Abstract
Transcription factor Prospero homeobox 1 (PROX1) is continuously expressed in the lymphatic endothelial cells, playing an essential role in their differentiation. Many reports have shown that PROX1 is implicated in cancer development and acts as an oncoprotein or suppressor in a tissue-dependent manner. Additionally, the PROX1 expression in many types of tumors has prognostic significance and is associated with patient outcomes. In our previous experimental studies, we showed that PROX1 is present in the thyroid cancer (THC) cells of different origins and has a high impact on follicular thyroid cancer (FTC) phenotypes, regulating migration, invasion, focal adhesion, cytoskeleton reorganization, and angiogenesis. Herein, we discuss the PROX1 transcript and protein structures, the expression pattern of PROX1 in THC specimens, and its epigenetic regulation. Next, we emphasize the biological processes and genes regulated by PROX1 in CGTH-W-1 cells, derived from squamous cell carcinoma of the thyroid gland. Finally, we discuss the interaction of PROX1 with other lymphatic factors. In our review, we aimed to highlight the importance of vascular molecules in cancer development and provide an update on the functionality of PROX1 in THC biology regulation.
1. Introduction
Transcription factor Prospero homeobox 1 (PROX1) is a homolog of the Prospero in Drosophila that regulates the development of various organs, including the central nervous system [1], lens, retina, [2], liver [3], heart [4], pancreas [5], and cell fate of lymphatic endothelial cells (LECs) [6]. The PROX1 gene encodes a protein belonging to the Homeobox family, which has a characteristic Prospero domain at the C-terminus [7].
It has recently been established that PROX1 has a variety of roles in cancerogenesis, and its functions may change according to the type of tissue. Thus, PROX1 acts as a tumor suppressor in hepatocellular carcinoma [8], esophageal cancer [9], pancreatic cancer [10], oral cancer [11], hematologic malignancy [12], sporadic breast cancer [13], carcinoma of the biliary system [14], and papillary thyroid cancer (PTC) [15]. On the other hand, PROX1 promotes aggressive behavior of colorectal cancer [16], kaposiform hemangioendothelioma [17], glioma [18,19], and our last observations point to its distinct oncogenic role in follicular thyroid cancer (FTC) [20,21,22].
Thyroid cancer (THC) is the most common malignancy of the endocrine system, which is clinically divided into categories: (1) well-differentiated thyroid cancer (DTC), including PTC and FTC carcinomas, (2) poorly differentiated thyroid cancer (PDTC) (3) undifferentiated–anaplastic thyroid cancer (ATC), and (4) neuroendocrine C-cell derived-medullary thyroid cancer (MTC) [23]. Squamous cell carcinoma of the thyroid gland (SCT) is an unusual neoplasm, which is thought to arise as a primary tumor or as a component of anaplastic or undifferentiated carcinoma [24].
Depending on the histological variant, THC can use the different vascular routes to metastasize. Here, PTC spreads preferentially to the lymph nodes via the lymphatic system, and the more aggressive types, FTC, MTC, ATC, and SCT, tend to metastasize to distant organs (such as lung and bone) through the bloodstream [25,26].
Recent data strongly indicates that the cancer cell road of metastasis is highly connected with active vascular factors in the microenvironment and their expression in the cancer cells [27]. In this scenario, many molecules associated with blood endothelial cells (such as CD44, Intercellular adhesion molecule 1; ICAM1, Vascular endothelial growth factor receptor 1; VEGFR-1 and Neutropilin-1) and LEC-specific proteins (including Podoplanin; PDPN, Lymphatic vessel endothelial hyaluronan receptor 1; LYVE-1, VEGFR-3, VEGFC, and PROX1) can be expressed in tumor cells and peri-/intra-tumoral vessels consequently regulating angiogenic potential of tumors. Furthermore, the altered expression and secretion of vascular molecules in the tumoral surrounding can change the behavior of cancer and stromal cells; as a result, increasing the invasiveness and metastasis of tumors [27].
Interestingly, VEGFR-1 is expressed in blood vessels of the tumor but not in those of the healthy tissue [28]. VEGFD can induce both intra- and peri-tumoral lymphatic vessel development; however, it is not involved in lymph node metastasis [29]. Finally, a series of lymphatic factors, including PROX1, VEGFC, PDPN, VEGFR-3, SOX18 (SRY-Box transcription factor 18), and COUP-TFII (Nuclear receptor subfamily 2 group F member 2) can be expressed in tumoral cells and, consequently, control their properties, such as invasion, migration, proliferation, survival, epithelial to mesenchymal transition (EMT), and adhesion [30,31,32,33,34,35].
In the presented review, we discuss the role of PROX1 in THC development and recent advances in this field. We describe the PROX1 mRNA / protein sequences, PROX1 expression pattern in THC and its epigenetic regulation. Next, we present the most important biological processes and genes regulated by PROX1 in CGTH-W-1 (squamous cell carcinoma of the thyroid derived; SCT) based on internal RNA sequencing analysis. Finally, the last segment of the review details the transcriptional interaction of PROX1 with other lymphatic markers, such as VEGFC, VEGFR-3, and PDPN.
2. Thyroid Cancer Classification
Most primary THCs are epithelial tumors that originate from thyroid follicular cells and can appear as three main histopathological types of carcinoma: PTC, FTC, and ATC. PTC accounts for 85%–90% of all thyroid cancer cases, followed by FTC; 5%–10% [23]. ATC accounts for less than 2% of thyroid cancers, and it is a lethal malignancy (survival ~6 months from diagnosis), typically arising in elderly patients [36]. Next, MTC, with estimated prevalence maximally 2% of THC cases, is a form of thyroid carcinoma originating from thyroid parafollicular (C) cells with the characteristic presence of numerous endocrine secretory granules containing calcitonin in the cytoplasm [37]. SCT is extremely rare (<1%) and carries the unfortunate prognosis of thyroid malignancy, which often mixes with heterogeneous elements and is associated with areas of well-differentiated PTC or FTC [24].
The recommended treatment for low-risk PTC patients (females < 45 years old with tumor limited to the thyroid gland) is thyroid lobectomy (removal one of two thyroid lobes) followed by thyroid stimulating hormone (TSH) suppressive therapy [38]. High-risk patients (males and women > 45 years of age with high-grade tumors) are subjected to total thyroidectomy (removal of the entire thyroid gland) followed by radioactive iodine (131I) ablation [39]. PTC spreads relatively easily to the neck, but distant metastases are found only in 1% of patients, mostly in lung and bones [40]. The 5-year survival rate for PTC patients accounts for 100% of patients with localized tumor, 99% for regionally spread cancer, and 78% for distant metastasizing, which is better than for FTC cases, where the rate is 100% for the localized tumor, 96% for regionally spread, and 63% for distant metastases. FTCs spread to remote sites, primarily to bones, and the most common treatment of FTC is total thyroidectomy [41].
In most aggressive types, the total thyroidectomy gives the best chance of cure for patients with MTC [42], and for ATC, the treatment is usually palliative with radiotherapy [43]. The 5-year survival rate for metastatic MTC and ATC is 39% and 4%, respectively [41].
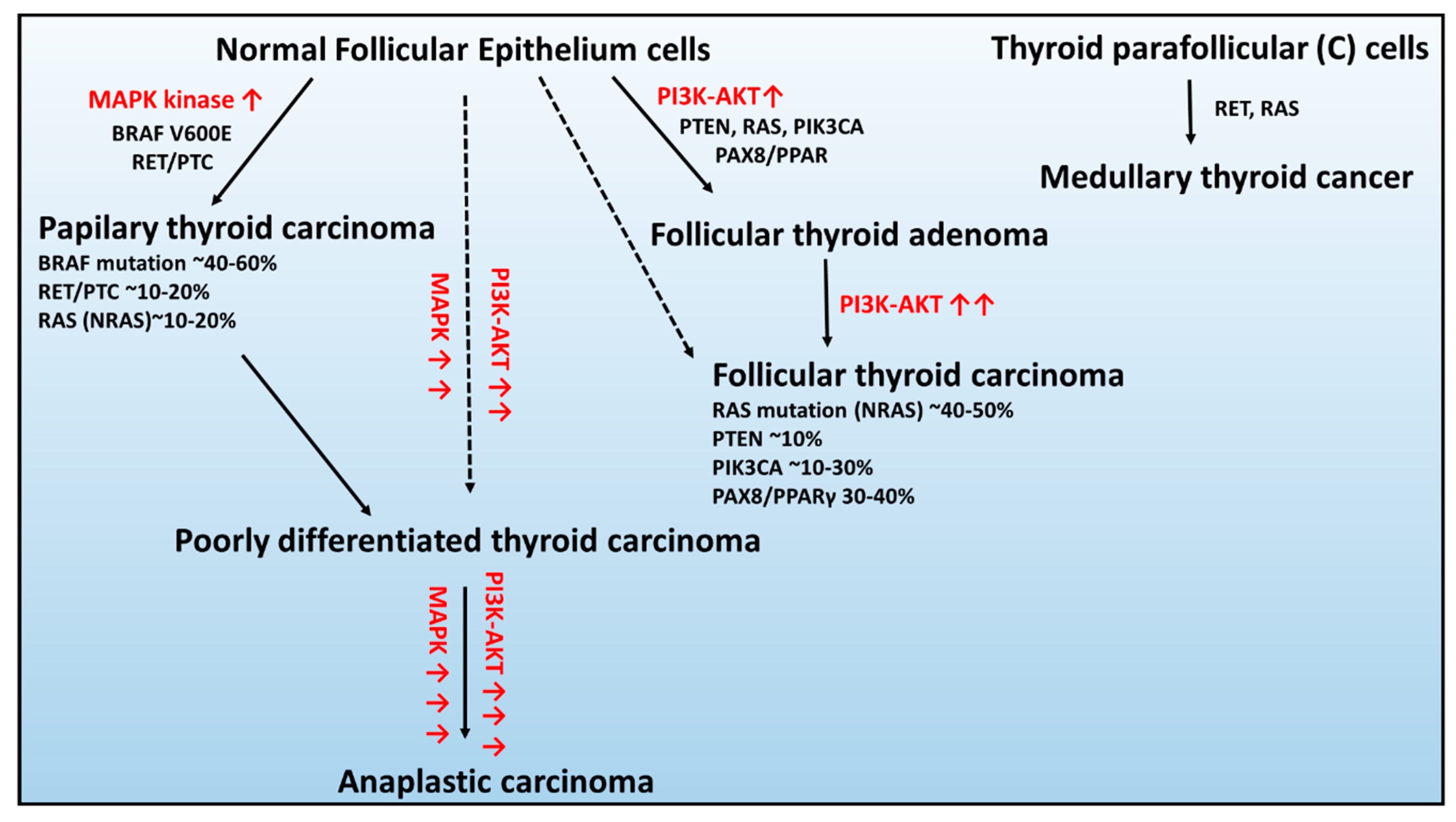
In the context of genetic alterations, the most frequent and mutually exclusive genetic changes in PTCs are BRAF V600E, RAS, and RET/PTC rearrangement, leading to constitutive activation of the signaling pathway of mitogen-activated kinases (MAPK) (Figure 1) [44].
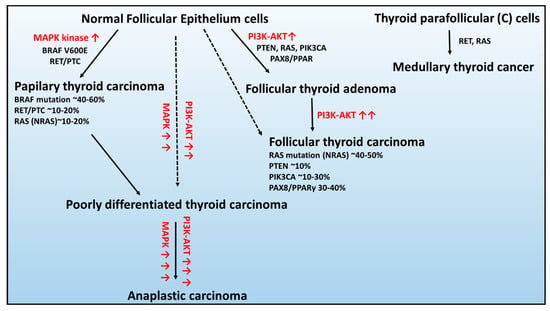
Figure 1.
Schematic of the genetic abnormalities associated with the development and progression of thyroid cancers developed from healthy epithelium cells: (1) follicular thyroid adenoma, (2) papillary thyroid carcinoma, (3) follicular thyroid carcinoma, and (4) anaplastic thyroid carcinoma and parafollicular thyroid cells: medullary thyroid carcinoma. Figure adapted from [45]. Red arrow signifies overactivation of signaling pathway.
BRAF occurs in 45% of PTCs, and in the majority, it is a substitution at the second position of codon 600 (V600E; GTG > GAG), c.1799 T > A) resulting in an amino acid change from valine to glutamic acid that leading to constitutive activation of serine/threonine kinase BRAF [46].
The RAS gene encodes a family of three highly homologous oncogenes: NRAS, HRAS, and KRAS, in which mutations occur in 10%–20% of PTCs [47]. RAS proteins transmit the signals from the receptors on cell membranes to several types of targets in the cell controlling MAPK and 3-phosphatidylinositol kinase PI kinase (PI3K) signaling pathways [47]. All point mutations of the RAS gene fix the protein activated states and, therefore, result in continuous stimulation of downstream targets of RAS [48].
The RET/PTC rearrangements occur in the chimeric oncogene RET/PTC, where the C-terminal kinase domain of the RET transmembrane tyrosine kinase receptor is fused to one of the different upstream partners, resulting in constitutive RET activity. At least 12 rearranged forms of the RET gene have been isolated and detected in 30% of the PTC cases, from which RET/PTC1 and RET/PTC3 are the most common [44,49].
In contrast, the characteristic genetic modifications that are mutually exclusive in FTC are changes in the RAS, PTEN, and PIK3CA genes, as well as rearrangement of PAX8-PPARγ, activating the 3-phosphatidylinositol kinase PI kinase (PI3K/Protein Kinase B–AKT) [44,50].
The RAS mutations were observed in approximately 40%–50% of FTC cases [51], and the modification was predominantly found in the NRAS codon 61, which positively associated with distant metastases of FTC [52].
Following, the mutation or deletion of the tumor suppressor gene – PTEN (phosphatase and tensin homolog) and PIK3CA transcript (coding the p110α catalytic subunit of PI3K) are the classical genetic alterations that activate the PI3K–AKT pathway in ~10% and 10%–30% of FTC cases, respectively [53,54].
The PAX8 gene encodes a transcription factor required for the generation of thyroid follicular cells and tissue-specific gene expression in the thyroid gland. The PAX8/PPAR fusion results in significant increases in expression of PAX8/PPAR chimeric protein and, as a result, inhibits the tumor suppressor activity of PPAR [55]. The PAX8/PPAR rearrangement presence was detected by real-time PCR in ~35% of FTC cases [56].
Consequently, the constitutive activation of pathways associated with the molecular changes in PTC and FTC can give rise to the formation of more aggressive forms PDTC and ATC [45]. The sporadic MTC development is mainly connected with RET receptor tyrosine kinase mutation, which has an essential role in cell survival, differentiation, and proliferation [37]. However, in ~10%–30% of patients, the RAS mutation was also found [57,58].
Additionally, through whole-genome sequencing, in many malignant THC cases, the mutations in the promoter region of telomerase reverse transcriptase (TERT) were found, contrary to the early stages of thyroid tumors [59]. TERT transcript is a 35 kb gene located on chromosome 5, which contains 16 exons and a 330 base pair promoter region. Two main TERT mutations: 1 295 228 C>T (C228T) and 1 295 250 C>T(C250T) can increase the TERT transcriptional activities. Particularly prevalent in THC is the C228T variant, which appeared in Liu X. et al.’s, 2013, analysis with 11.7% of PTCs, 11.4% of FTCs, 37.5% of PDTCs, and 42.6% of ATCs [59]. Moreover, the coexistence of TERT with BRAF or RAS alterations had a synergistic effect on poor clinicopathologic outcomes of PTCs, such as disease recurrence and patient mortality [60]. All data suggest that TERT promoter mutations may play a role in the THC de-differentiation, progression, and aggressive behavior [61]. Interestingly, Lee et al., 2019 correlated TERT and PROX1 mRNA expression levels in several cancer types, including melanoma, esophageal and head and neck, and lung cancer, with PROX1 downregulation indicating a poorer prognosis in melanoma [62]. The authors concluded that PROX1 perhaps regulates TERT in an activity-dependent manner with other genetic changes. The PROX1:TERT relation can be a new scientific aim, which has to be elucidated in THC research.
The establishment of molecular changes in THC and recent progress in this field provide unprecedented opportunities for the development of molecular-based diagnostic, prognostic, and therapeutic strategies for a different type of THC.
3. PROX1 mRNA/Protein Isoforms and Antisense of PROX1 Characterization
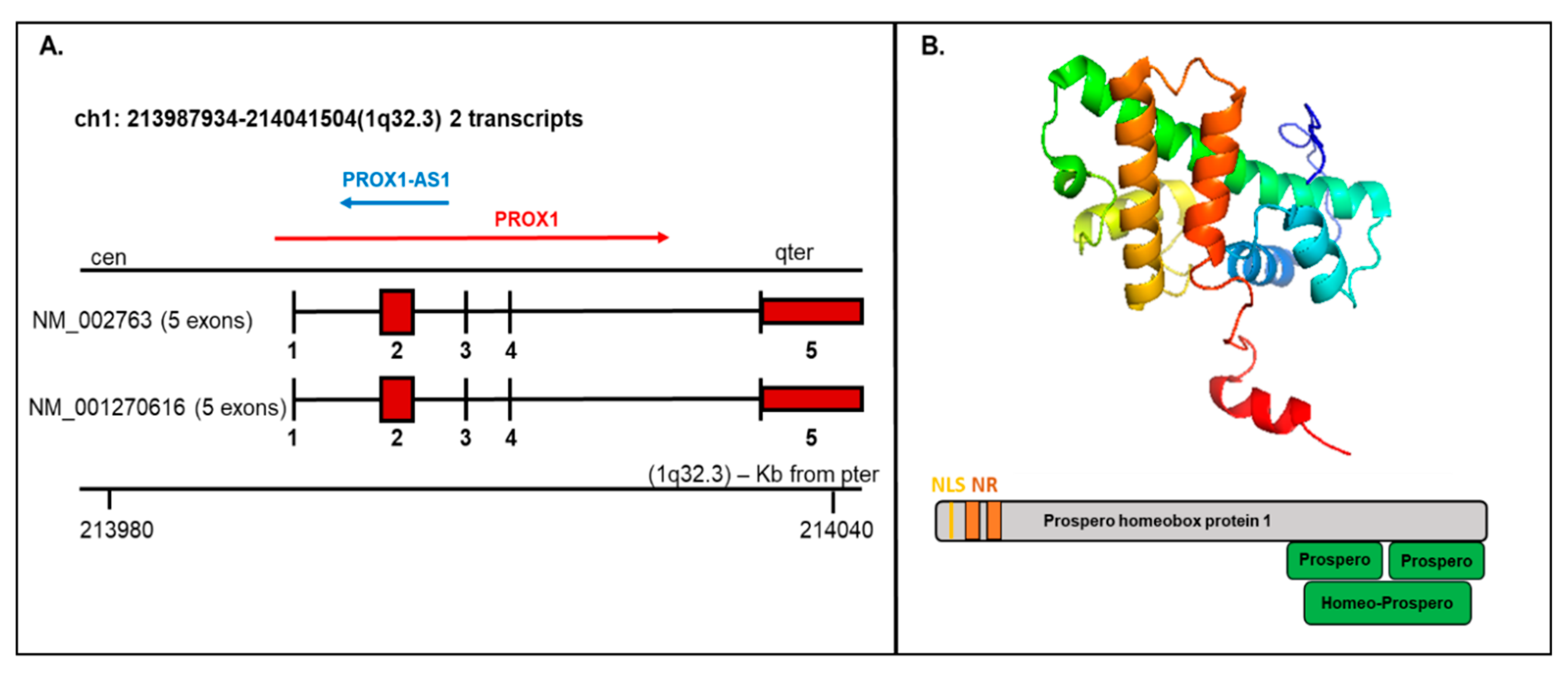
PROX1 is a transcription factor essential for the embryogenesis of a variety of organs. The human PROX1 transcript is located on chromosome 1q32.2–q32.3 composed of five exons and four introns and produces two variants: NM_002763 and NM_001270616, both encoding the same protein product, but the NM_002763 transcript is longer by 322 nucleotides [7]. Long noncoding transcript (PROX1-AS1) transcribed from the antisense strand of PROX1 is located on chromosome 1q32.3 with transcript length 3399 bp (Figure 2A).
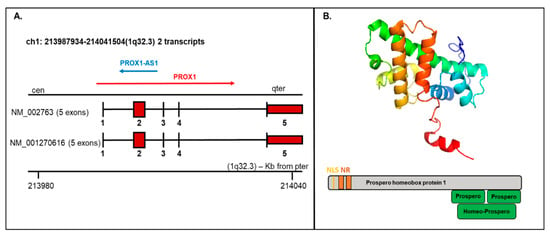
Figure 2.
Prospero homeobox 1 (PROX1) mRNA/protein structure. (A) The PROX1 transcript presents two variants (1-NM_002763 and 2-NM_001270616; the first variant represents the longer transcript; both options 1 and 2 encode the same protein); mRNA includes five exons and four introns. The structure is available on http://atlasgeneticsoncology.org/Genes/GC_PROX1.html (B) Top—Protein structure-based on the PDB model (ID: 2LMD; graphical visualization was made in PyMOL), bottom—the schematic protein structure with the nuclear localization signal (NLS) and nuclear receptor (NR) boxes at the N-terminus and prospero and homeobox domains on C-terminus.
The existence of different mRNA isoforms (7.9 kb and 2.9 kb) coding various PROX1 protein forms were presented by Zinovieva et al., 1996 and Dudas et al., 2008 using sequencing and hybridization [7,63]. In the performed research, the dominance of the 7.9 bp form was correlated with hepatocellular carcinoma samples, while the shorter isoform 2.9 kb was exclusively detected in cholangiocellular carcinoma [63].
The PROX1 protein contains 737 amino acids with a molecular weight of 82.3 kDa. Structurally, the PROX1 protein includes a unique homeodomain followed by a conserved prospero domain at the C-terminus and two nuclear receptor boxes (NR boxes) with nuclear localization signal (NLS) at the N-terminus (Figure 2B). PROX1 can act as a transcriptional activator, transcriptional repressor, or a transcriptional corepressor. While Prospero-/homeodomain and NLS are responsible for DNA binding, the NR boxes can interact with nuclear receptors, e.g., HNF4a/NR1A1 or SF-1/NR5A1 [64].
4. PROX1 Expression in Thyroid Cancer
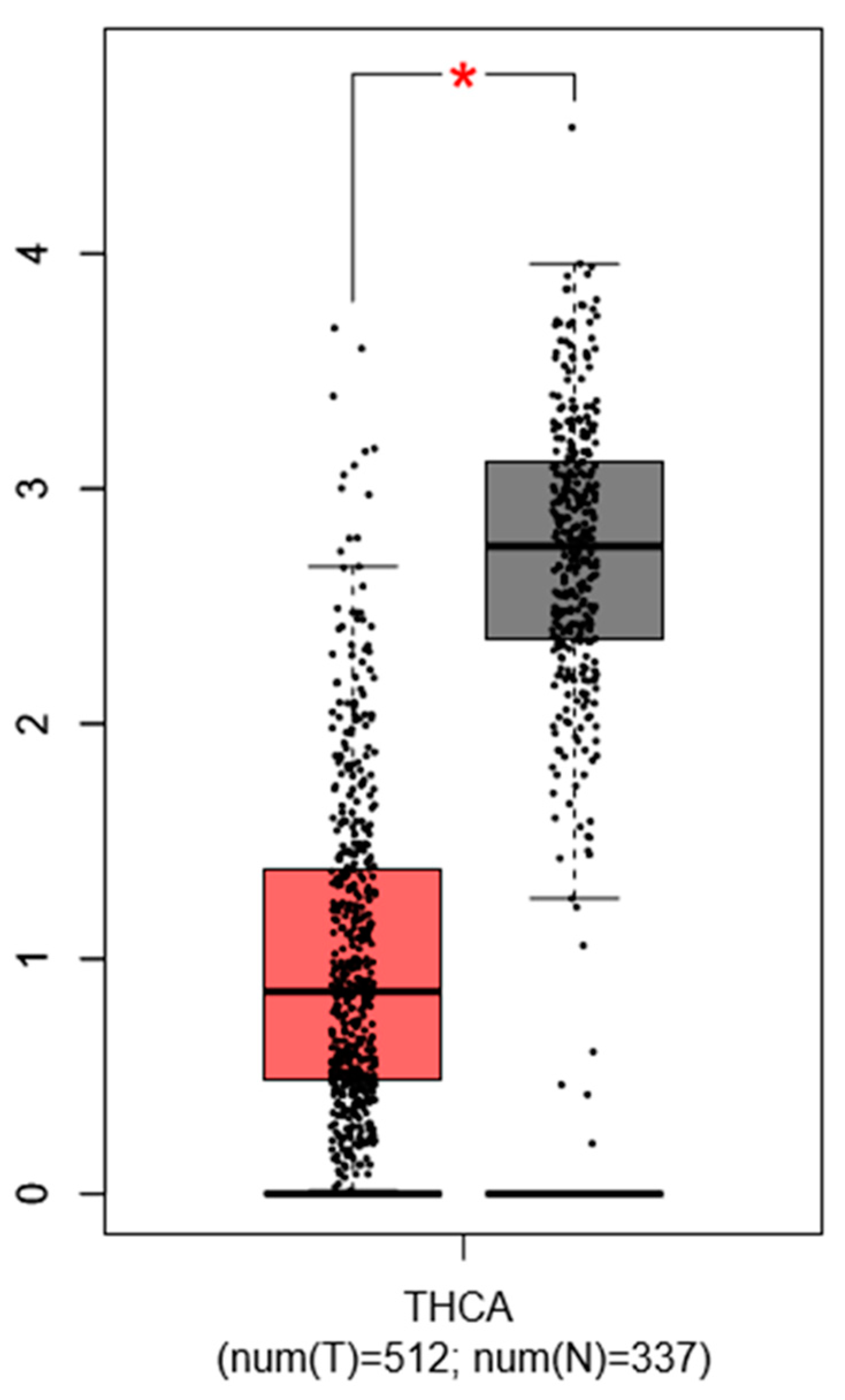
Choi D. et al., 2016 described that PTC specimens show a consistent downregulation of PROX1 by more than 2-fold (p < 1 × 10−4) compared to normal thyroid tissues, which was confirmed by our simulation (with GEPIA database) using another set of thyroid cancer gene profiling studies (Figure 3).
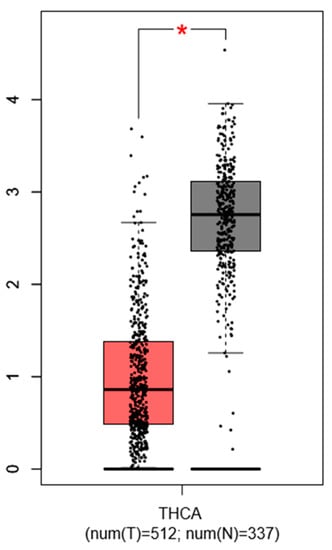
Figure 3.
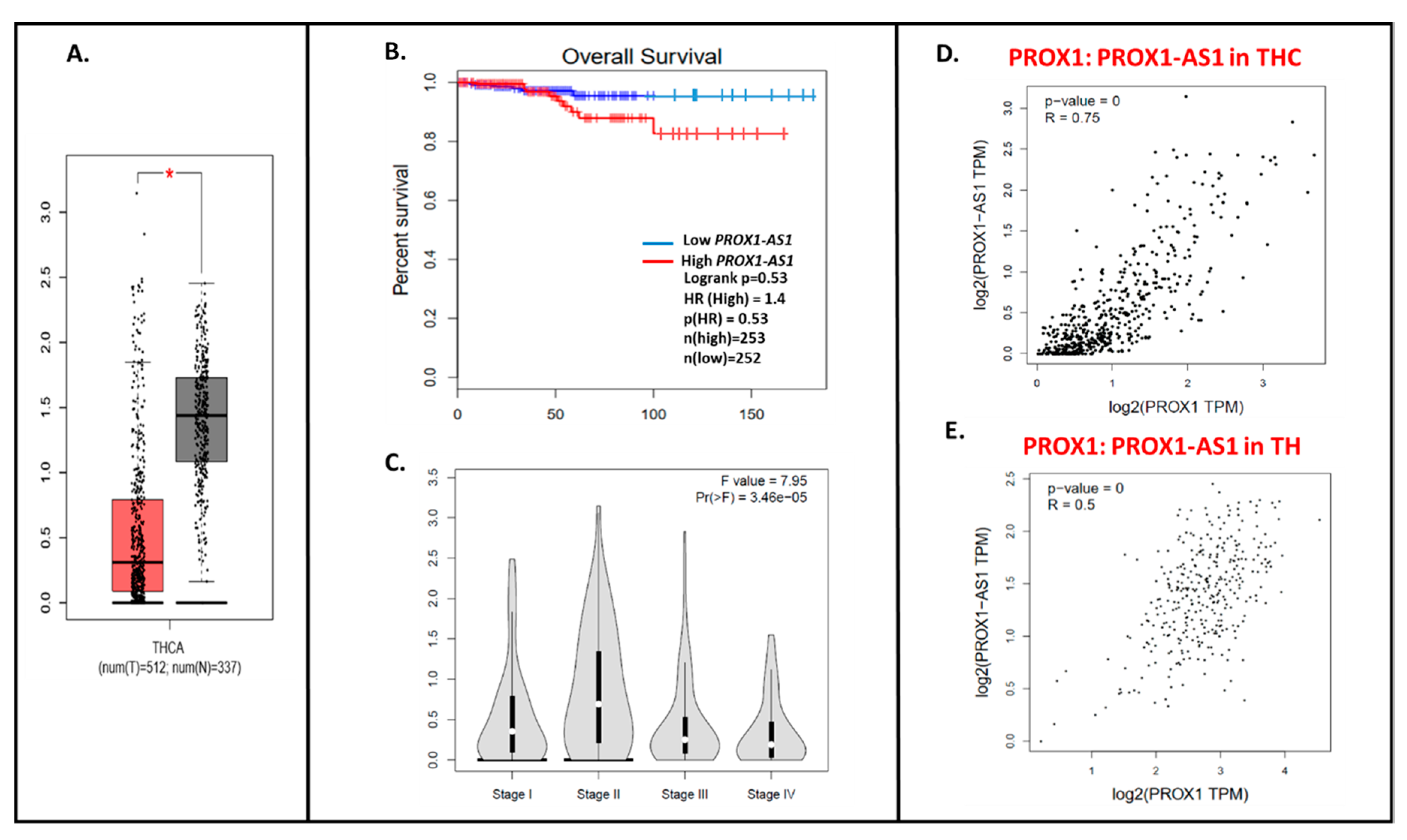
Expression pattern of PROX1 in thyroid cancer (THC) tissues. The red bar represents the PROX1 expression in papillary thyroid tumors (T; the number of cases = 512) and grey bar shows the PROX1 level in healthy tissues (N; the number of cases = 337), * p ≤ 0.05. The data are available on the GEPIA database.
Authors suggested that PROX1 downregulation can be already detectable in follicular and oncocytic adenomas, implying that this genetic event may happen in the early stage of follicular carcinogenesis [15].
Next, PROX1 downregulation in PTC-derived cells (BcPAP, TPC-1), normal thyroid cells (Nthy ori-3-1), ATC-derived cells (8505C), and two FTC-derived cell lines (FTC236 and FTC238) in comparison to FTC-derived cell line (FTC-133), and SCT cells (CGTH-W-1) was observed [22]. Moreover, as we found, lower PROX1 expression levels correlated with more prolonged survival and reduced disease severity, i.e., with I and II grades in comparison to III and IV stages. The switch of the PROX1 expression level in different cancer stages was suggested to be negatively regulated by fibroblast growth factor 2 (FGF2) [22]. In lens epithelial cells (LCs), the positive PROX1–FGFR signaling feedback loop was demonstrated, leading to PROX1 upregulation in response to FGF2 [65]. Additionally, the positive stimulation of PROX1 by FGF2 in CGTH-W-1 cells was noticed [22]. Interestingly, the lower expression of PROX1 mRNA in THC tissues and cultured cancer cells did not correspond to the protein level that revealed accumulation in the cytoplasm [15,20]. This phenomenon was connected with the higher stability of the cytoplasmic form of PROX1 protein. The experiments performed with Kaposi sarcoma cells showed that PROX1 mRNA contains a canonical AU-rich element (ARE) in 3’-untranslated region (3′-UTR) facilitating binding of RNA binding protein HuR which stabilizes the transcript [66].
5. Epigenetic Regulation of PROX1
Genetic and epigenetic mechanisms can be involved in PROX1 expression regulation [11,13]. Mutations [67], DNA methylation [11], and non-coding RNA [68] appear to be the major mechanisms modulating the PROX1 function.
5.1. Mutations and Methylation
Post-transcriptional RNA editing is a process in which the nucleotide sequence of a nuclear mRNA is changed from that encoded in genomic DNA. RNA editing occurs through base modification, by deamination of cytidine (C) to uridine (U) or by deamination of adenosine (A) to inosine (I), in nuclear mRNA. Uridine and inosine are recognized by translational apparatus as thymidine and guanosine, respectively, so the net effects are changes in C-to-T and A-to-G [69]. In this context, the A-to-G mutation affects PROX1 function in human specimens of pancreatic, colon, and esophageal cancers and A to I in esophageal cancer. All detected variations were observed in cDNA PROX1 but not at the genomic DNA level [67,70]. Still, no experimental data provide information on PROX1 mutations in THC and their effect on PROX1 function. Importantly, several single nucleotide polymorphisms (SNPs) present in intronic regions of PROX1 were suggested to modulate PROX1 expression levels with potential involvement in the pathogenesis of type 2 diabetes [71]. Therefore, it cannot be excluded that modulation of PROX1 expression levels by these SNPs will also influence THC pathogenesis and/-or aggressiveness.
DNA methylation is one of the most common epigenetic modifications in mammals, and in healthy cells, it ensures the proper regulation and stable gene silencing. DNA methylation that is catalyzed by DNA methyltransferases (DNMTs) is associated with the addition of a methyl group to cytosine residue present within CpG dinucleotides, which are concentrated in large clusters (CpG islands) [72]. It is commonly known that inactivation of specific tumor suppressor genes occurs as a consequence of hypermethylation within the promoter regions, and numerous studies have demonstrated a broad range of genes silenced by DNA methylation in different cancer types [72]. Epigenetic silencing is one of the mechanisms responsible for PROX1 inactivation in tumors. For example, hypermethylation of CpG islands was identified as a mechanism for PROX1 inactivation in breast, biliary system, and squamous cell carcinomas [11,13,14].
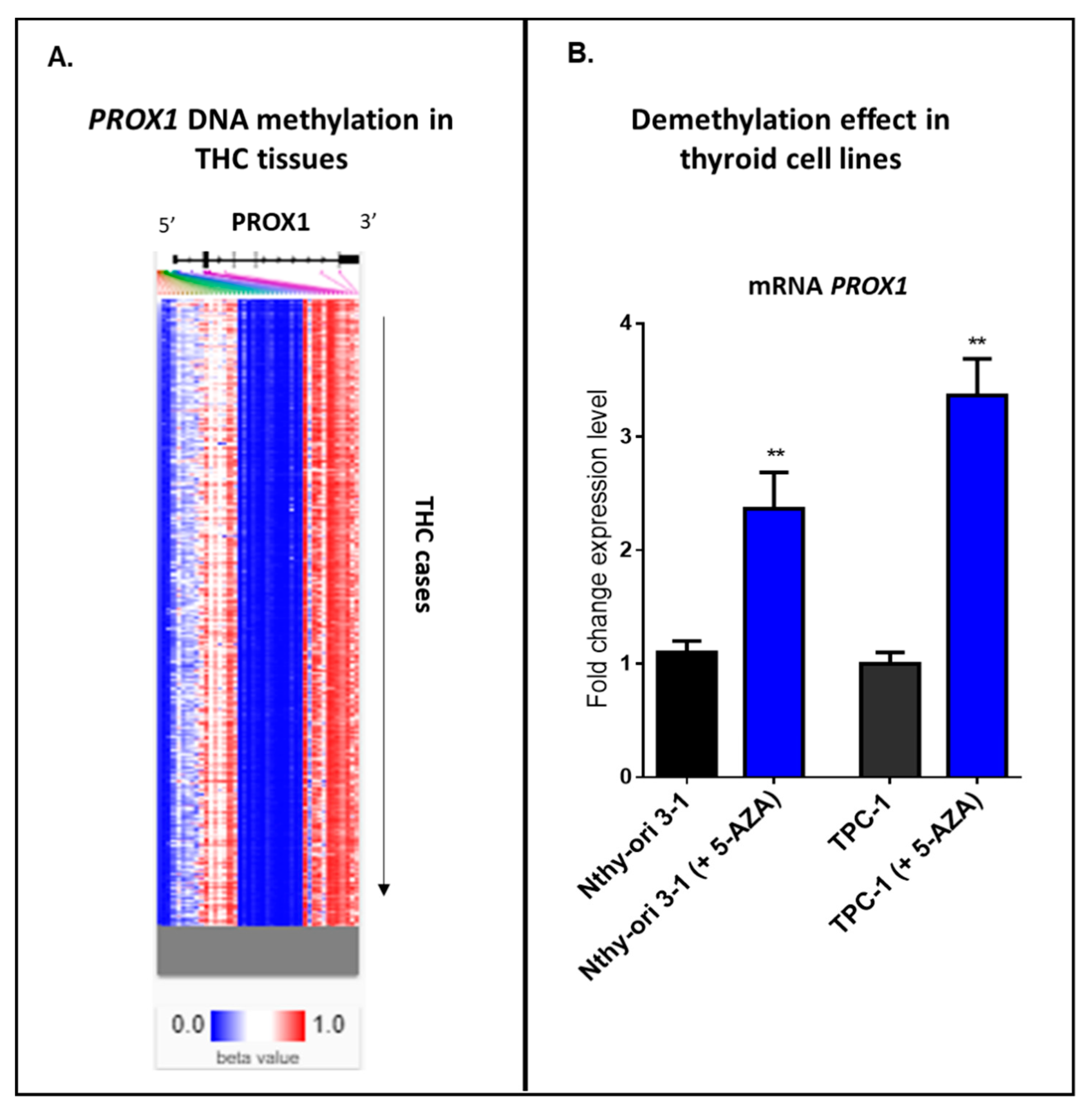
Our internal analysis using an online tool with clinical data—UCSC Xena database—has detected a heatmap showing relative methylation levels for the most variably methylated PROX1 promoter regions in THC tissues (Figure 4A). Hierarchical clustering (from top to down) provides different THC tissues, where blue indicates a low level or no methylation for the reported locus of PROX1, and red shows the high methylation. Furthermore, we provide our experimental data, where two cell lines, normal thyroid cells (Nthy ori-3-1) and PTC-derived cell line (TPC-1), showed a significant increase in PROX1 transcript after 24 h of treatment with a demethylating agent (5-aza-2’-deoxycytidine; 5 μM) (Figure 4B).
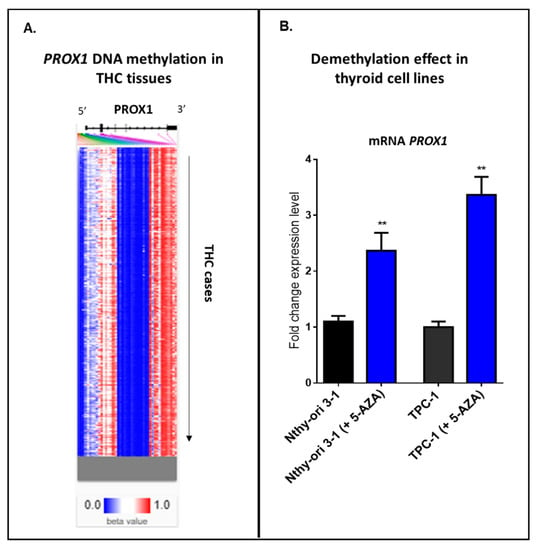
Figure 4.
Methylation in THC cases. A. Hierarchical clustering of THC tissues; blue indicates a low level or no methylation for PROX1; red indicates a high methylation level. The methylation analysis was studied using https://xenabrowser.net/heatmap/. B. Treatment of the immortalized normal thyroid cells (Nthy ori-3-1) and PTC-derived cell line (TPC-1) with 5 µM 5-aza-2’-deoxycytidine for 24 h, ** p ≤ 0.01.
Taken together, these data suggest that PROX1 expression could be regulated by DNA methylation status in thyroid cancer tissues and thyroid normal and cancer cells.
5.2. Non-Coding RNAs
Non-protein-coding RNAs (ncRNAs) have been associated with transcription/translation regulation and include microRNA (miRNA; approximate length 21–23 nucleotides) and non-protein-coding transcript (lncRNA; ≥ 200 nucleotides).
Several microRNAs have been shown to play critical roles in postnatal and pathologic angiogenesis [73] and pose attractive targets for the generation of novel therapeutic agents to treat vascular diseases and cancer [74].
It was demonstrated that the microRNA miR-181a is expressed in LEC cells and binds to the PROX1 3′-UTR, resulting in rapid and efficient transcript degradation and translational inhibition, which may have important implications for the control of PROX1 expression [68].
The miR-31 targets the 3’ UTR of PROX1 to suppress its expression in human LEC cells, and conversely, miR-31 overexpression led to defective lymphangiogenesis in Xenopus and Zebrafish embryos [75]. Next, using LEC cells and alkali burn corneal injury model, it was shown that miR-466 directly targets the 3’ UTR of PROX1, and similarly to other miRNAs, suppresses PROX1 expression resulting in inhibition of lymphangiogenesis [76].
According to the miRDB database (http://www.mirdb.org/index.html), for example, miR-10527-5p, miR-6867-5p, miR-4262, miR-4668-5p, and miR-3148 may still regulate PROX1 mRNA and thus can be the future research aim, especially in THC cases, due to the lack of published data.
The expression of PROX1 can be regulated by lncRNA (PROX1-AS1), which was also shown to be involved in THC biology [77]. According to research by Shen et al., 2018, PROX1-AS1 is expressed in PTC-derived cell lines and regulates their malignant behavior [77]. In detail, knockdown of PROX1-AS1 significantly inhibited proliferation, colony formation, migration, and invasion of PTC cells. Moreover, detected changes were associated with the mesenchymal-to-epithelial transition, where PROX1-AS1 downregulation lowered the expression of N-cadherin and Vimentin, while E-cadherin was enhanced [77].
Using the GEPIA database, we detected ~4× higher PROX1-AS1 expression in cancer samples (THC, n = 512) compared to healthy tissues (n = 337) (Figure 5A). Similarly to PROX1, the lower PROX1-AS1 expression is associated with a higher tendency to the longer survival time of THC patients (Figure 5B), and a higher expression level of PROX1-AS1 is observed in lower stages of the tumor (Figure 5C; [22]). The significant positive PROX1: PROX-AS1 correlation (0.75; p = 0) was observed for THC samples (Figure 5D) and healthy tissues (0.5; p = 0, Figure 5E).
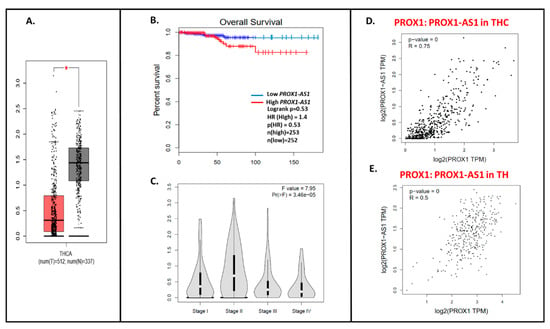
Figure 5.
The PROX1-AS1 expression pattern in thyroid cancer and correlation with PROX1. (A) Expression of PROX1-AS1 in thyroid cancer specimens (pictured on the red bar (T); the number of cases = 512) compared to non-cancerogenic thyroid tissues (pictured on the grey bar (N); the number of cases = 337), * p ≤ 0.05. (B) PROX1-AS1 relation to survival of patients with THC. (C) PROX1-AS1 expression compared to tumor stage I, II, III, and IV. (D,E) PROX1-AS1: PROX1 correlation in THC and healthy thyroid (TH) specimens, respectively.
6. The Role of the PROX1 in the Regulation of Cancer Biological Processes, Including Thyroid Cancer
PROX1 is involved in the stimulation of multiple intracellular signaling pathways regulating apoptosis, proliferation, lymph-/angiogenesis, and EMT of cancer cells. Thus, PROX1 in neuroblastomas and pancreas occurs in a subset of well-differentiated, high-grade tumors [78,79]. PROX1 overexpression enhanced the proliferation of glioblastoma cells and promoted the growth of glioblastoma xenograft tumors, and this invasiveness potential was regulated via activation of the NF-κB signaling pathway [79]. In esophageal squamous cell carcinoma cell lines, the PROX1 protein was expressed at a lower level compared with the healthy exocrine pancreas [10], and low expression was associated with poor patient survival. Contrary, in colorectal cancer, depletion of PROX1 in human hepatocellular carcinoma cell lines caused a significant increase in cell proliferation [80].
In colorectal cancer, PROX1 has been identified as a downstream target of the TCF/beta-catenin signaling pathway [30], and its lower expression in colon cancer cells was connected with estrogen receptor beta signaling [81]. While PROX1 does not appear to be responsible for the initiation of colon tumor cell growth, it promotes progression from a benign to a malignant phenotype [30,82]. Analysis of the PROX1 regulatory pathways showed that this phenotypical switch is most likely induced through alterations in cell polarity, extracellular matrix interactions, cell adhesion, and is associated with dysplasia and frequent mitotic figures [30].
A study of Kaposiform hemangioendothelioma revealed that overexpression of PROX1 facilitates a more aggressive behavior through induction of genes involved in cell adhesion, proteolysis, and migration, thereby enhancing cell invasion and migration into the surrounding tissue [17].
Overall, the examples mentioned above provide indirect evidence that PROX1 may regulate tumor progression by influencing cancer cell migration and invasion.
In the context of THC, PROX1 can be an essential regulator of secretory granules (SGs) formation in MTC. Its presence was observed in SGs in immunohistochemistry staining, and PROX1 gene depletion resulted in the reduced SG numbers and decreased expression of SG-related genes (Chromogranin A, Chromogranin B, Secretogranin II, Secretogranin III, Synaptophysin, and Carboxypeptidase E). Conversely, the introduction of a PROX1 transgene into a PTC and ATC cells induced the expression of SG-related transcripts [83].
The downregulation of PROX1 in PTC-derived cells was a consequence of aberrantly activated Notch signaling. Moreover, in PTC cells after transgenic PROX1 reexpression enhanced Wnt/β-catenin signaling was observed, coupled with and regulation of thyroid cancer 1 (TC-1) protein, Serpina 1, and Fatty acid-binding protein 4 (FABP4), that are known to be associated with PTC. Additionally, notch-induced PROX1 inactivation significantly promoted the malignant phenotype of thyroid cancer cells [15]. On the other hand, we observed that in FTC- and SCT-derived cells, PROX1 acts as an oncoprotein and supports malignant traits, including migration and invasion potential, anchorage-independent growth, that were accompanied by changes in focal adhesion force [20,21]. Furthermore, PROX1 knockdown increased the angiogenic potential of FTC- and SCT-derived cells by modulating the expression of genes involved in the angiogenic signaling pathway and was regulated in the opposite direction than pro-angiogenic factor FGF2 [22,84]. We can hypothesize that the discrepancy between the regulation of PTC and FTC and SCT by PROX1 may result from differences in the origin of cancer cells, mutations, as well as signaling pathways involved.
In our previous research, we analyzed the pattern of global gene expression in CGTH-W-1 cells lacking the PROX1 after transfection with RNA interference targeting of PROX1. We found that transcripts of many genes involved in migration, focal adhesion, invasion, cytoskeleton reorganization, and angiogenesis were regulated by PROX1 knockdown compared to control treated with negative siRNA. Studies examining biological processes have confirmed the involvement of selected factors as regulators of biological processes connected with cell–cell adhesion, cell migration, invasive behavior, and tube formation. Further, all molecular changes were rigorously confirmed in biological testing where cells after PROX1 knockdown revealed changed actin organization, showed the lower motility, increased invasive potential, and changed the tubularization of human umbilical endothelial cells cultivated in medium conditioned using CGTH-W-1 cells transfected with siPROX1.
Here, we provide a review of other biological processes (BPs) significantly changed by downregulated (Table 1) and upregulated (Table 2) genes in PROX1-silenced CGTH-W-1 cells, and 20 transcripts showing the strongest differential expression (Table 3). The altered BPs are strongly connected with the aspect of transcriptional and gene regulation, translation, and neuro-/morphogenesis control. Table 3 presents selected genes and their role in the physiological and tumorigenic context, including function in thyroid cancer development, if data are available. Among the listed genes: MMP1, VPS33A, CARNMT1, RASSF2, SOX2, MXRA5, SEPT3, LPAR1, FAM129A, FTH1, TUBA1 have already been connected with THC development, but the role of others remains unknown for THC biology. Therefore, the provided data can be a base for the perspective research focused on thyroid cancer and the PROX1.

Table 1.
Gene Ontology (biological process) analysis of up-regulated genes after Prospero homeobox 1 (PROX1) silencing in CGTH-W-1 cells.

Table 2.
Gene Ontology (biological process) analysis of down-regulated genes after PROX1 silencing in CGTH-W-1 cells.

Table 3.
List of 20 genes up- and downregulated in CGTH-W-1 cells upon PROX1-knockdown.
7. Relation of PROX1 with Other Lymphatic Factors
Although the importance of PROX1 in lymphangiogenesis is widely described, little is known about the mechanisms by which PROX1 expression is controlled in other cell types and how PROX1 affects other genes/proteins.
The road of lymphangiogenesis depends on VEGF-C and -D signaling pathways through VEGFR-2 and VEGFR-3, where especially VEGF-C and –D bind with VEGFR-3 and activate PROX1. In the cardinal vein, the PROX1-positive precursor cells differentiate into LEC cells. During asymmetrical division, a one daughter cell becomes lymphatic and progressively upregulates PROX1, and the other one downregulates PROX1 and stays in the vein [124]. In this process, VEGF-C controls the bipotential precursor division and mediates activation of VEGF-3, which next regulates PROX1 by establishing a feedback loop. Both VEGF-C and VEGFR-3 are required to PROX1-mediated cell fate reprogramming and to maintain the identity of LEC progenitors [125]. Additionally, the PROX1 activation of VEGFR-3 can be regulated by small ubiquitin-like modifier 1 (SUMO-1; sumoylation), which can reduce PROX1 transcriptional activity and consequently stops the lymphatic differentiation [126].
The PROX1/VEGF-C/VEGFR3 positive correlation was also detected in the analysis of human cervical neoplasia [127]. Furthermore, in vitro analysis demonstrated that PROX1 regulates cell growth, proliferation, invasion, and lymphangiogenesis by enhanced VEGFC expression in oral squamous cell carcinoma [128]. In thyroid cancer cell lines (FTC-133 and CGTH-W-1), PROX1 and VEGFC are expressed, and in CGTH-W-1 cells, VEGFC was oppositely regulated by PROX-1 knockdown, which enhanced the VEGFC expression level and its secretion [22].
On the other hand, PROX1 can be directly activated by the transcription factors SOX18 [129] and COUP-TFII [130,131], binding to the PROX1 promoter. Overexpression of SOX18 in blood endothelial cells induces them to express PROX1 and other lymphatic endothelial markers, while Sox18-null embryos show a complete blockade of LEC cells differentiation from the cardinal vein [129] and loss of PROX1 expression [132]. Furthermore, defects in the transcription factor SOX18 cause lymphatic dysfunction in the human syndrome hypotrichosis–lymphoedema–telangiectasia [133].
Throughout LECs development, COUP-TFII physically interacts with PROX1 to form a stable complex. As a result, COUP-TFII is a partner for PROX1 to control several genes, including VEGFR-3, FGFR-3, and neuropilin-1, required to LEC phenotype [134].
Similarly to PROX1, both SOX18 and COUP-TFII are associated with regulation in malignancy of various cancer types, such as gastric, breast, and lung cancers, and they are connected with vascularization of tumors [135,136,137,138].
PROX1 enables the reprogramming of vascular endothelial cells to become PDPN-expressing lymphatic endothelial cells [6,139,140]. PDPN—a mucin-type transmembrane protein—is a unique transmembrane glycoprotein receptor (38 to 50 kDa) with a heavily O-glycosylated amino-terminal extracellular domain. PDPN does not show enzymatic activity; to accomplish its biological functions, PDPN interacts with other proteins located in the same cell or neighbor cells [141]. Consequently, the binding of PDPN to its ligands leads to the modulation of signaling pathways that regulate proliferation, contractility, migration, EMT, and remodeling of the extracellular matrix [142]. In the PTC-derived cells, PDPN silencing reduces migration, invasion, and adhesion of tested cells through regulating the expression of the ezrin, radixin, and moesin proteins, MMP9 and MMP2 proteins [32,33].
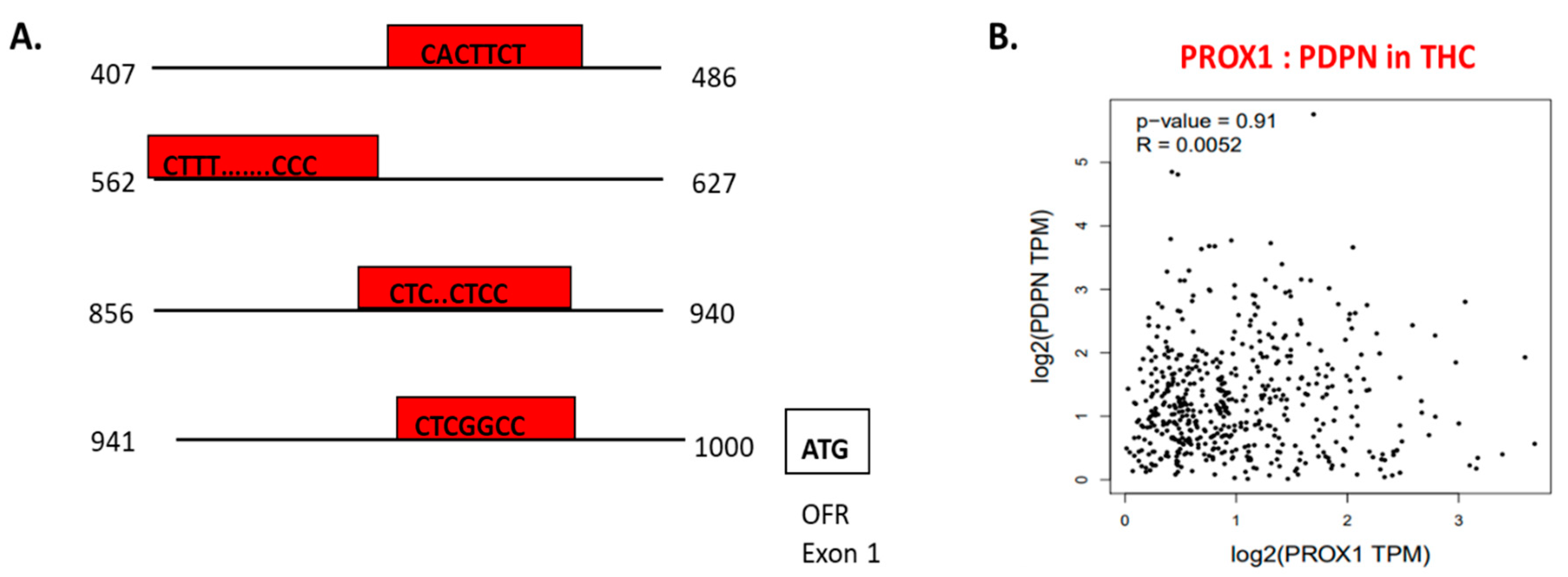
Using chromatin immunoprecipitation assay, performed on LEC cells, PROX1 binding to the 5′ regulatory sequence of PDPN regulating PDPN mRNA expression level was detected (Figure 6A) [105]. In the context of THC, PROX1 and PDPN expression is adjusted in opposite directions. PROX1 more highly expressed in FTC and SCT cells, whereas PDPN shows the upregulation in PTC cells [32]. Furthermore, in THC tissues, the positive co-expression of PROX1 and PDPN was on a slight level (= 0.0052) and statistically insignificant (Figure 6B).
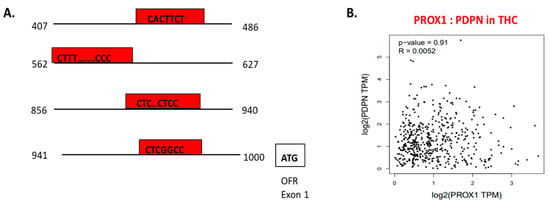
Figure 6.
The transcriptional function of PROX1 and interaction with other factors. A. PROX1 is a transcription factor of PDPN [143]; PROX1 binds to the 5’ regulatory sequence of PDPN and regulates PDPN expression. The graph presents a 5′ regulatory DNA region of the human PDPN gene and putative cis-elements (regions of non-coding DNA that binding to transcription factors; in red boxes) for PROX1. B. PROX1–PDPN relation in papillary thyroid cancer tissues. Data are available on https://www.grnpedia.org/trrust.
All the above observations can suggest that PROX1 relation with other vascular factors should be separately investigated in THC cases.
8. Conclusions and Perspectives
PROX1 shows a vital role in tumor progression and metastatic tumor growth through the impact on the aggressiveness of various cancer types, including thyroid cancer.
However, more profound knowledge regarding the molecular mechanisms, pathways, and targets of PROX1 in the various stages of thyroid tumor development remains to be obtained. Molecular regulation of PROX1 was deeply investigated in LEC cells, but it is unknown how PROX1 regulates and is regulated in other types of cells, especially under tumorigenic events.
Our work highlights the utility of PROX1 as a potential prognostic marker and adds biological insight to its role in different thyroid cancers, where it controls a gene expression profile involved in migration, invasion, adhesion, and vascularization. Still, further experiments are required to understand how the PROX1 epigenetic regulation and relation with other vascular molecules translate into a tumor setting and development. Considering the aggressive nature of THC and the minimal treatment window, there is an imperative need for novel molecular-based treatment strategies in which PROX1 can be an essential factor.
Author Contributions
Text, tables, and figures preparation, M.R.; supervision, B.C. All authors have read and agreed to the published version of the manuscript.
Funding
Work was supported by grants: 2013/11/N/NZ5/03394 (to MR) and 2012/07/B/ NZ5/02444 (to BC) from The National Science Centre, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| THC | Thyroid cancer |
| PROX1 | Prospero homeobox 1 |
| FTC | Follicular thyroid cancer |
| LEC | Lymphatic endothelial cell |
| PTC | Papillary thyroid cancer |
| SCT | Squamous cell carcinoma of the thyroid derived |
| DTC | Well-differentiated thyroid cancer |
| PDTC | Poorly differentiated thyroid cancer |
| ATC | Anaplastic thyroid cancer |
| MTC | Medullary thyroid cancer |
| ICAM1 | Intercellular adhesion molecule 1 |
| VEGFR-1 | Vascular endothelial growth factor receptor 1 |
| PDPN | Podoplanin |
| LYVE-1 | Lymphatic vessel endothelial hyaluronan receptor 1 |
| SOX18 | SRY-Box transcription factor 18 |
| COUP-TFII | Nuclear receptor subfamily 2 group F member 2 |
| EMT | Epithelial to mesenchymal transition |
| PI3K | 3-Phosphatidylinositol kinase PI kinase |
| NR | Nuclear receptor |
| NLS | Nuclear localization signal |
| FGF2 | Fibroblast growth factor 2 |
| ARE | AU-rich element |
| 3′-UTR | Three prime untranslated region |
| SNP | Nucleotide polymorphism |
| DNMT | DNA methyltransferase |
| ncRNA | Non-protein-coding RNA |
| SG | Secretory granule |
| TC-1 | Thyroid cancer 1 protein |
| FABP4 | Fatty acid-binding protein 4 |
| BP | Biological process |
| GO | Gene Ontology |
References
- Oliver, G.; Sosa-Pineda, B.; Geisendorf, S.; Spana, E.P.; Doe, C.Q.; Gruss, P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 1993, 44, 3–16. [Google Scholar] [CrossRef]
- Tomarev, S.I.; Sundin, O.; Banerjee-Basu, S.; Duncan, M.K.; Yang, J.M.; Piatigorsky, J. Chicken homeobox gene Prox 1 related to Drosophila prospero is expressed in the developing lens and retina. Dev. Dyn. 1996, 206, 354–367. [Google Scholar] [CrossRef]
- Sosa-Pineda, B.; Wigle, J.T.; Oliver, G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 2000, 25, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Risebro, C.A.; Searles, R.G.; Melville, A.A.D.; Ehler, E.; Jina, N.; Shah, S.; Pallas, J.; Hubank, M.; Dillard, M.; Harvey, N.L.; et al. Prox1 maintains muscle structure and growth in the developing heart. Development 2009, 136, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Kilic, G.; Aydin, M.; Burke, Z.; Oliver, G.; Sosa-Pineda, B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev. Biol. 2005, 286, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002, 225, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zinovieva, R.D.; Duncan, M.K.; Johnson, T.R.; Torres, R.; Polymeropoulos, M.H.; Tomarev, S.I. Structure and chromosomal localization of the human homeobox gene Prox 1. Genomics 1996, 35, 517–522. [Google Scholar] [CrossRef][Green Version]
- Shimoda, M.; Takahashi, M.; Yoshimoto, T.; Kono, T.; Ikai, I.; Kubo, H. A homeobox protein, Prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 6005–6011. [Google Scholar] [CrossRef]
- Akagami, M.; Kawada, K.; Kubo, H.; Kawada, M.; Takahashi, M.; Kaganoi, J.; Kato, S.; Itami, A.; Shimada, Y.; Watanabe, G.; et al. Transcriptional Factor Prox1 Plays an Essential Role in the Antiproliferative Action of Interferon-gamma in Esophageal Cancer Cells. Ann. Surg. Oncol. 2011, 18, 3868–3877. [Google Scholar] [CrossRef]
- Schneider, M.; Buchler, P.; Giese, N.; Giese, T.; Wilting, J.; Buchler, M.W.; Friess, H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int. J. Oncol. 2006, 28, 883–890. [Google Scholar] [CrossRef]
- Rodrigues, M.; Rodini, C.D.O.; Xavier, F.; Paiva, K.B.; Severino, P.; Moyses, R.A.; Lopez, R.M.; DeCicco, R.; Rocha, L.A.; Carvalho, M.B.; et al. PROX1 Gene is Differentially Expressed in Oral Cancer and Reduces Cellular Proliferation. Medicine 2014, 93. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Li, Y.H.; Hatano, S.; Toshihito, O.; Yuge, M.; Ito, E.; Utsumi, M.; Saito, H.; Kinoshita, T. Mutations and aberrant DNA methylation of the PROX1 gene in hematologic malignancies. Genes Chromosomes Cancer 2003, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Versmold, B.; Felsberg, J.; Mikeska, T.; Ehrentraut, D.; Kohler, J.; Hampl, J.A.; Rohn, G.; Niederacher, D.; Betz, B.; Hellmich, M.; et al. Epigenetic silencing of the candidate tumor suppressor gene PROX1 in sporadic breast cancer. Int. J.Cancer 2007, 121, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Laerm, A.; Helmbold, P.; Goldberg, M.; Dammann, R.; Holzhausen, H.J.; Ballhausen, W.G. Prospero-related homeobox 1 (PROX1) is frequently inactivated by genomic deletions and epigenetic silencing in carcinomas of the bilary system. J. Hepatol. 2007, 46, 89–97. [Google Scholar] [CrossRef]
- Choi, D.; Ramu, S.; Park, E.; Jung, E.; Yang, S.; Jung, W.; Choi, I.; Lee, S.; Kim, K.E.; Seong, Y.J.; et al. Aberrant Activation of Notch Signaling Inhibits PROX1 Activity to Enhance the Malignant Behavior of Thyroid Cancer Cells. Cancer Res. 2016, 76, 582–593. [Google Scholar] [CrossRef]
- Skog, M.; Bono, P.; Lundin, M.; Lundin, J.; Louhimo, J.; Linder, N.; Petrova, T.V.; Andersson, L.C.; Joensuu, H.; Alitalo, K.; et al. Expression and prognostic value of transcription factor PROX1 in colorectal cancer. Br. J. Cancer 2011, 105, 1346–1351. [Google Scholar] [CrossRef]
- Dadras, S.S.; Skrzypek, A.; Nguyen, L.; Shin, J.W.; Schulz, M.M.P.; Arbiser, J.; Mihm, M.C.; Detmar, M. Prox-1 Promotes Invasion of Kaposiform Hemangioendotheliomas. J. Investig. Dermatol. 2008, 128, 2798–2806. [Google Scholar] [CrossRef]
- Elsir, T.; Eriksson, A.; Orrego, A.; Lindstrom, M.S.; Nister, M. Expression of PROX1 Is a Common Feature of High-Grade Malignant Astrocytic Gliomas. J. Neuropathol. Exp. Neurol. 2010, 69, 129–138. [Google Scholar] [CrossRef]
- Elsir, T.; Qu, M.; Berntsson, S.G.; Orrego, A.; Olofsson, T.; Lindstrom, M.S.; Nister, M.; von Deimling, A.; Hartmann, C.; Ribom, D.; et al. PROX1 is a predictor of survival for gliomas WHO grade II. Br. J. Cancer 2011, 104, 1747–1754. [Google Scholar] [CrossRef]
- Rudzinska, M.; Ledwon, J.K.; Gawel, D.; Sikorska, J.; Czarnocka, B. The role of prospero homeobox 1 (PROX1) expression in follicular thyroid carcinoma cells. Oncotarget 2017, 8, 114136–114155. [Google Scholar] [CrossRef][Green Version]
- Rudzinska, M.; Grzanka, M.; Stachurska, A.; Mikula, M.; Paczkowska, K.; Stepien, T.; Paziewska, A.; Ostrowski, J.; Czarnocka, B. Molecular Signature of Prospero Homeobox 1 (PROX1) in Follicular Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2212. [Google Scholar] [CrossRef] [PubMed]
- Rudzinska, M.; Mikula, M.; Arczewska, K.D.; Gajda, E.; Sabalinska, S.; Stepien, T.; Ostrowski, J.; Czarnocka, B. Transcription Factor Prospero Homeobox 1 (PROX1) as a Potential Angiogenic Regulator of Follicular Thyroid Cancer Dissemination. Int. J. Mol. Sci. 2019, 20, 5619. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H.G. Current Thyroid Cancer Trends in the United States. Jama Otolaryngology-Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Basnet, A.; Pandita, A.; Fullmer, J.; Sivapiragasam, A. Squamous Cell Carcinoma of the Thyroid as a result of Anaplastic Transformation from BRAF-Positive Papillary Thyroid Cancer. Case Rep. Oncol. Med. 2017, 2017, 4276435. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.W. Lymph node and distant metastases of thyroid gland cancer. Metastases in the thyroid glands]. Pathologe 2015, 36, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Smit, J. Treatment of advanced medullary thyroid cancer. Thyroid Research 2013, 6, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paduch, R. The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell. Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef]
- Skobe, M.; Detmar, M. Structure, Function, and Molecular Control of the Skin Lymphatic System. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef]
- He, Y.; Kozaki, K.; Karpanen, T.; Koshikawa, K.; Yla-Herttuala, S.; Takahashi, T.; Alitalo, K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002, 94, 819–825. [Google Scholar] [CrossRef]
- Petrova, T.V.; Nykanen, A.; Norrmen, C.; Ivanov, K.I.; Andersson, L.C.; Haglund, C.; Puolakkainen, P.; Wempe, F.; von Melchner, H.; Gradwohl, G.; et al. Transcription factor PROM induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 2008, 13, 407–419. [Google Scholar] [CrossRef]
- Su, J.L.; Yang, P.C.; Shih, J.Y.; Yang, C.Y.; Wei, L.H.; Hsieh, C.Y.; Chou, C.H.; Jeng, Y.M.; Wang, M.Y.; Chang, K.J.; et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 2006, 9, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, M.; Gaweł, D.; Sikorska, J.; Karpińska, K.M.; Kiedrowski, M.; Stępień, T.; Marchlewska, M.; Czarnocka, B. The role of podoplanin in the biology of differentiated thyroid cancers. PLoS ONE 2014, 9, e96541. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, J.; Gawel, D.; Domek, H.; Rudzinska, M.; Czarnocka, B. Podoplanin (PDPN) affects the invasiveness of thyroid carcinoma cells by inducing ezrin, radixin and moesin (E/R/M) phosphorylation in association with matrix metalloproteinases. BMC Cancer 2019, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Deng, X.; Shuai, P.; Zeng, J. Upregulation of SOX18 in colorectal cancer cells promotes proliferation and correlates with colorectal cancer risk. Onco. Targets Ther. 2018, 11, 8481–8490. [Google Scholar] [CrossRef]
- Qin, J.; Tsai, S.Y.; Tsai, M.J. The critical roles of COUP-TFII in tumor progression and metastasis. Cell Biosci. 2014, 4. [Google Scholar] [CrossRef]
- Perri, F.; Lorenzo, G.D.; Scarpati, G.D.V.; Buonerba, C. Anaplastic thyroid carcinoma: A comprehensive review of current and future therapeutic options. World J. Clin. Oncol. 2011, 2, 150–157. [Google Scholar] [CrossRef]
- Thomas, C.; Asa, S.; Ezzat, S.; Sawka, A.; Goldstein, D. Diagnosis and pathologic characteristics of medullary thyroid carcinoma—review of current guidelines. Curr. Oncol. 2019, 26, 338–344. [Google Scholar] [CrossRef]
- Iñiguez-Ariza, N.M.; Brito, J.P. Management of low-risk papillary thyroid cancer. Endocrinol. Metab. 2018, 33, 185–194. [Google Scholar] [CrossRef]
- Andresen, N.S.; Buatti, J.M.; Tewfik, H.H.; Pagedar, N.A.; Anderson, C.M.; Watkins, J.M. Radioiodine ablation following thyroidectomy for differentiated thyroid cancer: Literature review of utility, dose, and toxicity. Eur. Thyroid. J. 2017, 6, 187–196. [Google Scholar] [CrossRef]
- Portela, R.A.; Choby, G.W.; Manni, A.; Campbell, D.; Crist, H.; Goldenberg, D. Unusual sites of metastasis of papillary thyroid cancer: Case series and review of the literature. ENT: Ear, Nose & Throat J. 2015, 94. [Google Scholar] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D. SEER Cancer Statistics Review, 1975-2016; National Cancer Institute: Bethesda, MD, USA, 2019; CRS section: 26.
- Al-Rawi, M.; Wheeler, M.H. Medullary thyroid carcinoma–update and present management controversies. Ann. Royal Coll. Surg. Engl. 2006, 88, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Lo, C.; Epstein, R.; Lam, A.; Wan, K.; Lang, B.H. Treatment outcomes in anaplastic thyroid carcinoma: Survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann. Surg. Oncol. 2008, 15, 2500. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ulisse, S.; Baldini, E.; Giannini, R.; Miccoli, P.; et al. Molecular testing in the diagnosis of differentiated thyroid carcinomas. Gland Surg. 2018, 7, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.Z. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Odolczyk, N.; Dremaux, J.; Toffin, J.; Regnier, A.; Sevestre, H.; Zielenkiewicz, P.; Arnault, J.P.; Gubler, B. The clinical response to vemurafenib in a patient with a rare BRAFV600DK601del mutation-positive melanoma. BMC Cancer 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.M.; Hodak, S.P.; Yip, L. RAS Mutations in Thyroid Cancer. Oncologist 2013, 18, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Omur, O.; Baran, Y. An update on molecular biology of thyroid cancers. Crit. Rev. Oncol. Hematol. 2014, 90, 233–252. [Google Scholar] [CrossRef]
- Henderson, Y.C.; Shellenberger, T.D.; Williams, M.D.; El-Naggar, A.K.; Fredrick, M.J.; Cieply, K.M.; Clayman, G.L. High Rate of BRAF and RET/PTC Dual Mutations Associated with Recurrent Papillary Thyroid Carcinoma. Clin. Cancer Res. 2009, 15, 485–491. [Google Scholar] [CrossRef]
- Hou, P.; Liu, D.X.; Shan, Y.; Hu, S.Y.; Studeman, K.; Condouris, S.; Wang, Y.G.; Trink, A.; El-Naggar, A.K.; Tallini, G.; et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin. Cancer Res. 2007, 13, 1161–1170. [Google Scholar] [CrossRef]
- Xing, M. Clinical utility of RAS mutations in thyroid cancer: A blurred picture now emerging clearer. BMC Med. 2016, 14. [Google Scholar] [CrossRef]
- Fukahori, M.; Yoshida, A.; Hayashi, H.; Yoshihara, M.; Matsukuma, S.; Sakuma, Y.; Koizume, S.; Okamoto, N.; Kondo, T.; Masuda, M. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid 2012, 22, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Ganapathi, S.; Comeras, I.; Peterson, C.; Orloff, M.; Porter, K.; Eng, C.; Ringel, M.D.; Kloos, R.T. Frequency of Germline PTEN Mutations in Differentiated Thyroid Cancer. Thyroid 2011, 21, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Genetic Alterations in the Phosphatidylinositol-3 Kinase/Akt Pathway in Thyroid Cancer. Thyroid 2010, 20, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.; Messina, M.; Gill, A.; Clarkson, A.; Learoyd, D.; Delbridge, L.; Wentworth, J.; Philips, J.; Clifton-Bligh, R.; Robinson, B.G. Detection of the PAX8-PPAR gamma fusion oncogene in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 2003, 88, 354–357. [Google Scholar] [CrossRef]
- Fagin, J.A.; Mitsiades, N. Molecular pathology of thyroid cancer: Diagnostic and clinical implications. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 955–969. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hedayati, M. A Brief Review on The Molecular Basis of Medullary Thyroid Carcinoma. Cell J 2017, 18, 485–492. [Google Scholar] [CrossRef]
- Oczko-Wojciechowska, M.; Pfeifer, A.; Rusinek, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Kowal, M.; Swierniak, M.; Krajewska, J.; Gawlik, T.; et al. The prevalence of somatic RAS mutations in medullary thyroid cancer - a Polish population study. Endokrynol. Pol. 2015, 66, 121–125. [Google Scholar] [CrossRef]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef]
- Shen, X.; Liu, R.; Xing, M. A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr. Relat. Cancer 2017, 24, 41–52. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2019, 67, 44–57. [Google Scholar] [CrossRef]
- Lee, J.H. Analysis of the cancer genome atlas data to determine the correlation between PROX1 and TERT in hepatocellular carcinoma. Int. J. Cancer. 2019, 144, 1752–1753. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.; Mansuroglu, T.; Moriconi, F.; Haller, F.; Wilting, J.; Lorf, T.; Füzesi, L.; Ramadori, G. Altered regulation of Prox1-gene-expression in liver tumors. BMC Cancer 2008, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Banerjee-Basu, S.; Landsman, D.; Baxevanis, A.D. Threading analysis of prospero-type homeodomains. Silico. Biol. 1999, 1, 163–173. [Google Scholar] [PubMed]
- Audette, D.S.; Anand, D.; So, T.; Rubenstein, T.B.; Lachke, S.A.; Lovicu, F.J.; Duncan, M.K. Prox1 and fibroblast growth factor receptors form a novel regulatory loop controlling lens fiber differentiation and gene expression. Development 2016, 143, 318–328. [Google Scholar] [CrossRef]
- Yoo, J.; Kang, J.; Lee, H.N.; Aguilar, B.; Kafka, D.; Lee, S.; Choi, I.; Lee, J.; Ramu, S.; Haas, J.; et al. Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi’s Sarcoma Herpes Virus. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Takahashi, M.; Yoshimoto, T.; Shimoda, M.; Kono, T.; Koizumi, M.; Yazumi, S.; Shimada, Y.; Doi, R.; Chiba, T.; Kubo, H. Loss of function of the candidate tumor suppressor prox1 by RNA mutation in human cancer cells. Neoplasia 2006, 8, 1003–1010. [Google Scholar] [CrossRef]
- Kazenwadel, J.; Michael, M.Z.; Harvey, N.L. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 2010, 116, 2395–2401. [Google Scholar] [CrossRef]
- Wang, Q.; Khillan, J.; Gadue, P.; Nishikura, K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science 2000, 290, 1765–1768. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Takahashi, M.; Nagayama, S.; Watanabe, G.; Shimada, Y.; Sakasi, Y.; Kubo, H. RNA mutations of prox1 detected in human esophageal cancer cells by the shifted termination assay. Biochem. Biophys. Res. Commun. 2007, 359, 258–262. [Google Scholar] [CrossRef]
- Lecompte, S.; Pasquetti, G.; Hermant, X.; Grenier-Boley, B.; Gonzalez-Gross, M.; De Henauw, S.; Molnar, D.; Stehle, P.; Béghin, L.; Moreno, L.A. Genetic and molecular insights into the role of PROX1 in glucose metabolism. Diabetes 2013, 62, 1738–1745. [Google Scholar] [CrossRef]
- Rodrigues, M.F.; Esteves, C.M.; Xavier, F.C.; Nunes, F.D. Methylation status of homeobox genes in common human cancers. Genomics 2016, 108, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Suárez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Pedrioli, D.M.; Karpanen, T.; Dabouras, V.; Jurisic, G.; van de Hoek, G.; Shin, J.W.; Marino, D.; Kalin, R.E.; Leidel, S.; Cinelli, P.; et al. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol. Cell Biol. 2010, 30, 3620–3634. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Choi, J.S.; Rho, C.R.; Joo, C.K.; Lee, S.K. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J. Biomed. Sci. 2015, 22. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xia, E.; Bhandari, A.; Wang, X.; Guo, G. LncRNA PROX1-AS1 promotes proliferation, invasion, and migration in papillary thyroid carcinoma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Saukkonen, K.; Hagström, J.; Mustonen, H.; Juuti, A.; Nordling, S.; Kallio, P.; Alitalo, K.; Seppänen, H.; Haglund, C. PROX1 and β-catenin are prognostic markers in pancreatic ductal adenocarcinoma. BMC Cancer 2016, 16, 472. [Google Scholar] [CrossRef]
- Xu, X.; Wan, X.; Wei, X. PROX1 promotes human glioblastoma cell proliferation and invasion via activation of the nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 2017, 15, 963–968. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, X.; Zhang, J.; Ouyang, H.; Shen, Z.; Wu, Y.; Wang, W.; Wu, J.; Tao, S.; Yang, X. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing β-catenin expression and nuclear translocation. Oncogene 2015, 34, 5524–5535. [Google Scholar] [CrossRef]
- Edvardsson, K.; Strom, A.; Jonsson, P.; Gustafsson, J.A.; Williams, C. Estrogen receptor beta induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol. Endocrinol. 2011, 25, 969–979. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Yazawa, T.; Chiba, T.; Shishido-Hara, Y.; Arimasu, Y.; Sato, H.; Kamma, H. PROX1 Promotes Secretory Granule Formation in Medullary Thyroid Cancer Cells. Endocrinology 2016, 157, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Dillard, M.E.; Baluk, P.; McDonald, D.M.; Harvey, N.L.; Frase, S.L.; Oliver, G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008, 22, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, N.; Kähäri, V. Matrix Metalloproteinases in Cancer Cell Invasion. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Patel, A.; Straight, A.M.; Mann, H.; Duffy, E.; Fenton, C.; Dinauer, C.; Tuttle, R.M.; Francis, G.L. Matrix metalloproteinase (MMP) expression by differentiated thyroid carcinoma of children and adolescents. J. Endocrinol. Invest. 2002, 25, 403–408. [Google Scholar] [CrossRef]
- Buergy, D.; Weber, T.; Maurer, G.D.; Mudduluru, G.; Medved, F.; Leupold, J.H.; Brauckhoff, M.; Post, S.; Dralle, H.; Allgayer, H. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int. J. Cancer 2009, 125, 894–901. [Google Scholar] [CrossRef]
- Kameyama, K. Expression of MMP-1 in the capsule of thyroid cancer--relationship with invasiveness. Pathol Res Pract 1996, 192, 20–26. [Google Scholar] [CrossRef]
- Somerville, T.D.D.; Xu, Y.; Wu, X.S.; Vakoc, C.R. ZBED2 is an antagonist of Interferon Regulatory Factor 1 and modulates cell identity in pancreatic cancer. BioRxiv 2019. [Google Scholar] [CrossRef]
- Nikolova, D.N.; Zembutsu, H.; Sechanov, T.; Vidinov, K.; Kee, L.S.; Ivanova, R.; Becheva, E.; Kocova, M.; Toncheva, D.; Nakamura, Y. Genome-wide gene expression profiles of thyroid carcinoma: Identification of molecular targets for treatment of thyroid carcinoma. Oncol. Rep. 2008, 20, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.C.; Wartosch, L.; Gray, S.R.; Scourfield, E.J.; Deane, J.E.; Luzio, J.P.; Owen, D.J. Structural basis of Vps33A recruitment to the human HOPS complex by Vps16. Proc. Natl. Acad. Sci. USA 2013, 110, 13345–13350. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 6352. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, X.; Guan, M.X.; Yan, Q. Pathogenic mutations of nuclear genes associated with mitochondrial disorders. Acta. Biochim. Biophys. Sin. 2009, 41, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Suhane, S.; Berel, D.; Ramanujan, V.K. Biomarker signatures of mitochondrial NDUFS3 in invasive breast carcinoma. Biochem. Biophys. Res. Commun. 2011, 412, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Namavar, Y.; Eggens, V.R.; Barth, P.G.; Baas, F. TSEN54-Related Pontocerebellar Hypoplasia. In GeneReviews; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- Blomen, V.A.; Májek, P.; Jae, L.T.; Bigenzahn, J.W.; Nieuwenhuis, J.; Staring, J.; Sacco, R.; van Diemen, F.R.; Olk, N.; Stukalov, A. Gene essentiality and synthetic lethality in haploid human cells. Science 2015, 350, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cui, D.; Ren, J.; Wang, K.; Zeng, T.; Gao, L. CACNA2D3 is downregulated in gliomas and functions as a tumor suppressor. Mol. Carcinog. 2017, 56, 945–959. [Google Scholar] [CrossRef]
- Kong, X.; Li, M.; Shao, K.; Yang, Y.; Wang, Q. Progesterone induces cell apoptosis via the CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol. Rep. 2020, 43, 121–132. [Google Scholar] [CrossRef]
- Williams, K.A.; Lee, M.; Winter, J.M.; Gildea, D.E.; Calagua, C.; Curry, N.L.; Lichtenberg, J.; Ye, H.; Crawford, N.P. Prostate cancer susceptibility gene HIST1H1A is a modulator of androgen receptor signaling and epithelial to mesenchymal transition. Oncotarget 2018, 9, 28532–28546. [Google Scholar] [CrossRef][Green Version]
- Lu, X.M.; Long, H. Nicotinamide N-methyltransferase as a potential marker for cancer. Neoplasma 2018, 65, 656–663. [Google Scholar] [CrossRef]
- Xu, J.; Moatamed, F.; Caldwell, J.S.; Walker, J.R.; Kraiem, Z.; Taki, K.; Brent, G.A.; Hershman, J.M. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2003, 88, 4990–4996. [Google Scholar] [CrossRef][Green Version]
- Ban, M.J.; Ji, S.H.; Lee, C.K.; Bae, S.B.; Kim, H.J.; Ahn, T.S.; Lee, M.S.; Baek, M.J.; Jeong, D. Solute carrier organic anion transporter family member 4A1 (SLCO4A1) as a prognosis marker of colorectal cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1437–1447. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, R.; Qiu, Y.; Lv, Y.; Zhang, J.; Qin, G.; Yuan, C.; Liu, Z.; Li, Y.; Zou, D.; et al. Expression and gene regulation network of RBM8A in hepatocellular carcinoma based on data mining. Aging 2019, 11, 423–447. [Google Scholar] [CrossRef]
- Schagdarsurengin, U.; Richter, A.M.; Hornung, J.; Lange, C.; Steinmann, K.; Dammann, R.H. Frequent epigenetic inactivation of RASSF2 in thyroid cancer and functional consequences. Mol. Cancer 2010, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Huser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gauchotte, G.; Philippe, C.; Lacomme, S.; Leotard, B.; Wissler, M.P.; Allou, L.; Toussaint, B.; Klein, M.; Vignaud, J.M.; Bressenot, A. BRAF, p53 and SOX2 in anaplastic thyroid carcinoma: Evidence for multistep carcinogenesis. Pathology 2011, 43, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Sanz, A.B.; Fernandez-Fernandez, B.; Carrasco, S.; Ruiz-Ortega, M.; Cannata-Ortiz, P.; Ortiz, A.; Sanchez-Nino, M.D. MXRA5 is a TGF-beta1-regulated human protein with anti-inflammatory and anti-fibrotic properties. J. Cell Mol. Med. 2017, 21, 154–164. [Google Scholar] [CrossRef]
- Chakladar, J.; Li, W.T.; Bouvet, M.; Chang, E.Y.; Wang-Rodriguez, J.; Ongkeko, W.M. Papillary Thyroid Carcinoma Variants are Characterized by Co-dysregulation of Immune and Cancer Associated Genes. Cancers 2019, 11, 1179. [Google Scholar] [CrossRef]
- Wang, X.; Fei, F.; Qu, J.; Li, C.; Li, Y.; Zhang, S. The role of septin 7 in physiology and pathological disease: A systematic review of current status. J. Cell Mol. Med. 2018, 22, 3298–3307. [Google Scholar] [CrossRef]
- Ward, Y.; Lake, R.; Martin, P.L.; Killian, K.; Salerno, P.; Wang, T.; Meltzer, P.; Merino, M.; Cheng, S.-y.; Santoro, M.; et al. CD97 amplifies LPA receptor signaling and promotes thyroid cancer progression in a mouse model. Oncogene 2012, 32, 2726–2738. [Google Scholar] [CrossRef]
- Adachi, H.; Majima, S.; Kon, S.; Kobayashi, T.; Kajino, K.; Mitani, H.; Hirayama, Y.; Shiina, H.; Igawa, M.; Hino, O. Niban gene is commonly expressed in the renal tumors: A new candidate marker for renal carcinogenesis. Oncogene 2004, 23, 3495–3500. [Google Scholar] [CrossRef]
- Nozima, B.H.; Mendes, T.B.; Pereira, G.; Araldi, R.P.; Iwamura, E.S.M.; Smaili, S.S.; Carvalheira, G.M.G.; Cerutti, J.M. FAM129A regulates autophagy in thyroid carcinomas in an oncogene-dependent manner. Endocr. Relat. Cancer 2019, 26, 227–238. [Google Scholar] [CrossRef]
- Sigstad, E.; Paus, E.; Bjøro, T.; Berner, A.; Grøholt, K.K.; Jørgensen, L.H.; Sobrinho-Simões, M.; Holm, R.; Warren, D.J. The new molecular markers DDIT3, STT3A, ARG2 and FAM129A are not useful in diagnosing thyroid follicular tumors. Mod. Pathol. 2012, 25, 537–547. [Google Scholar] [CrossRef]
- Cerutti, J.M.; Oler, G.; Delcelo, R.; Gerardt, R.; Michaluart, P.; de Souza, S.J.; Galante, P.A.; Huang, P.; Riggins, G.J. PVALB, a New Hürthle Adenoma Diagnostic Marker Identified through Gene Expression. J. Clin. Endocrinol. Metab. 2020, 96. [Google Scholar] [CrossRef]
- Lobello, N.; Biamonte, F.; Pisanu, M.E.; Faniello, M.C.; Jakopin, Ž.; Chiarella, E.; Giovannone, E.D.; Mancini, R.; Ciliberto, G.; Cuda, G.; et al. Ferritin heavy chain is a negative regulator of ovarian cancer stem cell expansion and epithelial to mesenchymal transition. Oncotarget 2016, 7, 62019–62033. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.J.; Kwok, Z.H.; Chew, X.H.; Zhang, B.; Liu, C.; Soong, T.W.; Yang, H.; Tay, Y. A FTH1 gene:pseudogene:microRNA network regulates tumorigenesis in prostate cancer. Nucleic. Acids. Res. 2018, 46, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Fontaine, J.-F.; Franc, B.; Mirebeau-Prunier, D.; Triau, S.; Savagner, F.; Malthiery, Y. Death-associated protein 3 is overexpressed in human thyroid oncocytic tumours. Br. J. Cancer 2009, 101, 132–138. [Google Scholar] [CrossRef]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. α-tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion and invasive migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef]
- Takano, T.; Hasegawa, Y.; Miyauchi, A.; Matsuzuka, F.; Yoshida, H.; Kuma, K.; Amino, N. Overexpression of kalpha1 tubulin mRNA in thyroid anaplastic carcinoma. Cancer Lett. 2001, 168, 51–55. [Google Scholar] [CrossRef]
- Sofiadis, A.; Becker, S.; Hellman, U.; Hultin-Rosenberg, L.; Dinets, A.; Hulchiy, M.; Zedenius, J.; Wallin, G.; Foukakis, T.; Höög, A.; et al. Proteomic profiling of follicular and papillary thyroid tumors. Eur. J. Endocrinol. 2012, 166, 657–667. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pang, S.W.; Tan, K.O. PNMA2 mediates heterodimeric interactions and antagonizes chemo-sensitizing activities mediated by members of PNMA family. Biochem. Biophys. Res. Commun. 2016, 473, 224–229. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, D.; Hu, B.; Wang, J.; Shen, X.; Xiao, W. FBXO32 Targets c-Myc for Proteasomal Degradation and Inhibits c-Myc Activity. J. Biol. Chem. 2015, 290, 16202–16214. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Zhu, R.; Ding, F.; Wan, Y.; Li, Y.; Liu, Z. FBXO32 suppresses breast cancer tumorigenesis through targeting KLF4 to proteasomal degradation. Oncogene 2017, 36, 3312–3321. [Google Scholar] [CrossRef]
- Wigle, J.T.; Harvey, N.; Detmar, M.; Lagutina, I.; Grosveld, G.; Gunn, M.D.; Jackson, D.G.; Oliver, G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002, 21, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Escobedo, N.; Yang, Y.; Interiano, A.; Dillard, M.E.; Finkelstein, D.; Mukatira, S.; Gil, H.J.; Nurmi, H.; Alitalo, K.; et al. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-R.; Chang, T.-M.; Chang, H.-C.; Su, J.-L.; Wang, H.-W.; Hung, W.-C. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J. Cell Sci. 2009, 122, 3358–3364. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Mazuru, V.; Saptefrati, L.; Ceausu, R.; Raica, M. Prox 1, VEGF-C and VEGFR3 expression during cervical neoplasia progression as evidence of an early lymphangiogenic switch. Histol. Histopathol. 2012, 27, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Ueda, N.; Yamamoto, K.; Kurihara, M.; Matsushima, S.; Bhawal, U.K.; Kirita, T.; Kuniyasu, H. Prox1 and FOXC2 Act as Regulators of Lymphangiogenesis and Angiogenesis in Oral Squamous Cell Carcinoma. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Francois, M.; Caprini, A.; Hosking, B.; Orsenigo, F.; Wilhelm, D.; Browne, C.; Paavonen, K.; Karnezis, T.; Shayan, R.; Downes, M.; et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008, 456, 643–647. [Google Scholar] [CrossRef]
- Srinivasan, R.S.; Geng, X.; Yang, Y.; Wang, Y.D.; Mukatira, S.; Studer, M.; Porto, M.P.R.; Lagutin, O.; Oliver, G. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 2010, 24, 696–707. [Google Scholar] [CrossRef]
- Aranguren, X.L.; Beerens, M.; Coppiello, G.; Wiese, C.; Vandersmissen, I.; Lo Nigro, A.; Verfaillie, C.M.; Gessler, M.; Luttun, A. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or hetero-dimerisation with PROX1. J. Cell Sci. 2013, 126, 1164–1175. [Google Scholar] [CrossRef]
- Watabe, T. Roles of transcriptional network during the formation of lymphatic vessels. J. Biochem. 2018, 152, 213–220. [Google Scholar] [CrossRef]
- Irrthum, A.; Devriendt, K.; Chitayat, D.; Matthijs, G.; Glade, C.; Steijlen, P.M.; Fryns, J.P.; Van Steensel, M.A.; Vikkula, M. Mutations in the Transcription Factor Gene SOX18 Underlie Recessive and Dominant Forms of Hypotrichosis-Lymphedema-Telangiectasia. Am. J. Hum. Genet. 2003, 72, 1470–1478. [Google Scholar] [CrossRef]
- Lee, S.; Kang, J.; Yoo, J.; Ganesan, S.K.; Cook, S.C.; Aguilar, B.; Ramu, S.; Lee, J.; Hong, Y.K. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 2009, 113, 1856–1859. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, L.M.; Klinge, C.M. Multiple roles of COUP-TFII in cancer initiation and progression. J. Mol. Endocrinol. 2012, 49, R135–R148. [Google Scholar] [CrossRef] [PubMed]
- Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Role of the SOX18 protein in neoplastic processes. Oncol. Lett. 2018, 16, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Overman, J.; Fontaine, F.; Wylie-Sears, J.; Moustaqil, M.; Huang, L.; Meurer, M.; Chiang, I.K.; Lesieur, E.; Patel, J.; Zuegg, J.; et al. R-propranolol is a small molecule inhibitor of the SOX18 transcription factor in a rare vascular syndrome and hemangioma. Elife 2019, 6, 8. [Google Scholar] [CrossRef]
- Kim, I.; Koh, G.Y. Tumor Angiogenesis: Taking aim at Sox18. Elife 2017, 6. [Google Scholar] [CrossRef]
- Petrova, T.V.; Makinen, T.; Makela, T.P.; Saarela, J.; Virtanen, I.; Ferrell, R.E.; Finegold, D.N.; Kerjaschki, D.; Yla-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo. J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef]
- Hirakawa, S.; Hong, Y.K.; Harvey, N.; Schacht, V.; Matsuda, K.; Libermann, T.; Detmar, M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 2003, 162, 575–586. [Google Scholar] [CrossRef]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.W.; Yoneda, K.; Asai, J. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef]
- Raica, M.; Cimpean, A.M.; Ribatti, D. The role of podoplanin in tumor progression and metastasis. Anticancer. Res. 2008, 28, 2997–3006. [Google Scholar] [PubMed]
- Pan, Y.F.; Wang, W.D.; Yago, T. Transcriptional regulation of podoplanin expression by Prox1 in lymphatic endothelial cells. Microvasc. Res. 2014, 94, 96–102. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).