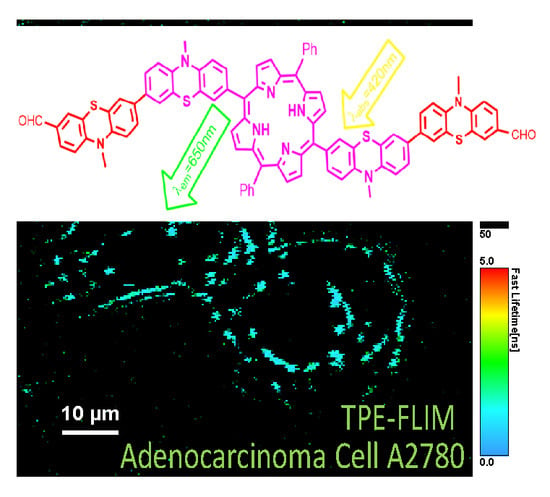
Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines
Abstract
1. Introduction
2. Results
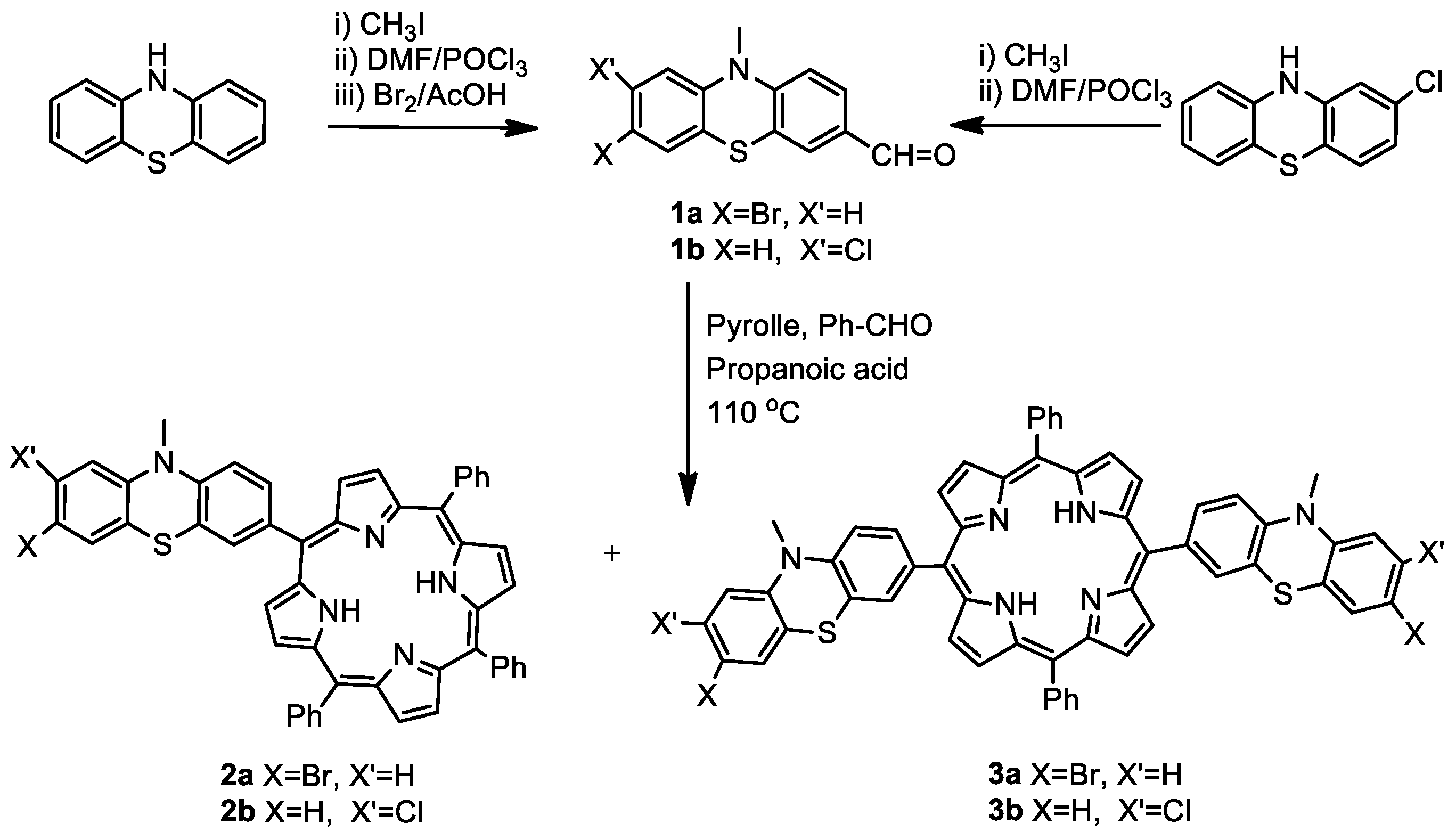
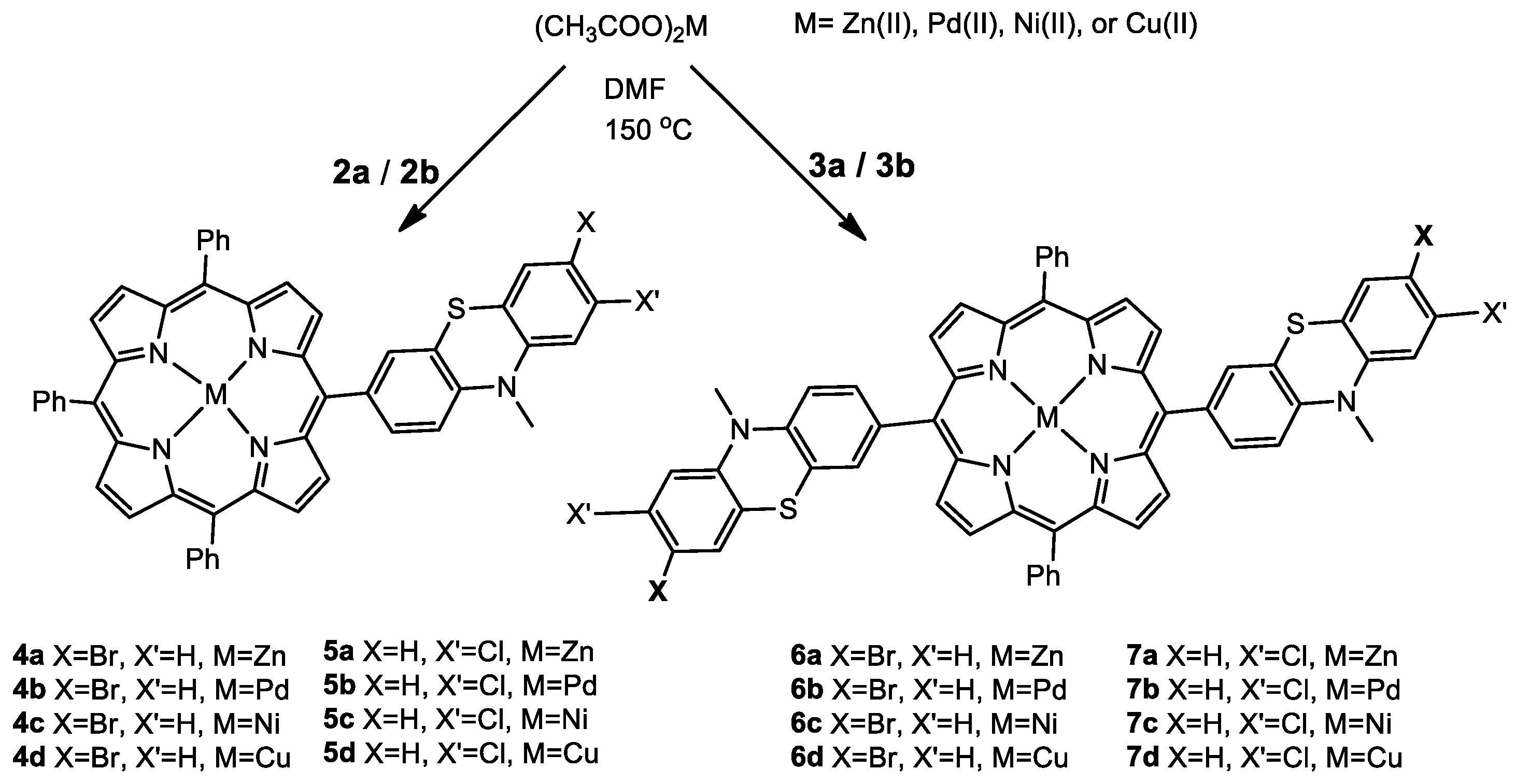
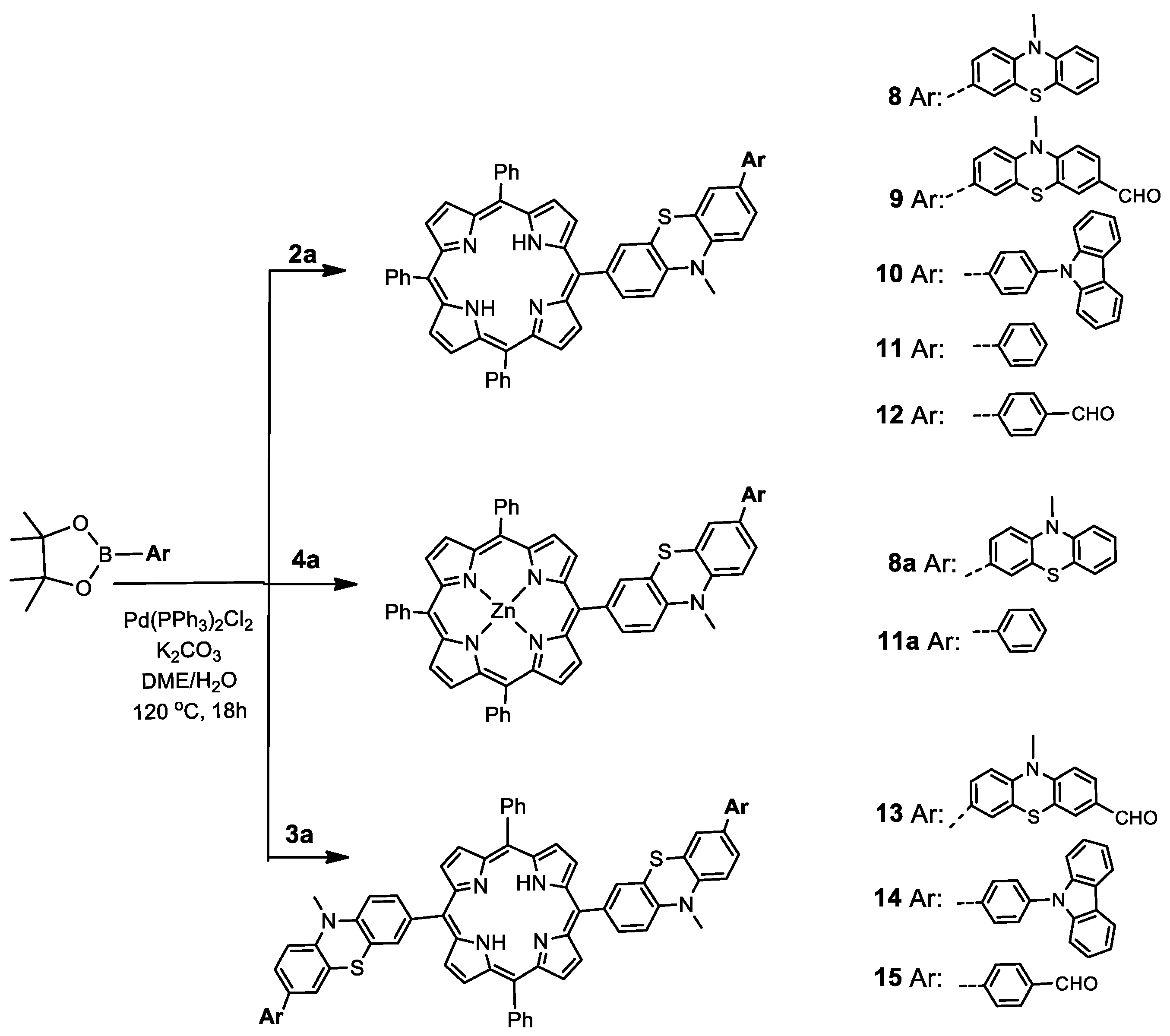
2.1. Synthesis of Novel MPP Dyes
2.1.1. Meso-(Halogenophenothiazinyl-Phenyl)Porphyrins
2.1.2. Meso-(Halogenophenothiazinyl-Phenyl)Porphyrin Metal Complexes
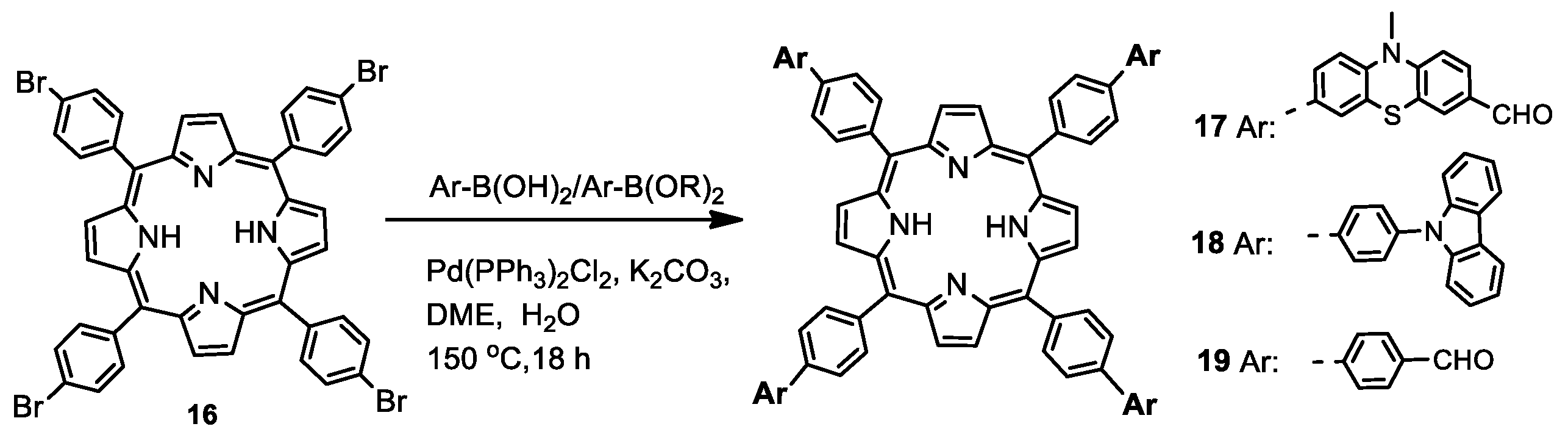
2.1.3. Phenothiazine-Bridged Porphyrin-(Hetero)Aryl Dyads
2.1.4. Phenylene-Bridged Porphyrin-(Hetero)Aryl Dyads
2.2. Optical Properties of Novel MPP Dyes
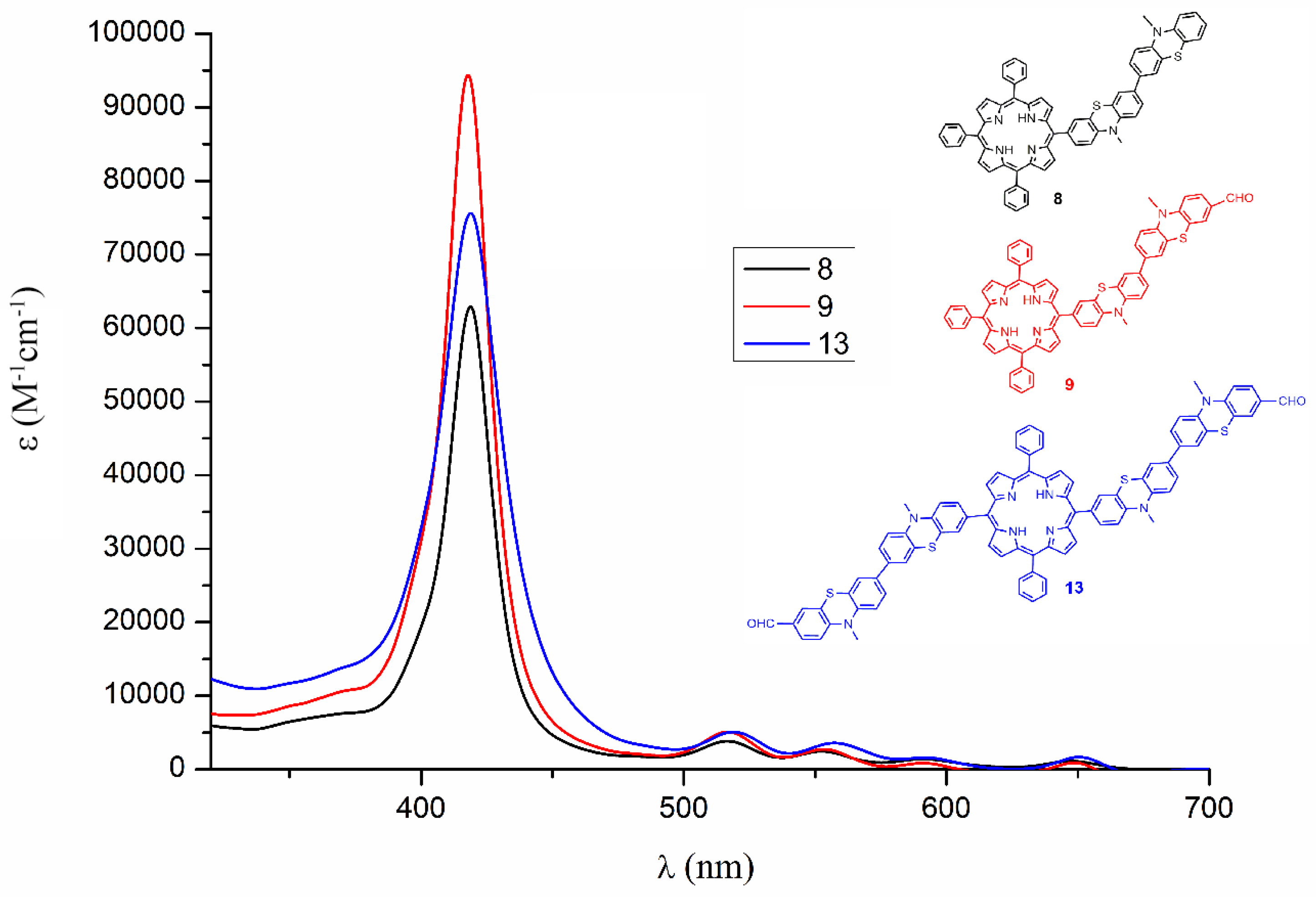
2.2.1. UV–Vis Absorption Spectra
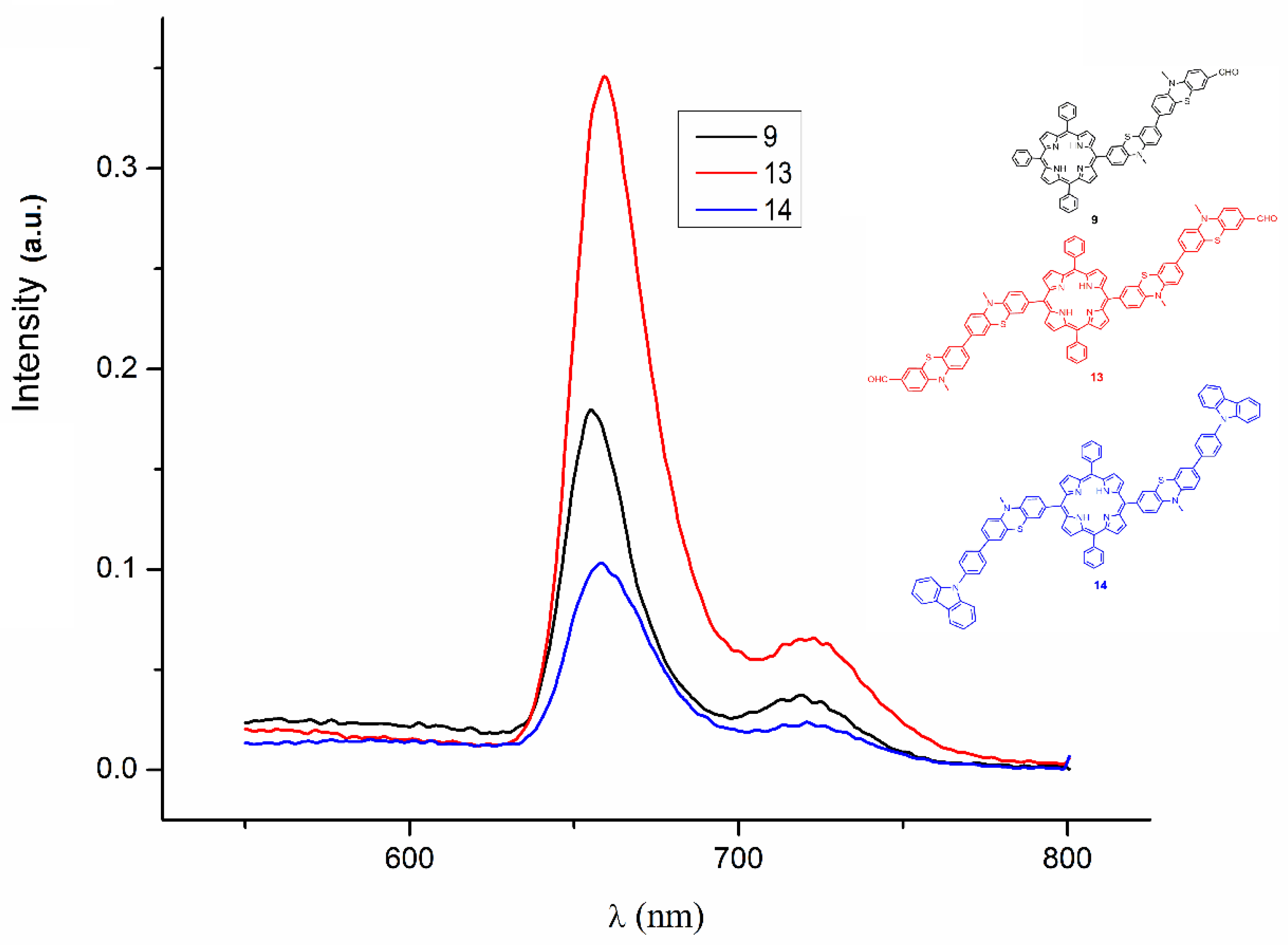
2.2.2. UV–Vis Fluorescence Emission Spectra
2.3. Biological Activity Evaluation of Phenothiazine-Bridged Porphyrin-Phenothiazine Dyad Fluorescent Dyes 9, 13 and 15
2.3.1. MTT Colorimetric Assay
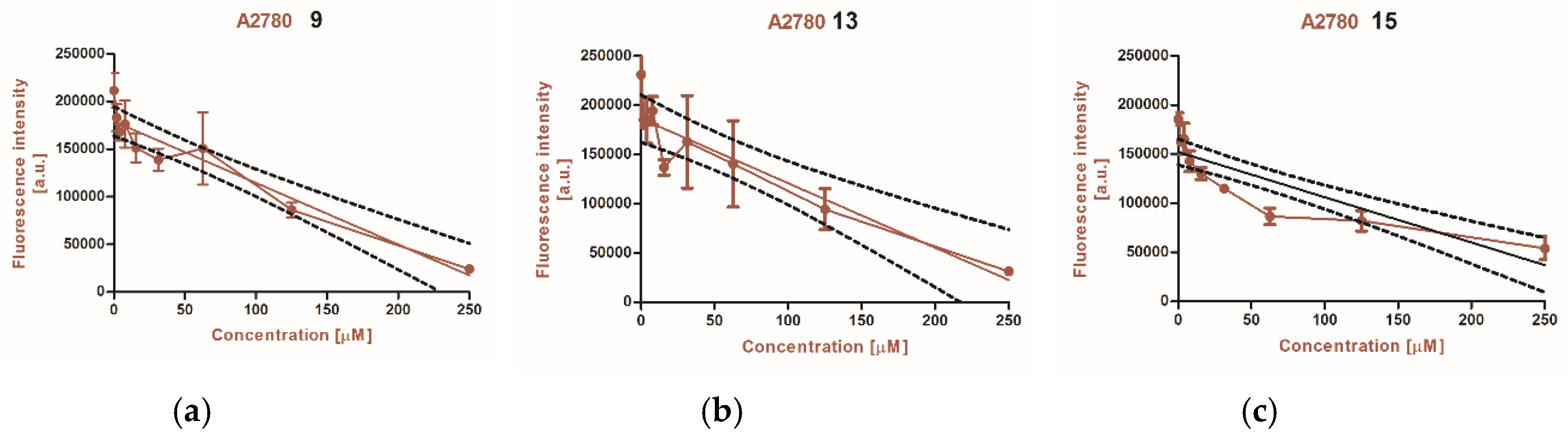
2.3.2. Alamar Blue Assay
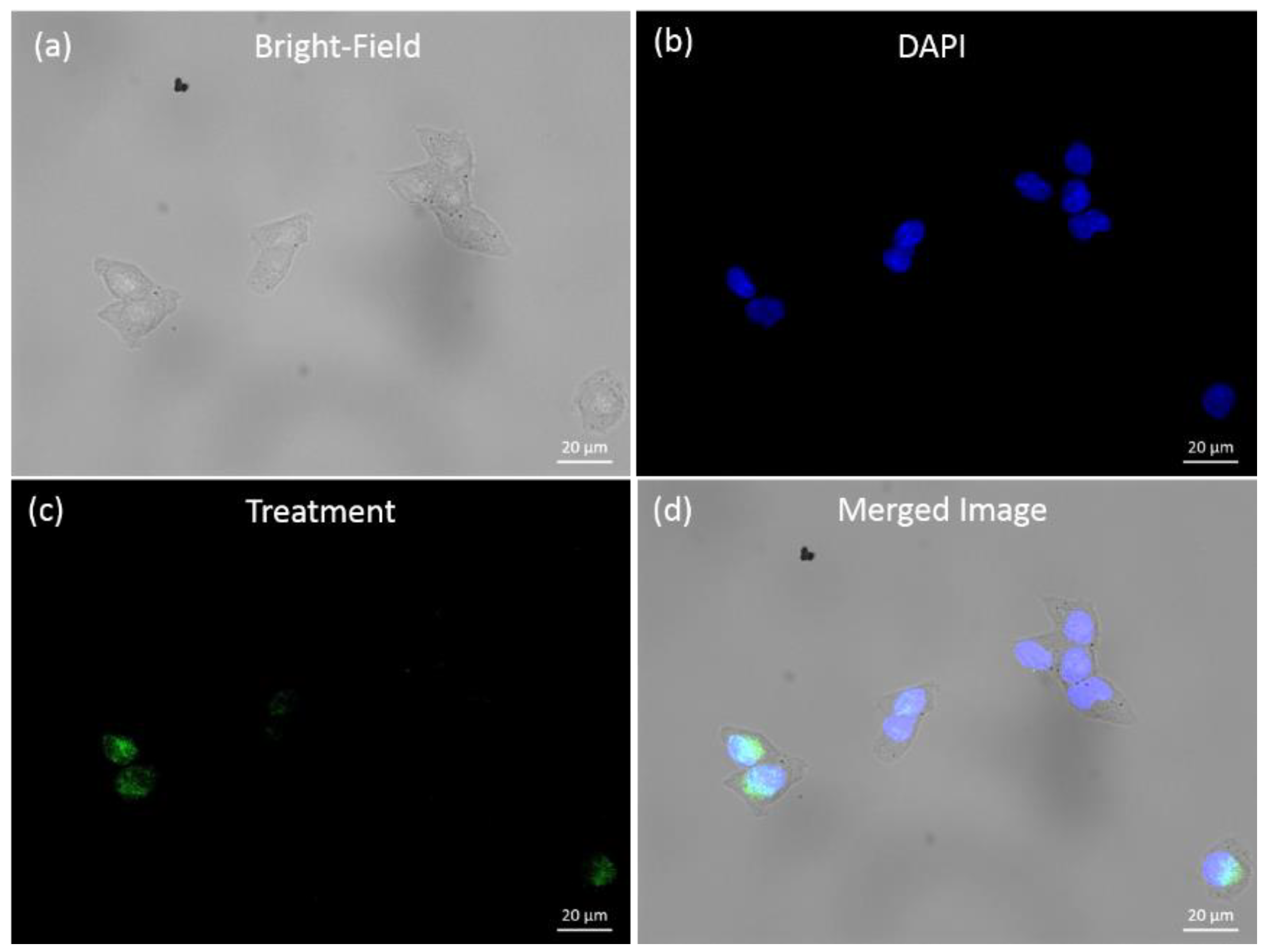
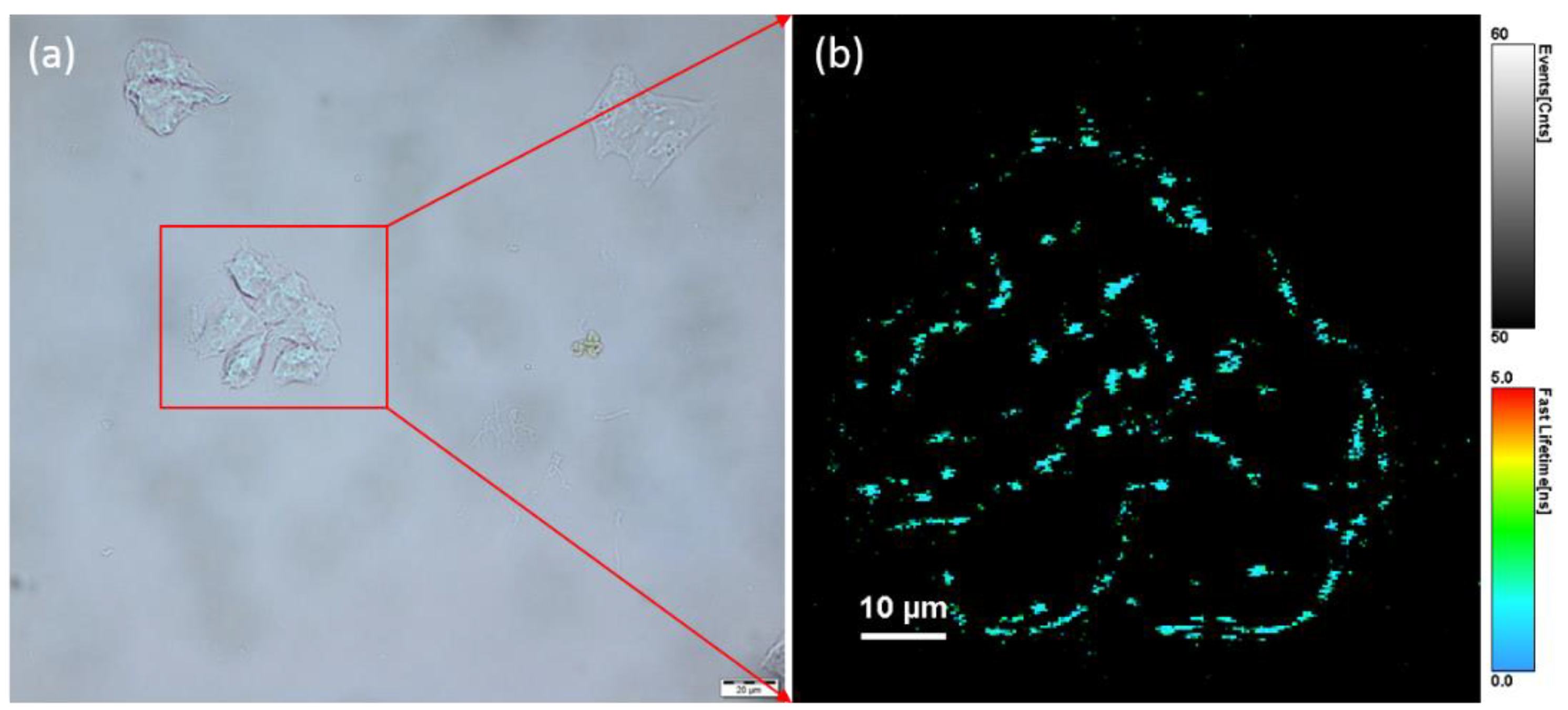
2.3.3. Cell Fluorescence Imaging
3. Materials and Methods
3.1. Spectral Measurements
3.2. Reagents
3.2.1. General Procedure for the Synthesis of AB3 and A2B2-Type Meso-Halogenophenothiazinyl-Porphyrins (2 and 3)
3.2.2. General Procedure for the Preparation of Metallo-Phenothiazinyl-Porphyrins
3.2.3. General Procedure for the Synthesis of Suzuki Cross-Coupling Products
3.3. In Vitro Biological Assay
3.4. Fluorescence Imaging of Human Ovarian Tumor Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MPP | meso-phenothiazinyl-phenyl porphyrin |
| BrMPP | meso-bromophenothiazinyl-phenyl porphyrin |
| BrTPP | meso-tetrakis(4-bromo-phenyl)porphyrin |
References
- Qiu, X.; Lu, R.; Zhou, H.; Zhang, X.; Xu, T.; Liu, X.; Zhao, Y. Synthesis of phenothiazine-functionalized porphyrins with high fluorescent quantum yields. Tetrahedron Lett. 2008, 49, 7446–7449. [Google Scholar] [CrossRef]
- Prakash, K.; Alsaleh, A.Z.; Rathi, P.; Sharma, A.; Sankar, M.; D’Souza, F. Synthesis, Spectral, Electrochemical and Photovoltaic Studies of A3B Porphyrinic Dyes having Peripheral Donors. ChemPhysChem 2019, 20, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sudhakar, V.; Prakash, K.; Krishnamoorthy, K.; Sankar, M. Tuning the Photovoltaic Performance of DSSCs by Appending Various Donor Groups on trans-Dimesityl Porphyrin Backbone. ACS Appl. Energy Mater. 2018, 1, 2793–2801. [Google Scholar] [CrossRef]
- Zeng, K.; Tang, W.; Li, C.; Chen, Y.; Zhao, S.; Liu, Q.; Xie, Y. Systematic optimization of the substituents on the phenothiazine donor of doubly strapped porphyrin sensitizers: An efficiency over 11% unassisted by any cosensitizer or coadsorbent. J. Mater. Chem. A 2019, 7, 20854–20860. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Ågren, H.; Xie, Y. Branched and linear alkoxy chains-wrapped push-pull porphyrins for developing efficient dye-sensitized solar cells. Dyes Pigments 2017, 137, 421–429. [Google Scholar] [CrossRef]
- Abu-Melha, S. Efficient synthesis of meso-substituted porphyrins andmolecular docking as potential new antioxidant andcytotoxicity agents. Arch. Pharm. Chem. Life Sci. 2019, 352, e1800221. [Google Scholar] [CrossRef]
- Gal, E.; Brem, B.; Pereţeanu, I.; Gǎinǎ, L.; Lovasz, T.; Perde-Schrepler, M.; Silaghi-Dumitrescu, L.; Cristea, C.; Silaghi-Dumitrescu, L. Novel meso-phenothiazinylporphyrin dyes: Synthesis, optical, electrochemical properties and PDT assay. Dyes Pigments 2013, 99, 144–153. [Google Scholar] [CrossRef]
- Brem, B.; Gal, E.; Gaina, L.; Cristea, C.; Gabudean, A.M.; Astilean, S.; Silaghi-Dumitrescu, L. Metallo complexes of meso-phenothiazinylporphyrins: Synthesis, linear and nonlinear optical properties. Dyes Pigments 2015, 123, 386–395. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynylhalides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Suzuki, A. Cross coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Suzuki, A. Organoboranes in organic synthesis including Suzuki coupling reaction. Heterocycles 2010, 80, 15–43. [Google Scholar] [CrossRef]
- Kingston, J.V.; Verkade, J.G. Synthesis and Characterization of R2PN=P(iBuNCH2CH2)3N: A New Bulky Electron-Rich Phosphine for Efficient Pd-Assisted Suzuki−Miyaura Cross-Coupling Reactions. J. Org. Chem. 2007, 72, 2816–2822. [Google Scholar] [CrossRef]
- Dolliver, D.; Bhattarai, B.T.; Pandey, A.; Lanier, M.L.; Bordelon, A.S.; Adhikari, S.; Dinser, J.A.; Flowers, P.F.; Wills, V.S.; Schneider, C.L.; et al. Stereospecific Suzuki, Sonogashira, and Negishi Coupling Reactions of N-Alkoxyimidoyl Iodides and Bromides. J. Org. Chem. 2013, 78, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Saito, B.; Fu, G.C. Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc. 2007, 129, 9602–9603. [Google Scholar] [CrossRef] [PubMed]
- Baillie, C.; Zhang, L.; Xiao, J. Ferrocenyl Monophosphine Ligands: Synthesis and Applications in the Suzuki–Miyaura Coupling of Aryl Chlorides. J. Org. Chem. 2004, 69, 7779–7782. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Petersen, T.B.; Abela, A.R. Room-Temperature Suzuki–Miyaura Couplings in Water Facilitated by Nonionic Amphiphiles. Org. Lett. 2008, 10, 1333–1336. [Google Scholar] [CrossRef]
- Krämer, C.S.; Zimmermann, T.J.; Sailer, M.; Müller, T.J.J. Syntheses of Phenothiazinylboronic Acid Derivatives—Suitable Starting Points for the Construction of Redox Active Materials. Synthesis 2002, 9, 1163–1170. [Google Scholar] [CrossRef]
- Onoabedje, E.A.; Uchechukwu, C.O.; Knight, D.W.; Sarkar, A. Fuctionalization of Linear and Angular Phenothiazine and Phenoxazine Ring Systems via Pd(0)/XPhos Mediated Suzuki-Miyaura Cross-coupling Reactions. J. Heterocyclic Chem. 2016, 53, 1787–1794. [Google Scholar] [CrossRef]
- Grisorio, R.; Roose, B.; Colella, S.; Listorti, A.; Suranna, G.P.; Abate, A. Molecular Tailoring of Phenothiazine-Based Hole-Transporting Materials for High- Performing Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1029–1034. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.; Chen, Q.; Shan, H.; Liu, C.; Xu, J.; Chen, Z.K.; Huang, W.; Xu, Z.X. Facile synthesis of a dopant-free hole transporting material with a phenothiazine core for planar perovskite solar cells. RSC Adv. 2017, 7, 53604–53610. [Google Scholar] [CrossRef]
- Chao, P.; Gu, R.; Ma, X.; Wang, T.; Zhaod, Y. Thiophene-substituted phenothiazine-based photosensitisers for radical and cationic photopolymerization reactions under visible laser beams (405 and 455 nm). Polym. Chem. 2016, 7, 5147–5156. [Google Scholar] [CrossRef]
- Vaz, B.; Alvarez, R.; Nieto, M.; Paniello, A.I.; de Lera, A.R. Suzuki cross-coupling of meso-dibromoporphyrins for the synthesis of functionalized A2B2 porphyrins. Tetrahedron Lett. 2001, 42, 7409–7412. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A. Synthesis of meso-meso Linked Hybrid Porphyrin Arrays by Pd-Catalyzed Cross-Coupling Reaction. Org. Lett. 2001, 3, 4213–4216. [Google Scholar] [CrossRef] [PubMed]
- Bakar, M.A.; Sergeeva, N.N.; Juillard, T.; Senge, M.O. Synthesis of Ferrocenyl Porphyrins via Suzuki Coupling and Their Photophysical Properties. Organometallics 2011, 30, 3225–3228. [Google Scholar] [CrossRef]
- Diev, V.V.; Schlenker, C.W.; Hanson, K.; Zhong, Q.; Zimmerman, J.D.; Forrest, S.R.; Thompson, M.E. Porphyrins Fused with Unactivated Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2012, 77, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.S.; Zeitler, K.; Mu1ller, T.J.J. Synthesis of Functionalized Ethynylphenothiazine Fluorophores. Org. Lett. 2000, 2, 3723–3726. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the preparation of metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Gong, J.; Han, J.; Liu, Q.; Ren, X.; Wei, P.; Yang, L.; Zhang, Y.; Liu, J.; Dong, Y.Q.; Wang, Y.; et al. An ideal platform of light-emitting materials from phenothiazine: Facile preparation, tunable red/NIR fluorescence, bent geometry-promoted AIE behaviour and selective lipid-droplet (LD) tracking ability. J. Mater. Chem. C 2019, 7, 4185–4190. [Google Scholar] [CrossRef]
- Boșca, B.A.; Ilea, A.; Sorițău, O.; Tatomir, C.; Miklášová, N.; Pârvu, A.E.; Mihu, C.M.; Melincovici, C.S.; Fischer-Fodor, E. Modulatory effect of curcumin analogs on the activation of metalloproteinases in human periodontal stem cells. Eur. J. Oral Sci. 2019, 127, 304–312. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzău, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef]
- Shashkova, S.; Leake, M.C. Single-molecule fluorescence microscopy review: Shedding new light on old problems. Biosci. Rep. 2017, 37, 37. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, S.; Han, C.; Guo, H.; Yang, F. High solid fluorescence of novel tetraphenylethene-porphyrin. J. Lumin. 2020, 220, 117017. [Google Scholar] [CrossRef]
- Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J.D.; Kohl, M.; Anderson, H.L. Porphyrin Dyes for Nonlinear Optical Imaging of Live Cells. iScience 2018, 4, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Su, C.; Xing, P.; Zhou, Y.; Gong, L.; Zhang, J.; Du, H.; Bian, Y.; Jiang, J. An AceDAN–porphyrin(Zn) dyad for fluorescence imaging and photodynamic therapy via two-photon excited FRET. Inorg. Chem. Front. 2018, 5, 3061–3066. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Zhou, Y.; Xing, P.; Gong, L.; Su, C.; Qi, D.; Du, H.; Bian, Y.; Jiang, J. Two-Photon Excited FRET Dyads for Lysosome-Targeted Imaging and Photodynamic Therapy. Inorg. Chem. 2018, 57, 11537–11542. [Google Scholar] [CrossRef]
- Lia, S.; Zhanga, Y.; Hea, X.-W.; Li, W.-Y.; Zhangab, Y.-K. Multifunctional mesoporous silica nanoplatform based on silicon nanoparticles for targeted two-photon-excited fluorescence imaging-guided chemo/photodynamic synergetic therapy in vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Klymchenko, A.S.; Skripka, A.; Richert, L.; Steponkiene, S.; Streckyte, G.; Mély, Y.; Rotomskis, R. A non-covalent complex of quantum dots and chlorin e 6: Efficient energy transfer and remarkable stability in living cells revealed by FLIM. RSC Adv. 2014, 4, 52270–52278. [Google Scholar] [CrossRef]
- Prashanth, K.P.; Maiya, B.G. Aluminium(III) porphyrin based dimers and trimers: Synthesis, spectroscopy and photochemistry. New J. Chem. 2003, 27, 619–625. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, X.; Cao, L.; Tong, Y.; Wu, G. Design of Bodipy based organic dyes for high-efficient dye-sensitized solar cells employing double electron acceptor. Dyes Pigments 2015, 117, 28–36. [Google Scholar] [CrossRef]
- Wang, P.; Fan, S.; Liang, J.; Ying, L.; You, J.; Wang, S.; Li, X. Carbazole-diphenylimidazole based bipolar material and its application in blue, green and red single layer OLEDs by solution processing. Dyes Pigments 2017, 142, 175–182. [Google Scholar] [CrossRef]
- Kurniawan, F.; Miura, Y.; Kartasasmita, R.E.; Mutalib, A.; Yoshioka, N.; Tjahjono, D.H. In Silico Study, Synthesis, and Cytotoxic Activities of Porphyrin Derivatives. Pharmaceuticals 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Suarasan, S.; Craciun, A.-M.; Licarete, E.; Focsan, M.; Magyari, K.; Astilean, S. Intracellular Dynamic Disentangling of Doxorubicin Release from Luminescent Nanogold Carriers by Fluorescence Lifetime Imaging Microscopy (FLIM) under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2019, 11, 7812–7822. [Google Scholar] [CrossRef] [PubMed]
| Cpd | λabs [nm] | ||
|---|---|---|---|
| Ptz | Soret ε [M−1 cm−1] | Q4,3,2,1 | |
| 2a | 259 | 420 (369256) | 517, 554, 592, 652 |
| 2b | 259 | 421 (186680) | 519, 555, 592, 651 |
| 3a | 259 | 422 (134911) | 519, 555, 592, 651 |
| 3b | 257 | 422 (193193) | 519,555, 598, 655 |
| 8 | 259 | 422 (61409) | 519, 555, 598, 655 |
| 8a | 260 | 422 (58049) | 548, 585 |
| 9 | 250 | 420 (96961) | 515, 555, 590, 650 |
| 10 | 250 | 420 (79805) | 515, 554, 590, 650 |
| 11 | 250 | 418 (125483) | 514, 552, 590, 655 |
| 11a | 253 | 422 (72411) | 548, 587 |
| 12 | 245 | 420 (191988) | 515, 555, 585, 645 |
| 13 | 250 | 420 (78229) | 520, 555, 590, 650 |
| 14 | 245 | 420 (182215) | 520, 555, 590, 650 |
| 15 | 240 | 420 (95928) | 520, 555, 590, 650 |
| 17 | - | 423 (174035) | 519, 548, 592, 652 |
| 18 | - | 420 (660743) | 515, 550, 590, 645 |
| 19 | - | 427 (78383) | 516, 555, 592, 642 |
| Cpd * | Phenothiazine | Porphyrin | |
|---|---|---|---|
| λ [nm] | λ [nm] | λ [nm] | |
| Soret ε [M−1 cm−1] | Qalfa, beta | ||
| 4a | 255 | 425 (146756) | 550, 595 |
| 4b | 261 | 418 (215712) | 524, 589 |
| 4c | 255 | 425 (156953) | 555, 595 |
| 4d | 257 | 416 (277707) | 538, 583 |
| 5a | 256 | 422 (222108) | 539, 589 |
| 5b | 257 | 418 (73993) | 524, 589 |
| 5c | 256 | 416 (201024) | 538, 583 |
| 5d | 256 | 416 (160106) | 539, 583 |
| 6a | 250 | 425 (108260) | 555, 600 |
| 6b | 259 | 422 (212575) | 526 |
| 6c | 260 | 425 (235777) | 550, 595 |
| 6d | 259 | 419 (207318) | 542, 587 |
| 7a | 257 | 425 (193910) | 552, 593 |
| 7b | 256 | 417 (208454) | 524 |
| 7c | 257 | 418 (310212) | 531 |
| 7d | 257 | 418 (219339) | 526 |
| Compounds | λema [nm] | Stokes Shift [cm−1] | ϕFb |
|---|---|---|---|
| 9 | 655 | 8542 | 0.16 |
| 10 | 657 | 8588 | 0.14 |
| 12 | 654 | 8519 | 0.18 |
| 13 | 659 | 8635 | 0.19 |
| 14 | 652 | 8472 | 0.08 |
| 15 | 658 | 8612 | 0.15 |
| Compound | 9 | |||||||
| Conc. [µM] | 240.00 | 120.00 | 60.00 | 30.00 | 15.00 | 7.50 | 3.75 | 1.88 |
| % cell survival A2780 | 59.40 ± 2.40 | 80.83 ± 3.28 | 77.56 ± 3.56 | 79.87 ± 4.60 | 89.87 ± 3.76 | 91.30 ± 3.47 | 90.60 ± 4.35 | 94.00 ± 4.51 |
| A2780cis | 90.38 ± 2.74 | 92.45 ± 2.58 | 92.96 ± 1.45 | 102.00 ± 1.55 | 95.00 ± 3.11 | 99.27 ± 2.39 | 92.46 ± 3.72 | 89.30 ± 1.20 |
| OVCAR 3 | 56.94 ± 3.61 | 69.58 ± 3.68 | 83.68 ± 4.50 | 76.36 ± 4.17 | 83.02 ± 3.58 | 82.89 ± 4.18 | 80.82 ± 5.12 | 87.81 ± 4.14 |
| Compound | 13 | |||||||
| Conc. [µM] | 210.00 | 105.00 | 52.50 | 26.25 | 13.13 | 6.56 | 3.28 | 1.64 |
| % cell survival A2780 | 62.00 ± 0.95 | 75.77 ± 1.04 | 93.07 ± 3.10 | 95.51 ± 2.36 | 93.57 ± 2.92 | 100.06 ± 1.60 | 98.43 ± 3.36 | 100.23 ± 0.39 |
| A2780cis | 94.06 ± 3.72 | 99.75 ± 1.42 | 98.00 ± 2.30 | 100.37 ± 4.15 | 97.12 ± 3.46 | 95.04 ± 3.39 | 101.00 ± 1.73 | 100.85 ± 2.54 |
| OVCAR 3 | 44.14 ± 3.46 | 66.89 ± 4.35 | 73.07 ± 2.80 | 75.21 ± 4.09 | 82.19 ± 4.91 | 82.65 ± 2.45 | 85.37 ± 4.18 | 89.92 ± 2.27 |
| Compound | 15 | |||||||
| Conc.[µM] | 250.00 | 125.00 | 62.50 | 31.25 | 15.63 | 7.81 | 3.91 | 1.95 |
| % cell survival A2780 | 66.46 ± 1.78 | 82.30 ± 3.62 | 87.73 ± 1.74 | 92.17 ± 0.64 | 92.03 ± 1.17 | 95.26 ± 5.13 | 94.13 ± 3.25 | 98.34 ± 2.11 |
| A2780cis | 93.38 ± 3.18 | 96.29 ± 1.45 | 92.00 ± 1.15 | 102.00 ± 2.88 | 97.05 ± 1.16 | 99 ± 2.31 | 86.85 ± 1.15 | 89 ± 2.52 |
| OVCAR 3 | 44.51 ± 3.67 | 64.41 ± 4.84 | 80.26 ± 3.75 | 83.20 ± 5.65 | 79.65 ± 3.38 | 85.89 ± 6.53 | 101.45 ± 5.59 | 94.11 ± 6.73 |
| Cpd | Cell Lines | Linear Regression Data | |||
|---|---|---|---|---|---|
| Slope | Goodness of Fit (R2) | p Value | Deviation from Zero | ||
| 9 | OVCAR-3 | −344.8 ± 30.66 | 0.83 | <0.0001 | Significant |
| A2780 | −646.9 ± 77.62 | 0.74 | <0.0001 | Significant | |
| A2780cis | −459.7 ± 42.01 | 0.86 | <0.0001 | Significant | |
| 13 | OVCAR-3 | −275.7 ± 32.17 | 0.75 | <0.0001 | Significant |
| A2780 | −655.4 ± 119.1 | 0.56 | <0.0001 | Significant | |
| A2780cis | −567.1 ± 58.13 | 0.82 | <0.0001 | Significant | |
| 15 | OVCAR-3 | −169.0 ± 23.17 | 0.68 | <0.0001 | Significant |
| A2780 | −460.7 ± 64.12 | 0.69 | <0.0001 | Significant | |
| A2780cis | −361.1 ± 53.16 | 0.71 | <0.0001 | Significant | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, E.; Gal, E.; Gaina, L.; Cristea, C.; Fischer-Fodor, E.; Perde-Schrepler, M.; Achimas-Cadariu, P.; Focsan, M.; Silaghi-Dumitrescu, L. Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines. Int. J. Mol. Sci. 2020, 21, 3178. https://doi.org/10.3390/ijms21093178
Molnar E, Gal E, Gaina L, Cristea C, Fischer-Fodor E, Perde-Schrepler M, Achimas-Cadariu P, Focsan M, Silaghi-Dumitrescu L. Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines. International Journal of Molecular Sciences. 2020; 21(9):3178. https://doi.org/10.3390/ijms21093178
Chicago/Turabian StyleMolnar, Eva, Emese Gal, Luiza Gaina, Castelia Cristea, Eva Fischer-Fodor, Maria Perde-Schrepler, Patriciu Achimas-Cadariu, Monica Focsan, and Luminita Silaghi-Dumitrescu. 2020. "Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines" International Journal of Molecular Sciences 21, no. 9: 3178. https://doi.org/10.3390/ijms21093178
APA StyleMolnar, E., Gal, E., Gaina, L., Cristea, C., Fischer-Fodor, E., Perde-Schrepler, M., Achimas-Cadariu, P., Focsan, M., & Silaghi-Dumitrescu, L. (2020). Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines. International Journal of Molecular Sciences, 21(9), 3178. https://doi.org/10.3390/ijms21093178