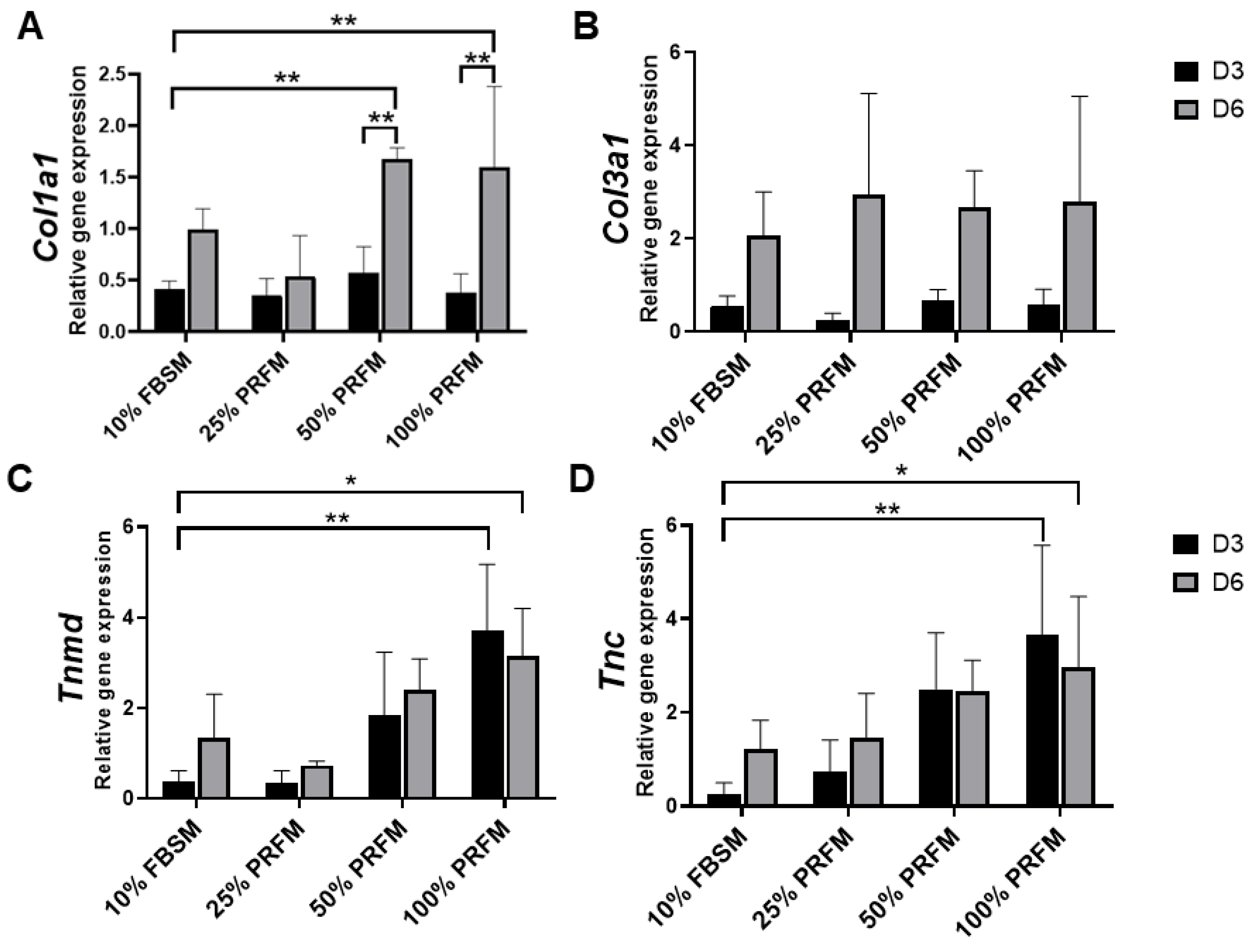
2.1.4. PRF Promotes Tenogenic Gene Expression
2.1.5. Sonographic Findings
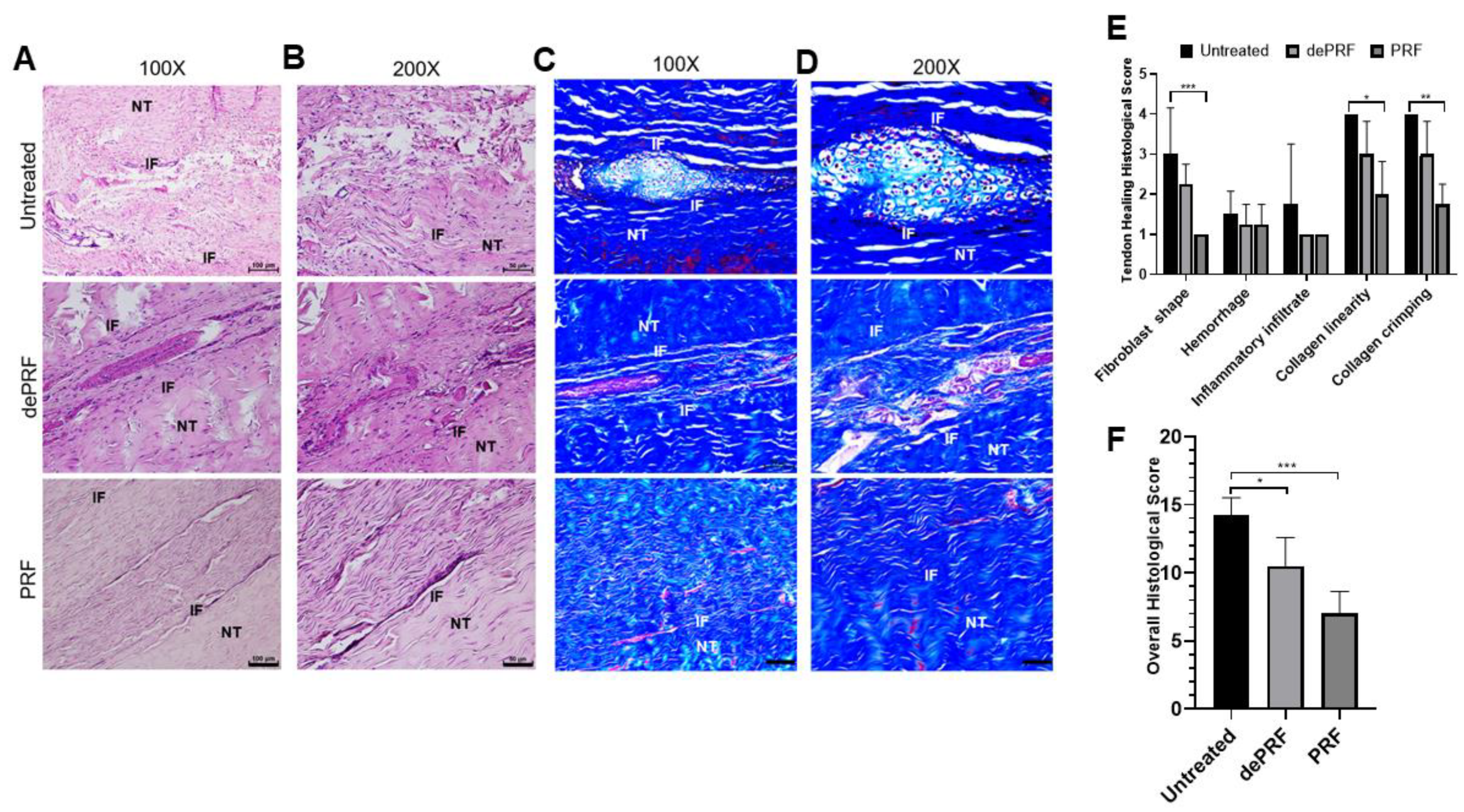
2.1.6. Histological Analysis of Tendon Healing
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethics Statement
4.2. In Vitro
4.2.1. Preparation of Platelet-Rich Fibrin (PRF)
4.2.2. Detection of local adenosine triphosphate release from activated platelets
4.2.3. Cytokine Quantitation and Time-Sequential Release Kinetics
4.2.4. Preparation of PRF-Conditioned Medium
4.2.5. Isolation and Culture of Rabbit Tenocytes
4.2.6. Cell Viability of Rabbit Tenocytes
4.2.7. Tenogenic Gene Expression of Cultured Tenocytes
4.3. In Vivo
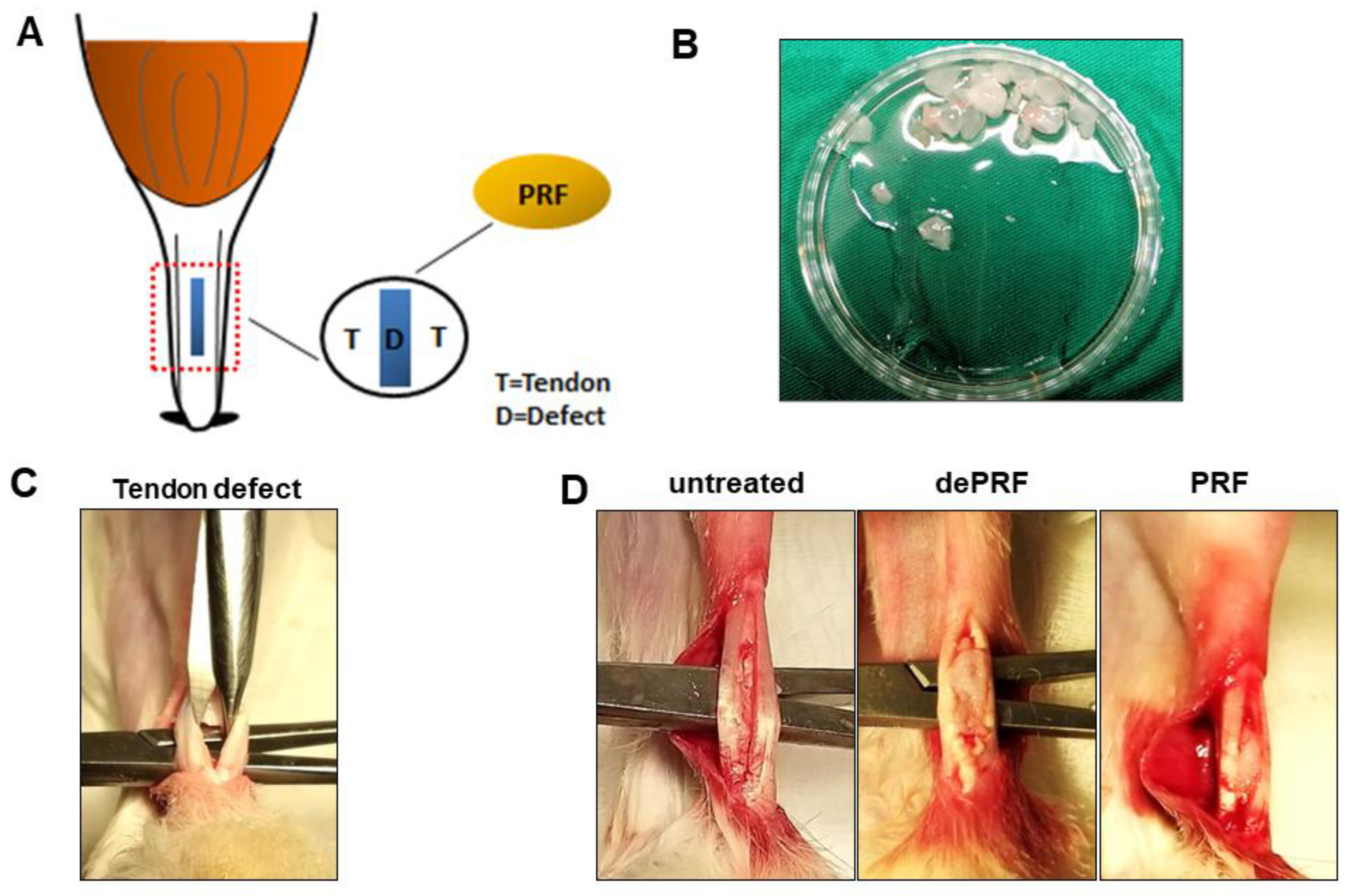
4.3.1. Surgical Procedure to Create a Tendon Defect
4.3.2. Sonographic Evaluation
4.3.3. Histological Analysis
4.3.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Komi, P.V. Relevance of in vivo force measurements to human biomechanics. J. Biomech. 1990, 23 (Suppl. 1), 23–34. [Google Scholar] [CrossRef]
- Muller, S.A.; Todorov, A.; Heisterbach, P.E.; Martin, I.; Majewski, M. Tendon healing: An overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair-A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Walden, G.; Liao, X.; Donell, S.; Raxworthy, M.J.; Riley, G.P.; Saeed, A. A Clinical, Biological, and Biomaterials Perspective into Tendon Injuries and Regeneration. Tissue Eng. Part B: Rev. 2017, 23, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999, 28, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Heisterbach, P.; Jaquiery, C.; Durselen, L.; Todorov, A.; Martin, I.; Evans, C.H.; Muller, S.A. Improved tendon healing using bFGF, BMP-12 and TGFbeta1 in a rat model. Eur. Cells Mater. 2018, 35, 318–334. [Google Scholar] [CrossRef]
- Muller, S.A.; Quirk, N.P.; Muller-Lebschi, J.A.; Heisterbach, P.E.; Durselen, L.; Majewski, M.; Evans, C.H. Response of the Injured Tendon to Growth Factors in the Presence or Absence of the Paratenon. Am. J. Sports Med. 2019, 47, 462–467. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Takamura, M.; Yasuda, T.; Nakano, A.; Shima, H.; Neo, M. The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop. Traumatol. Turc. 2017, 51, 65–72. [Google Scholar] [CrossRef]
- Sadoghi, P.; Rosso, C.; Valderrabano, V.; Leithner, A.; Vavken, P. The role of platelets in the treatment of Achilles tendon injuries. J. Orthop. Res. Publ. Orthop. Res. Soc. 2013, 31, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019, 192, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Bai, M.Y.; Chuang, M.H.; Lin, M.F.; Tang, S.L.; Wong, C.C.; Chan, W.P. Relationships of Age and Sex with Cytokine Content and Distribution in Human Platelet Fibrin Gels. Sci. Rep. 2018, 8, 10642. [Google Scholar] [CrossRef]
- Wong, C.C.; Chen, C.H.; Chan, W.P.; Chiu, L.H.; Ho, W.P.; Hsieh, F.J.; Chen, Y.T.; Yang, T.L. Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds With Autologous Cartilaginous Grafts. Am. J. Sports Med. 2017, 45, 3128–3142. [Google Scholar] [CrossRef]
- Khiste, S.V.; Naik Tari, R. Platelet-Rich Fibrin as a Biofuel for Tissue Regeneration. ISRN Biomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Wong, C.C.; Kuo, T.F.; Yang, T.L.; Tsuang, Y.H.; Lin, M.F.; Chang, C.H.; Lin, Y.H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci. 2017, 18, 1722. [Google Scholar] [CrossRef]
- Wong, C.C.; Ou, K.L.; Lin, Y.H.; Lin, M.F.; Yang, T.L.; Chen, C.H.; Chan, W.P. Platelet-Rich Fibrin Facilitates One-Stage Cartilage Repair by Promoting Chondrocytes Viability, Migration, and Matrix Synthesis. Int. J. Mol. Sci. 2020, 21, 577. [Google Scholar] [CrossRef]
- Dietrich, F.; Duré, G.L.; Klein, C.P.; Bampi, V.F.; Padoin, A.V.; Silva, V.D.; Jefferson, B.-S. Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J. Plast. Surg. 2015, 4, 101–109. [Google Scholar]
- Metineren, H.; Dülgeroğlu, T.; Metineren, M.; Aydın, E.; Koçak, C. Effectiveness of platelet-rich fibrin on tendon healing: Experimental study in a rat model. Int. J. Clin. Exp. Med. 2016, 7, 14260–14265. [Google Scholar]
- Alviti, F.; Gurzi, M.; Santilli, V.; Paoloni, M.; Padua, R.; Bernetti, A.; Bernardi, M.; Mangone, M. Achilles Tendon Open Surgical Treatment With Platelet-Rich Fibrin Matrix Augmentation: Biomechanical Evaluation. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2017, 56, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Keene, D.J.; Harrison, P.; Hulley, P.; Wagland, S.; Thompson, J.Y.; Parsons, S.R.; Byrne, C.; Schlussel, M.M.; O’Connor, H.M.; et al. Efficacy and Mechanism Evaluation. In Platelet-Rich Plasma Injection for Adults with Acute Achilles Tendon Rupture: The PATH-2 RCT; NIHR Journals Library: Southampton, UK, 2019. [Google Scholar]
- Beigi, R.; Kobatake, E.; Aizawa, M.; Dubyak, G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 1999, 276, C267–C278. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Jeon, S.H.; Park, J.Y.; Chung, J.H.; Choung, Y.H.; Choung, H.W.; Kim, E.S.; Choung, P.H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthr. Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef]
- Halpern, B.C.; Chaudhury, S.; Rodeo, S.A. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J. 2012, 8, 137–145. [Google Scholar] [CrossRef]
- Siess, W. Molecular mechanisms of platelet activation. Physiol. Rev. 1989, 69, 58–178. [Google Scholar] [CrossRef]
- Bai, M.Y.; Wang, C.W.; Wang, J.Y.; Lin, M.F.; Chan, W.P. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo) 2017, 72, 116–124. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Zaegel, M.; Das, R.; Harwood, F.L.; Silva, M.J.; Amiel, D.; Sakiyama-Elbert, S.; Gelberman, R.H. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J. Orthop. Res. 2007, 25, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, J.; Dong, S.; Huangfu, X.; Li, B.; Yang, H.; Zhao, J.; Cui, W. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014, 9, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.N.; Kim, H.M.; Sakiyama-Elbert, S.; Galatz, L.M.; Havlioglu, N.; Thomopoulos, S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J. Orthop. Res. 2011, 29, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Dex, S.; Alberton, P.; Willkomm, L.; Sollradl, T.; Bago, S.; Milz, S.; Shakibaei, M.; Ignatius, A.; Bloch, W.; Clausen-Schaumann, H.; et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. EBioMedicine 2017, 20, 240–254. [Google Scholar] [CrossRef]
- Dex, S.; Lin, D.; Shukunami, C.; Docheva, D. Tenogenic modulating insider factor: Systematic assessment on the functions of tenomodulin gene. Gene 2016, 587, 1–17. [Google Scholar] [CrossRef]
- Docheva, D.; Hunziker, E.B.; Fassler, R.; Brandau, O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol. Cell. Biol. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Martin, J.A.; Mehr, D.; Pardubsky, P.D.; Buckwalter, J.A. The role of tenascin-C in adaptation of tendons to compressive loading. Biorheology 2003, 40, 321–329. [Google Scholar]
- Docheva, D.; Muller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug. Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Rosenbaum, A.J.; Wicker, J.F.; Dines, J.S.; Bonasser, L.; Razzano, P.; Dines, D.M.; Grande, D.A. Histologic stages of healing correlate with restoration of tensile strength in a model of experimental tendon repair. HSS J. 2010, 6, 164–170. [Google Scholar] [CrossRef]
- Nixon, A.J.; Dahlgren, L.A.; Haupt, J.L.; Yeager, A.E.; Ward, D.L. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Vet. Res. 2008, 69, 928–937. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | Size (basepair) |
|---|---|---|
| Col1a1 XM_017348831.1 | F:ACCTGGTCCCCAAGGTTTCCAA R:CTTGGCACCATCCAAACCACTG | 247 |
| Col3a1 XM_002712333.3 | F:TGGTCTTCCTGGTGAAAACGGA R:TTCACCCTTAGCACCAGGGGAT | 196 |
| Tnmd NM_001109818.1 | F:GAACAAAATGAGCAGTGGGTGGTC R:TTGCAAGGCATGATGACACGACAG | 272 |
| Tenascin FJ480400.1 | F:AGGGCTTTGAGGAAAGTGAACC R:TCAGAGCATACTCCACCGTGTT | 216 |
| β-actin NM_001101683.1 | F:CAACTGGGACGACATGGAGAAG R:TGAACGTCTCGAACATGATCTG | 152 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.-C.; Huang, Y.-M.; Chen, C.-H.; Lin, F.-H.; Yeh, Y.-Y.; Bai, M.-Y. Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing. Int. J. Mol. Sci. 2020, 21, 3221. https://doi.org/10.3390/ijms21093221
Wong C-C, Huang Y-M, Chen C-H, Lin F-H, Yeh Y-Y, Bai M-Y. Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing. International Journal of Molecular Sciences. 2020; 21(9):3221. https://doi.org/10.3390/ijms21093221
Chicago/Turabian StyleWong, Chin-Chean, Yu-Min Huang, Chih-Hwa Chen, Feng-Huei Lin, Yi-Yen Yeh, and Meng-Yi Bai. 2020. "Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing" International Journal of Molecular Sciences 21, no. 9: 3221. https://doi.org/10.3390/ijms21093221
APA StyleWong, C.-C., Huang, Y.-M., Chen, C.-H., Lin, F.-H., Yeh, Y.-Y., & Bai, M.-Y. (2020). Cytokine and Growth Factor Delivery from Implanted Platelet-Rich Fibrin Enhances Rabbit Achilles Tendon Healing. International Journal of Molecular Sciences, 21(9), 3221. https://doi.org/10.3390/ijms21093221







