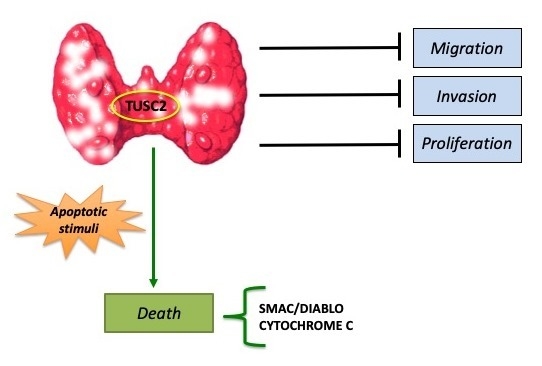
The TUSC2 Tumour Suppressor Inhibits the Malignant Phenotype of Human Thyroid Cancer Cells via SMAC/DIABLO Protein
Abstract
1. Introduction
2. Results
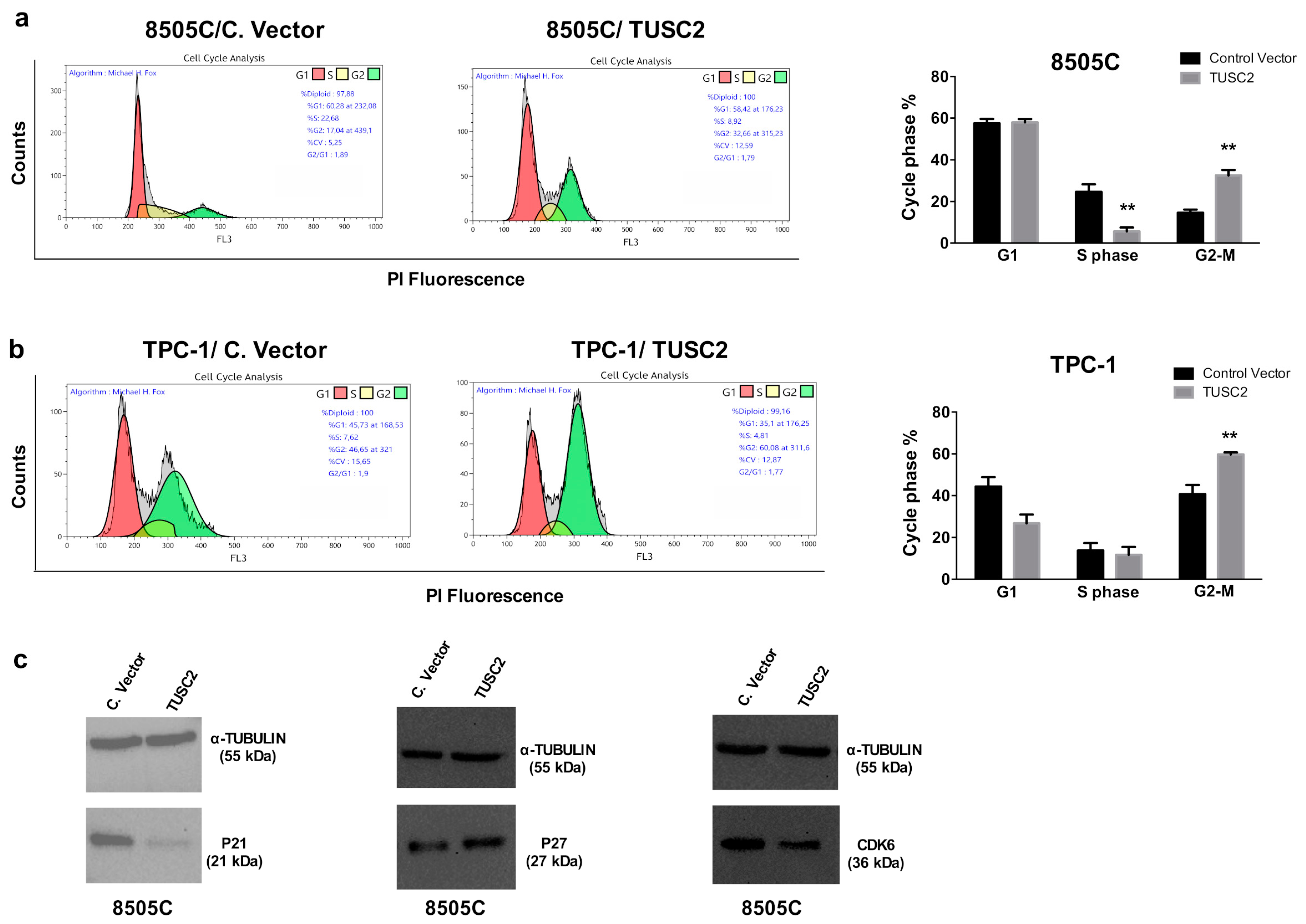
2.1. TUSC2 Forced Expression Decreased Cell Proliferation in Thyroid Cancer Cells
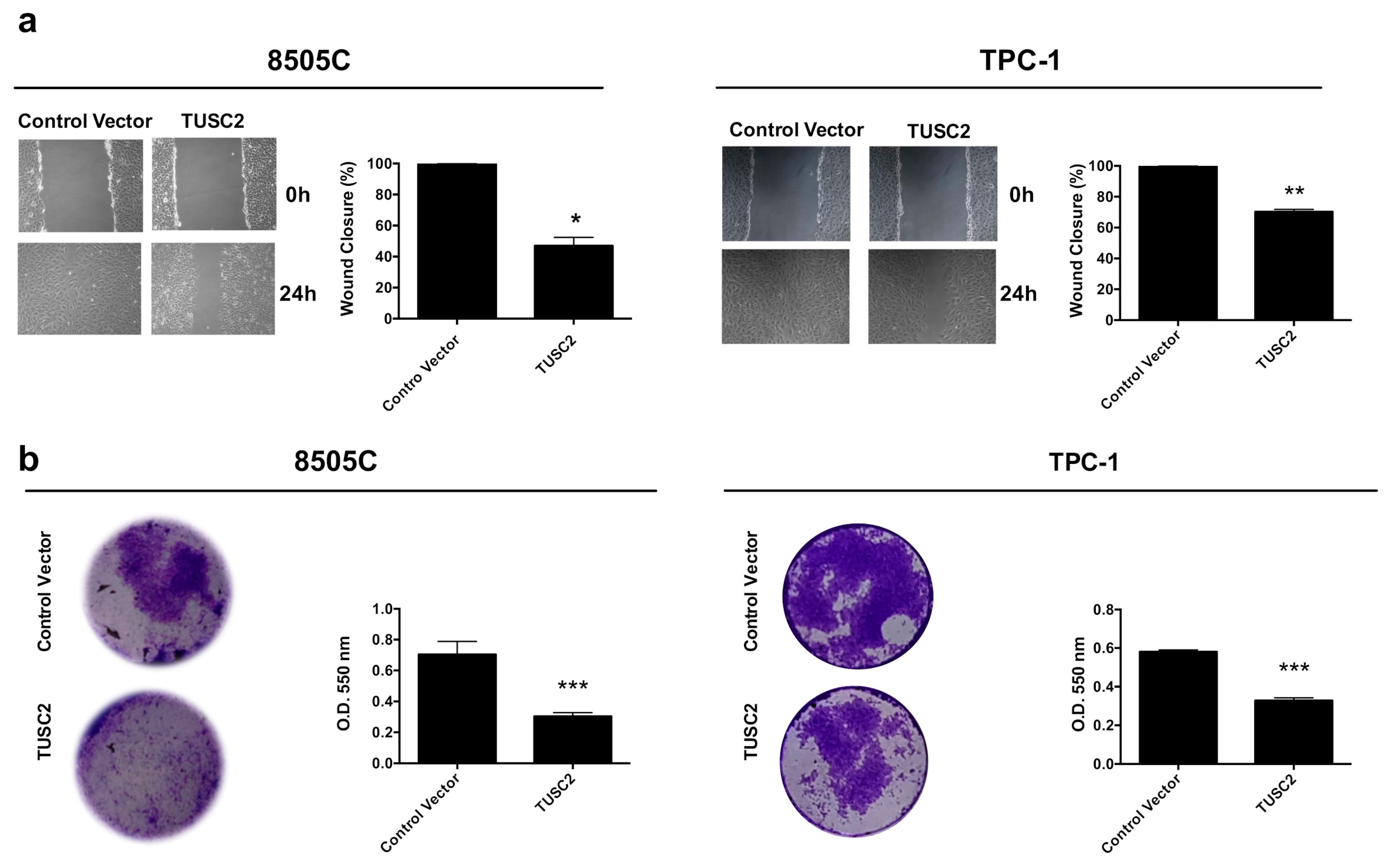
2.2. TUSC2 Forced Expression Decreased the Migration and Invasion of Thyroid Cancer Cells
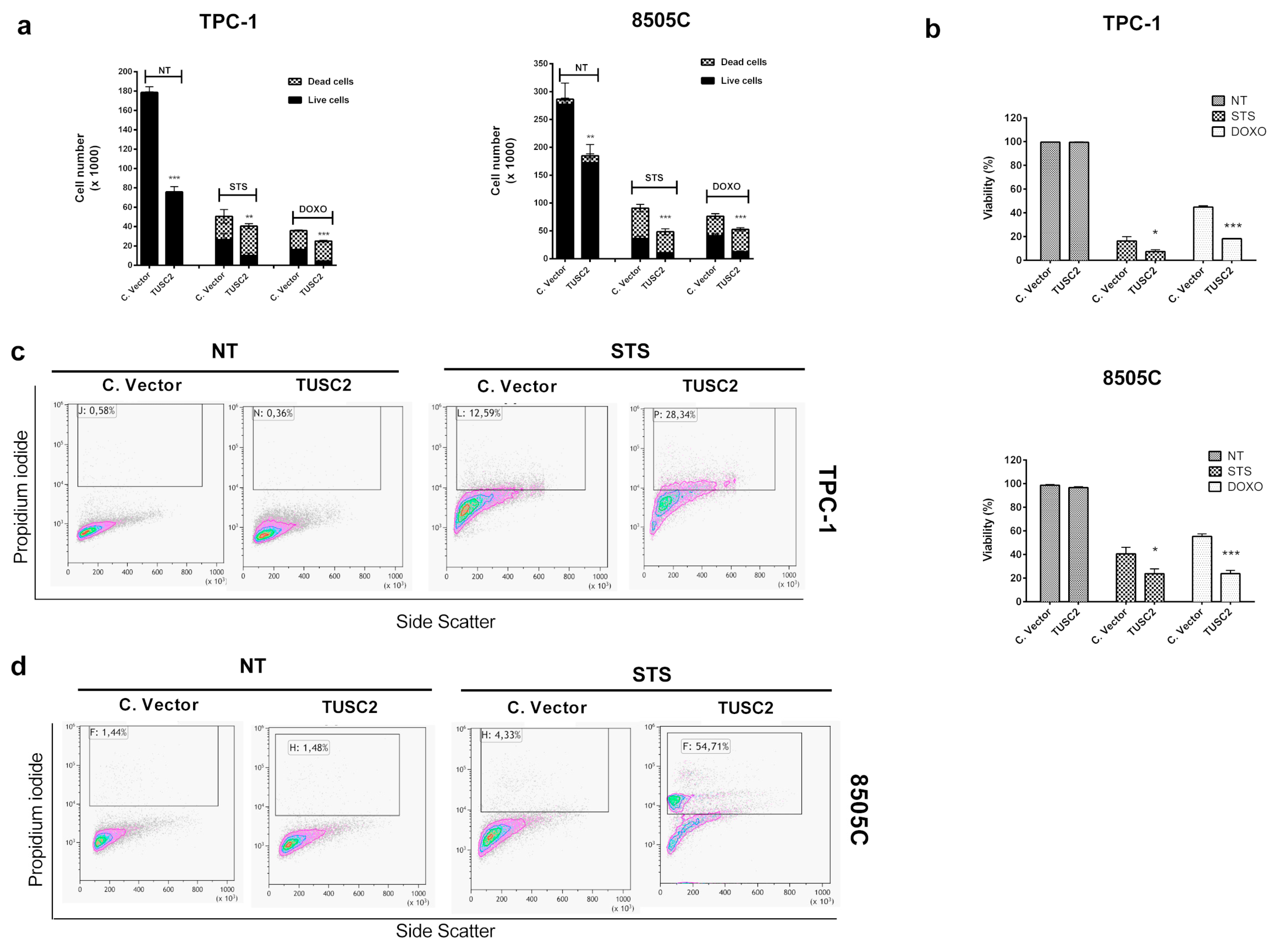
2.3. TUSC2 Forced Expression Increased Sensitivity to Apoptosis Induced by Doxorubicin and Staurosporine in Thyroid Cancer Cells
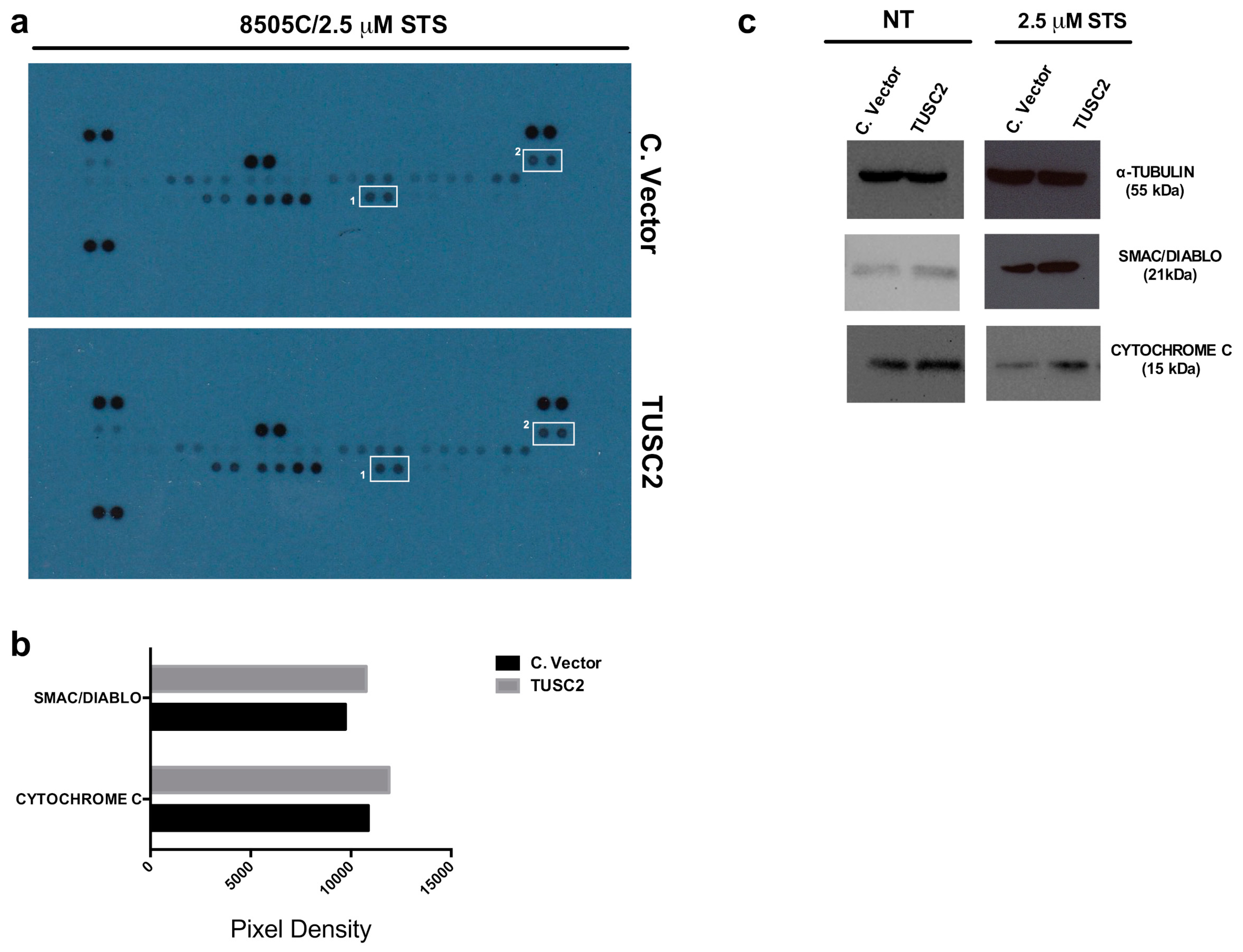
2.4. TUSC2 Increased SMAC/DIABLO and CYTOCHROME C Protein Expression in Response to Apoptotic Stimuli in Thyroid Cancer Cells
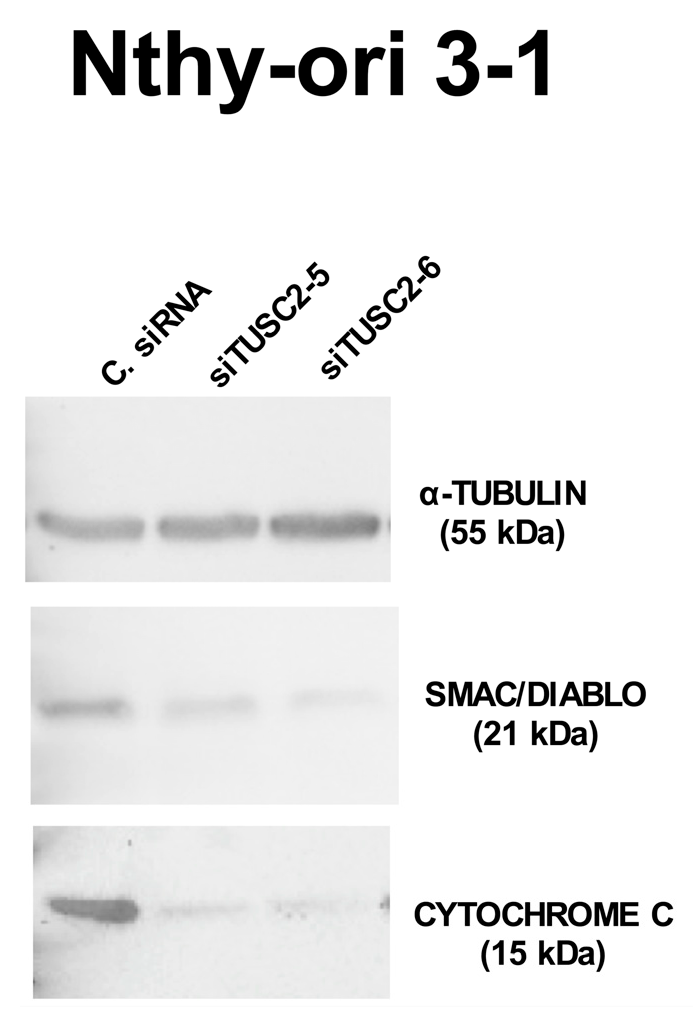
2.5. TUSC2 Silencing Increased the Malignant Phenotype of the Nthy-ori 3-1 Cell Line
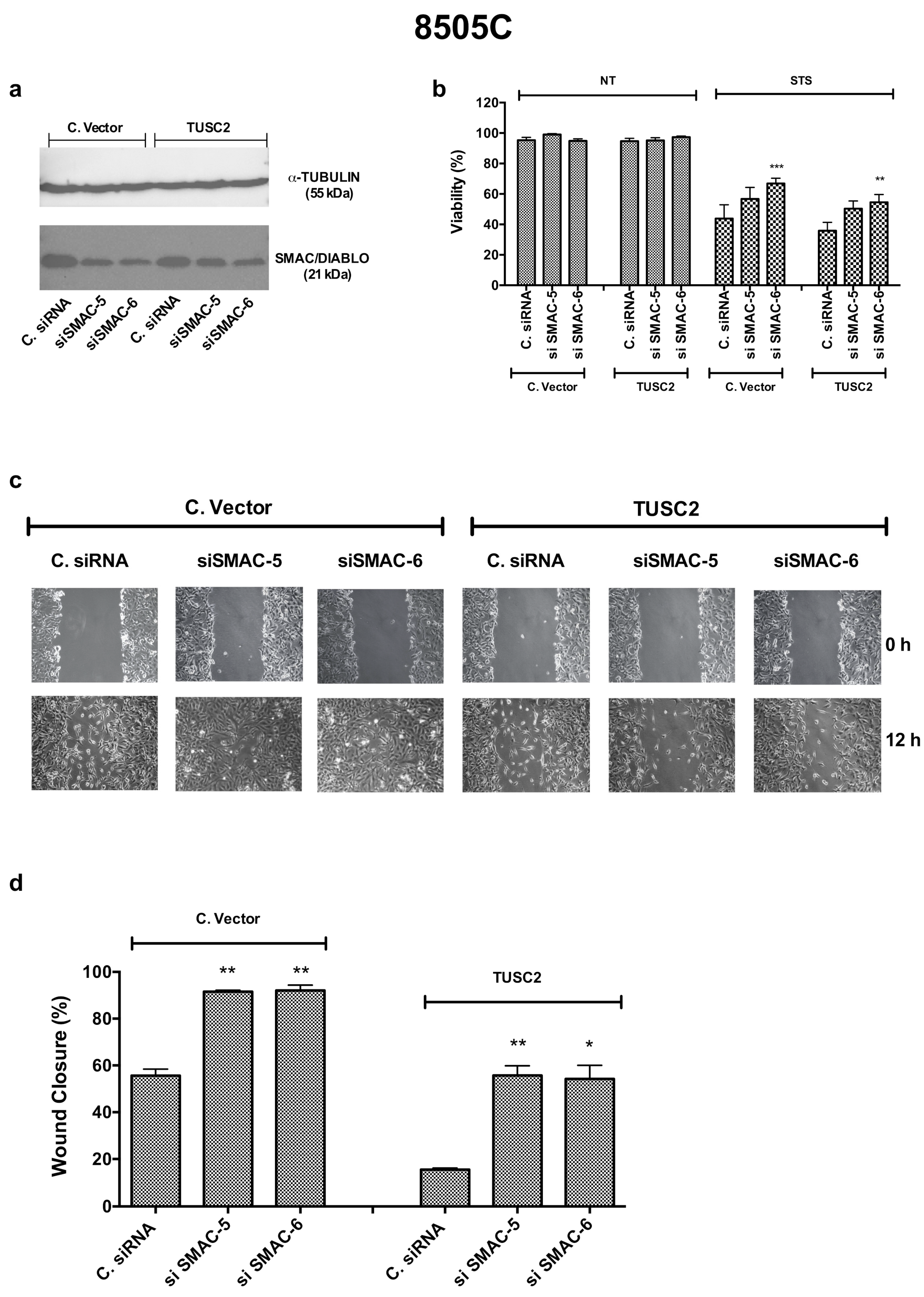
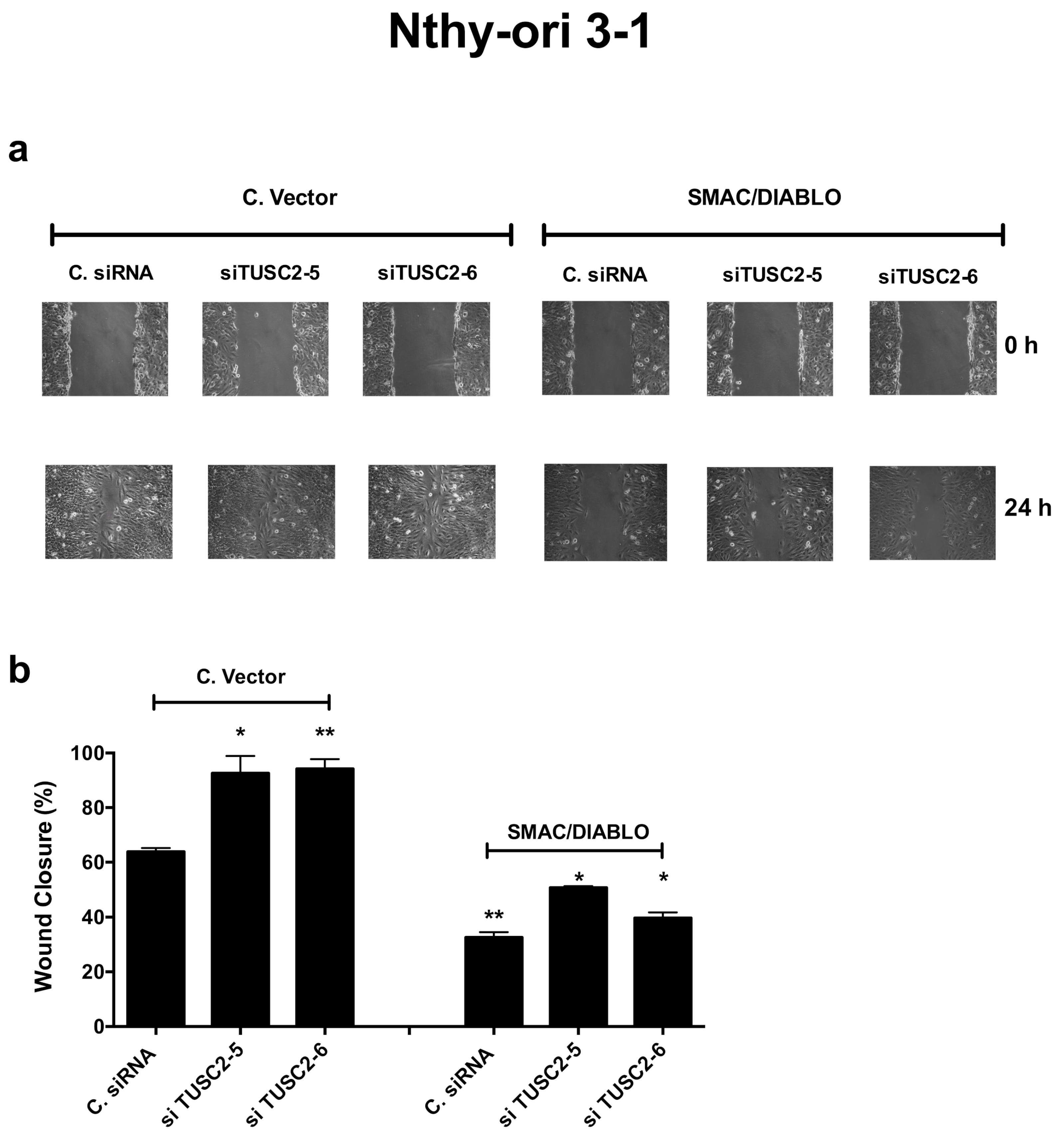
2.6. TUSC2 Effects Are Partially Mediated by SMAC/DIABLO
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cell Transfections
4.3. Western Blot
4.4. Migration and Invasion Assays
4.5. MTS Assay
4.6. Trypan Blue Assay
4.7. Anchorage-Independent Cell Growth in Soft Agar
4.8. Flow Cytometry
4.9. Human Apoptosis Array
4.10. Proximity Ligation Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lerman, M.I.; Minna, J.D. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: Identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000, 60, 6116–6133. [Google Scholar] [PubMed]
- Rimkus, T.; Sirkisoon, S.; Harrison, A.; Lo, H.W. Tumor suppressor candidate 2 (TUSC2, FUS-1) and human cancers. Discov. Med. 2017, 23, 325–330. [Google Scholar] [PubMed]
- Ji, L.; Roth, J.A. Tumor suppressor FUS1 signaling pathway. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2008, 3, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Prudkin, L.; Behrens, C.; Liu, D.D.; Zhou, X.; Ozburn, N.C.; Bekele, B.N.; Minna, J.D.; Moran, C.; Roth, J.A.; Ji, L.; et al. Loss and reduction of FUS1 protein expression is a frequent phenomenon in the pathogenesis of lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Ivanov, S.V.; Prudkin, L.; Nonaka, D.; Liu, Z.; Tsao, A.; Wistuba, I.; Roth, J.; Pass, H.I. Mechanisms of FUS1/TUSC2 deficiency in mesothelioma and its tumorigenic transcriptional effects. Mol. Cancer 2009, 8, 91. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Qi, Z.; Peng, L.; Qiu, B.; Huo, X. The FUS1 gene inhibits EC109 cell growth mediated by a lentivirus vector. Br. J. Biomed. Sci. 2013, 70, 22–26. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, X.K.; Xin, D.Y.; Li, X.F.; Sun, D.K.; Ma, Y.Y.; Tian, L.Q. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol. Rep. 2015, 34, 868–876. [Google Scholar] [CrossRef]
- Li, G.; Kawashima, H.; Ji, L.; Ogose, A.; Ariizumi, T.; Umezu, H.; Xu, Y.; Hotta, T.; Endo, N. Frequent absence of tumor suppressor FUS1 protein expression in human bone and soft tissue sarcomas. Anticancer Res. 2011, 31, 11–21. [Google Scholar]
- Deng, W.G.; Wu, G.; Ueda, K.; Xu, K.; Roth, J.A.; Ji, L. Enhancement of antitumor activity of cisplatin in human lung cancer cells by tumor suppressor FUS1. Cancer Gene Ther. 2008, 15, 29–39. [Google Scholar] [CrossRef][Green Version]
- Dai, B.; Yan, S.; Lara-Guerra, H.; Kawashima, H.; Sakai, R.; Jayachandran, G.; Majidi, M.; Mehran, R.; Wang, J.; Bekele, B.N.; et al. Exogenous Restoration of TUSC2 Expression Induces Responsiveness to Erlotinib in Wildtype Epidermal Growth Factor Receptor (EGFR) Lung Cancer Cells through Context Specific Pathways Resulting in Enhanced Therapeutic Efficacy. PLoS ONE 2015, 10, e0123967. [Google Scholar] [CrossRef]
- Meng, J.; Majidi, M.; Fang, B.; Ji, L.; Bekele, B.N.; Minna, J.D.; Roth, J.A. The tumor suppressor gene TUSC2 (FUS1) sensitizes NSCLC to the AKT inhibitor MK2206 in LKB1-dependent manner. PLoS ONE 2013, 8, e77067. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Ji, L.; Tanaka, F.; Saito, Y.; Gopalan, B.; Branch, C.D.; Xu, K.; Atkinson, E.N.; Bekele, B.N.; Stephens, L.C.; et al. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Ther. 2004, 11, 733–739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, C.; Stewart, D.J.; Lee, J.J.; Ji, L.; Ramesh, R.; Jayachandran, G.; Nunez, M.I.; Wistuba, I.I.; Erasmus, J.J.; Hicks, M.E.; et al. Phase I clinical trial of systemically administered TUSC2(FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS ONE 2012, 7, e34833. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, L.; Duval, M.; Benini, A.F.; Ferreira, C.V.; Maia, A.L. Medullary thyroid carcinoma beyond surgery: Advances, challenges, and perspectives. Endocr. Relat. Cancer 2019, 26, R499–R518. [Google Scholar] [CrossRef]
- Saini, S.; Tulla, K.; Maker, A.V.; Burman, K.D.; Prabhakar, B.S. Therapeutic advances in anaplastic thyroid cancer: A current perspective. Mol. Cancer 2018, 17, 154. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Wang, T.S.; Sosa, J.A. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat. Rev. Endocrinol. 2018, 14, 670–683. [Google Scholar] [CrossRef]
- Orlandella, F.M.; Di Maro, G.; Ugolini, C.; Basolo, F.; Salvatore, G. TWIST1/miR-584/TUSC2 pathway induces resistance to apoptosis in thyroid cancer cells. Oncotarget 2016, 7, 70575–70588. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA: Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Stuschke, M.; Weber, F.; Dralle, H.; Moss, L.; Fuhrer, D. Anaplastic thyroid carcinoma: Review of treatment protocols. Endocr. Relat. Cancer 2018, 25, R153–R161. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, C.; Ren, J.; Ye, S.; Ou, W.; Wang, Y.; Yang, W.; Zhong, G.; Chen, X.; Shi, H.; et al. Synergistic effects of eukaryotic coexpression plasmid carrying LKB1 and FUS1 genes on lung cancer in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2014, 140, 895–907. [Google Scholar] [CrossRef]
- Kondo, M.; Ji, L.; Kamibayashi, C.; Tomizawa, Y.; Randle, D.; Sekido, Y.; Yokota, J.; Kashuba, V.; Zabarovsky, E.; Kuzmin, I.; et al. Overexpression of candidate tumor suppressor gene FUS1 isolated from the 3p21.3 homozygous deletion region leads to G1 arrest and growth inhibition of lung cancer cells. Oncogene 2001, 20, 6258–6262. [Google Scholar] [CrossRef]
- Ren, J.; Yu, C.; Wu, S.; Peng, F.; Jiang, Q.; Zhang, X.; Zhong, G.; Shi, H.; Chen, X.; Su, X.; et al. Cationic liposome mediated delivery of FUS1 and hIL-12 coexpression plasmid demonstrates enhanced activity against human lung cancer. Curr. Cancer Drug Targets 2014, 14, 167–180. [Google Scholar] [CrossRef]
- Yazlovitskaya, E.M.; Voziyan, P.A.; Manavalan, T.; Yarbrough, W.G.; Ivanova, A.V. Cellular oxidative stress response mediates radiosensitivity in Fus1-deficient mice. Cell Death Dis. 2015, 6, e1652. [Google Scholar] [CrossRef][Green Version]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef]
- Tirro, E.; Consoli, M.L.; Massimino, M.; Manzella, L.; Frasca, F.; Sciacca, L.; Vicari, L.; Stassi, G.; Messina, L.; Messina, A.; et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res. 2006, 66, 4263–4272. [Google Scholar] [CrossRef]
- Bai, L.; Smith, D.C.; Wang, S. Small-molecule SMAC mimetics as new cancer therapeutics. Pharmacol. Ther. 2014, 144, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Singh, S.; Haq, W. IAP Proteins Antagonist: An Introduction and Chemistry of Smac Mimetics under Clinical Development. Curr. Med. Chem. 2018, 25, 3768–3795. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Schageman, J.J.; Subauste, M.C.; Saber, B.; Hammond, S.M.; Prudkin, L.; Wistuba, I.I.; Ji, L.; Roth, J.A.; Minna, J.D.; et al. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol. Cancer Res. MCR 2009, 7, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, N.; Deng, Y.; Chen, L.; Zhang, Y.; Zheng, Z.; Luo, W.; Lv, Z.; Li, S.; Xu, T. miR-663b promotes tumor cell proliferation, migration and invasion in nasopharyngeal carcinoma through targeting TUSC2. Exp. Ther. Med. 2017, 14, 1095–1103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, H.H.; Huan, W.T.; Han, J.Q.; Ren, W.R.; Yang, L.H. MicroRNA-663 facilitates the growth, migration and invasion of ovarian cancer cell by inhibiting TUSC2. Biol. Res. 2019, 52, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Deng, Z.; Wang, C.H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhou, J.; Zhang, J.; Zhou, L.Y.; Zhai, L.L.; Vanessa, M.E.; Yi, J.; Yi, Y.Y.; Lin, J.; Deng, Z.Q. Low Expression of FUS1 Is Negatively Correlated with miR-378 and May Predict Adverse Prognoses in Acute Myeloid Leukemia. Acta Haematol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Orlandella, F.M.; Mariniello, R.M.; Iervolino, P.L.C.; Auletta, L.; De Stefano, A.E.; Ugolini, C.; Greco, A.; Mirabelli, P.; Pane, K.; Franzese, M.; et al. Junctional adhesion molecule-A is down-regulated in anaplastic thyroid carcinomas and reduces cancer cell aggressiveness by modulating p53 and GSK3 alpha/beta pathways. Mol. Carcinog. 2019, 58. [Google Scholar] [CrossRef]
- Fox, M.H. A model for the computer analysis of synchronous DNA distributions obtained by flow cytometry. Cytometry 1980, 1, 71–77. [Google Scholar] [CrossRef]
- Lof, L.; Arngarden, L.; Olsson-Stromberg, U.; Siart, B.; Jansson, M.; Dahlin, J.S.; Thorn, I.; Christiansson, L.; Hermansson, M.; Larsson, A.; et al. Flow Cytometric Measurement of Blood Cells with BCR-ABL1 Fusion Protein in Chronic Myeloid Leukemia. Sci. Rep. 2017, 7, 623. [Google Scholar] [CrossRef]
- Cilloni, D.; Martinelli, G.; Messa, F.; Baccarani, M.; Saglio, G. Nuclear factor kB as a target for new drug development in myeloid malignancies. Haematologica 2007, 92, 1224–1229. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariniello, R.M.; Maria Orlandella, F.; De Stefano, A.E.; Iervolino, P.L.C.; Smaldone, G.; Luciano, N.; Cervone, N.; Munciguerra, F.; Esposito, S.; Mirabelli, P.; et al. The TUSC2 Tumour Suppressor Inhibits the Malignant Phenotype of Human Thyroid Cancer Cells via SMAC/DIABLO Protein. Int. J. Mol. Sci. 2020, 21, 702. https://doi.org/10.3390/ijms21030702
Mariniello RM, Maria Orlandella F, De Stefano AE, Iervolino PLC, Smaldone G, Luciano N, Cervone N, Munciguerra F, Esposito S, Mirabelli P, et al. The TUSC2 Tumour Suppressor Inhibits the Malignant Phenotype of Human Thyroid Cancer Cells via SMAC/DIABLO Protein. International Journal of Molecular Sciences. 2020; 21(3):702. https://doi.org/10.3390/ijms21030702
Chicago/Turabian StyleMariniello, Raffaela Mariarosaria, Francesca Maria Orlandella, Anna Elisa De Stefano, Paola Lucia Chiara Iervolino, Giovanni Smaldone, Neila Luciano, Nara Cervone, Francesco Munciguerra, Silvia Esposito, Peppino Mirabelli, and et al. 2020. "The TUSC2 Tumour Suppressor Inhibits the Malignant Phenotype of Human Thyroid Cancer Cells via SMAC/DIABLO Protein" International Journal of Molecular Sciences 21, no. 3: 702. https://doi.org/10.3390/ijms21030702
APA StyleMariniello, R. M., Maria Orlandella, F., De Stefano, A. E., Iervolino, P. L. C., Smaldone, G., Luciano, N., Cervone, N., Munciguerra, F., Esposito, S., Mirabelli, P., & Salvatore, G. (2020). The TUSC2 Tumour Suppressor Inhibits the Malignant Phenotype of Human Thyroid Cancer Cells via SMAC/DIABLO Protein. International Journal of Molecular Sciences, 21(3), 702. https://doi.org/10.3390/ijms21030702