Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics
Abstract
1. Introduction
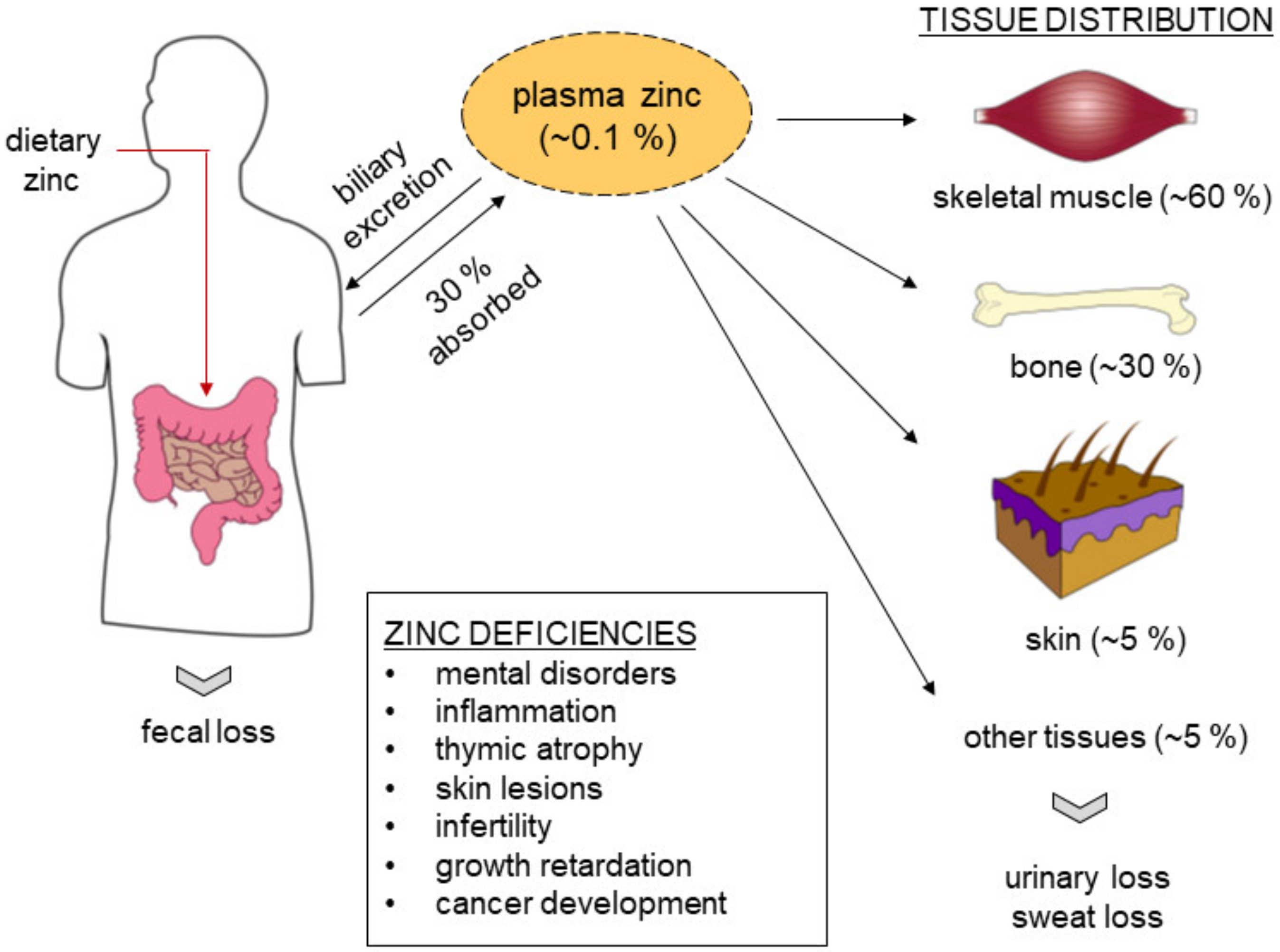
2. Zinc Biology
3. Zinc and Zinc Transporters in Prostate Cancer
4. Zinc and Zinc Transporters in Other Cancers
5. Zinc as an Agent for Treatment of Prostate Cancer
6. Perspective
Funding
Conflicts of Interest
References
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. et Biophys. Acta 2006, 1763, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef]
- John, E.; Laskow, T.C.; Buchser, W.J.; Pitt, B.R.; Basse, P.H.; Butterfield, L.H.; Kalinski, P.; Lotze, M.T. Zinc in innate and adaptive tumor immunity. J. Transl. Med. 2010, 8, 118. [Google Scholar] [CrossRef]
- Duncan, J.R.; Hurley, L.S. Thymidine kinase and DNA polymerase activity in normal and zinc deficient developing rat embryos. Proc. Soc. Exp. Biol. Med. 1978, 159, 39–43. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wu, C.W. Zinc in DNA replication and transcription. Annu. Rev. Nutr. 1987, 7, 251–272. [Google Scholar] [CrossRef]
- Song, Y.; Leonard, S.W.; Traber, M.G.; Ho, E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses and DNA repair in rats. J. Nutr. 2009, 139, 1626–1631. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Sliwinski, T.; Czechowska, A.; Kolodziejczak, M.; Jajte, J.; Wisniewska-Jarosinska, M.; Blasiak, J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol. Int. 2009, 33, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Song, Y.; Wong, C.P.; Hardin, K.; Ho, E. Zinc deficiency alters DNA damage response genes in normal human prostate epithelial cells. J. Nutr. 2008, 138, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.P.; Kirchgessner, M. Influence of alimentary zinc deficiency on the concentration of growth hormone (gh), insulin-like growth factor i (igf-i) and insulin in the serum of force-fed rats. Horm. Metab. Res. 1994, 26, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Mukherjee, J.J.; Chung, T.; Crilly, K.S.; Kiss, Z. Extracellular calcium stimulates DNA synthesis in synergism with zinc, insulin and insulin-like growth factor i in fibroblasts. Eur. J. Biochem. 1999, 266, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Chesters, J.K.; Boyne, R. Nature of the zn2+ requirement for DNA synthesis by 3t3 cells. Exp. Cell Res. 1991, 192, 631–634. [Google Scholar] [CrossRef]
- Wong, S.H.; Shih, R.S.; Schoene, N.W.; Lei, K.Y. Zinc-induced g2/m blockage is p53 and p21 dependent in normal human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2008, 294, C1342–C1349. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Slininger, P.J.; Bothast, R.J. Effects of zinc, iron, cobalt and manganese on fusarium moniliforme nrrl 13616 growth and fusarin c biosynthesis in submerged cultures. Appl. Environ. Microbiol. 1989, 55, 649–655. [Google Scholar] [CrossRef]
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of zip and znt zinc transporters in human health and diseases. Cell Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Cousins, R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004, 24, 151–172. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal. Transduct Target. Ther. 2017, 2. [Google Scholar] [CrossRef]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. (Landmark Ed.) 2017, 22, 623–643. [Google Scholar] [CrossRef]
- Maret, W. Zinc and human disease. Met. Ions Life Sci. 2013, 13, 389–414. [Google Scholar]
- Danscher, G.; Stoltenberg, M. Zinc-specific autometallographic in vivo selenium methods: Tracing of zinc-enriched (zen) terminals, zen pathways and pools of zinc ions in a multitude of other zen cells. J. Histochem. Cytochem. 2005, 53, 141–153. [Google Scholar] [CrossRef]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian zinc transport, trafficking and signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human zip1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg Biochem. 2003, 96, 435–442. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. The human zip1 transporter mediates zinc uptake in human k562 erythroleukemia cells. J. Biol. Chem. 2001, 276, 22258–22264. [Google Scholar] [CrossRef]
- Milon, B.; Dhermy, D.; Pountney, D.; Bourgeois, M.; Beaumont, C. Differential subcellular localization of hzip1 in adherent and non-adherent cells. FEBS Lett. 2001, 507, 241–246. [Google Scholar] [CrossRef]
- Cao, J.; Bobo, J.A.; Liuzzi, J.P.; Cousins, R.J. Effects of intracellular zinc depletion on metallothionein and zip2 transporter expression and apoptosis. J. Leukoc. Biol. 2001, 70, 559–566. [Google Scholar]
- Gaither, L.A.; Eide, D.J. Functional expression of the human hzip2 zinc transporter. J. Biol. Chem. 2000, 275, 5560–5564. [Google Scholar] [CrossRef]
- Peters, J.L.; Dufner-Beattie, J.; Xu, W.; Geiser, J.; Lahner, B.; Salt, D.E.; Andrews, G.K. Targeting of the mouse slc39a2 (zip2) gene reveals highly cell-specific patterns of expression and unique functions in zinc, iron and calcium homeostasis. Genesis 2007, 45, 339–352. [Google Scholar] [CrossRef]
- Costello, L.C.; Zou, J.; Desouki, M.M.; Franklin, R.B. Evidence for changes in rreb-1, zip3 and zinc in the early development of pancreatic adenocarcinoma. J. Gastrointest Cancer 2012, 43, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; Huang, Z.L.; Geiser, J.; Xu, W.; Andrews, G.K. Generation and characterization of mice lacking the zinc uptake transporter zip3. Mol. Cell Biol. 2005, 25, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; Langmade, S.J.; Wang, F.; Eide, D.; Andrews, G.K. Structure, function and regulation of a subfamily of mouse zinc transporter genes. J. Biol. Chem. 2003, 278, 50142–50150. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kim, B.E.; Dufner-Beattie, J.; Petris, M.J.; Andrews, G.; Eide, D.J. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse zip4 zinc transporter. Human Mol. Genet. 2004, 13, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, B.; Kuo, Y.M.; Zemansky, J.; Gitschier, J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Human Genet. 2002, 71, 66–73. [Google Scholar] [CrossRef]
- Dufner-Beattie, J.; Kuo, Y.M.; Gitschier, J.; Andrews, G.K. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters zip4 and zip5. J. Biol. Chem. 2004, 279, 49082–49090. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.E.; Petris, M.J.; Eide, D.J. The mammalian zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J. Biol. Chem. 2004, 279, 51433–51441. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Cui, X.; Yao, W.; Yu, X.; Cen, P.; Hodges, S.E.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr. Mol. Med. 2013, 13, 401–409. [Google Scholar]
- Croxford, T.P.; McCormick, N.H.; Kelleher, S.L. Moderate zinc deficiency reduces testicular zip6 and zip10 abundance and impairs spermatogenesis in mice. J. Nutr. 2011, 141, 359–365. [Google Scholar] [CrossRef]
- Liu, Y.; Batchuluun, B.; Ho, L.; Zhu, D.; Prentice, K.J.; Bhattacharjee, A.; Zhang, M.; Pourasgari, F.; Hardy, A.B.; Taylor, K.M.; et al. Characterization of zinc influx transporters (zips) in pancreatic beta cells: Roles in regulating cytosolic zinc homeostasis and insulin secretion. J. Biol. Chem. 2015, 290, 18757–18769. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for zip7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Zhang, Y.; Yu, Y.Y. The zip7 gene (slc39a7) encodes a zinc transporter involved in zinc homeostasis of the golgi apparatus. J. Biol. Chem. 2005, 280, 15456–15463. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of hke4, a member of the new liv-1 subfamily of zinc transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef]
- Begum, N.A.; Kobayashi, M.; Moriwaki, Y.; Matsumoto, M.; Toyoshima, K.; Seya, T. Mycobacterium bovis bcg cell wall and lipopolysaccharide induce a novel gene, bigm103, encoding a 7-tm protein: Identification of a new protein family having zn-transporter and zn-metalloprotease signatures. Genomics 2002, 80, 630–645. [Google Scholar] [CrossRef]
- Ryu, M.S.; Lichten, L.A.; Liuzzi, J.P.; Cousins, R.J. Zinc transporters znt1 (slc30a1), zip8 (slc39a8) and zip10 (slc39a10) in mouse red blood cells are differentially regulated during erythroid development and by dietary zinc deficiency. J. Nutr. 2008, 138, 2076–2083. [Google Scholar] [CrossRef]
- Matsuura, W.; Yamazaki, T.; Yamaguchi-Iwai, Y.; Masuda, S.; Nagao, M.; Andrews, G.K.; Kambe, T. Slc39a9 (zip9) regulates zinc homeostasis in the secretory pathway: Characterization of the zip subfamily i protein in vertebrate cells. Biosci. Biotechnol. Biochem. 2009, 73, 1142–1148. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the zip9 zinc transporter subfamily: Ii. Role of human zip9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef]
- Kagara, N.; Tanaka, N.; Noguchi, S.; Hirano, T. Zinc and its transporter zip10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007, 98, 692–697. [Google Scholar] [CrossRef]
- Kaler, P.; Prasad, R. Molecular cloning and functional characterization of novel zinc transporter rzip10 (slc39a10) involved in zinc uptake across rat renal brush-border membrane. Am. J. Physiol. Renal Physiol. 2007, 292, F217–F229. [Google Scholar] [CrossRef][Green Version]
- Pawan, K.; Neeraj, S.; Sandeep, K.; Kanta Ratho, R.; Rajendra, P. Upregulation of slc39a10 gene expression in response to thyroid hormones in intestine and kidney. Biochim. Biophys. Acta 2007, 1769, 117–123. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Velasquez, V.; Croxford, T.P.; McCormick, N.H.; Lopez, V.; MacDavid, J. Mapping the zinc-transporting system in mammary cells: Molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation. J. Cell. Physiol. 2012, 227, 1761–1770. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, A.; Zhang, Z.; Yan, G.; Zhang, F.; Zhang, L.; Shen, X.; Hu, R.; Zhang, Y.; Zhang, K.; et al. Characterization of the gufa subfamily member slc39a11/zip11 as a zinc transporter. J. Nutr. Biochem. 2013, 24, 1697–1708. [Google Scholar] [CrossRef]
- Chowanadisai, W.; Graham, D.M.; Keen, C.L.; Rucker, R.B.; Messerli, M.A. Neurulation and neurite extension require the zinc transporter zip12 (slc39a12). Proc. Natl. Acad. Sci. USA 2013, 110, 9903–9908. [Google Scholar] [CrossRef]
- Zhao, L.; Oliver, E.; Maratou, K.; Atanur, S.S.; Dubois, O.D.; Cotroneo, E.; Chen, C.N.; Wang, L.; Arce, C.; Chabosseau, P.L.; et al. The zinc transporter zip12 regulates the pulmonary vascular response to chronic hypoxia. Nature 2015, 524, 356–360. [Google Scholar] [CrossRef]
- Bin, B.H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human zip13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic ehlers-danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter slc39a13/zip13 is required for connective tissue development; its involvement in bmp/tgf-beta signaling pathways. PloS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef]
- Tominaga, K.; Kagata, T.; Johmura, Y.; Hishida, T.; Nishizuka, M.; Imagawa, M. Slc39a14, a lzt protein, is induced in adipogenesis and transports zinc. FEBS J. 2005, 272, 1590–1599. [Google Scholar] [CrossRef]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. Zrt/irt-like protein 14 (zip14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Troche, C.; Kim, M.H.; Cousins, R.J. Hepatic zip14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J. Biol. Chem. 2016, 291, 23939–23951. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the slc30 family of solute carriers. Pflugers Archiv.Eur. J. Physiol. 2004, 447, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lichten, L.A.; Ryu, M.S.; Liuzzi, J.P.; Wang, F.; Cousins, R.J. Stat5-glucocorticoid receptor interaction and mtf-1 regulate the expression of znt2 (slc30a2) in pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Blanchard, R.K.; Cousins, R.J. Differential regulation of zinc transporter 1, 2 and 4 mrna expression by dietary zinc in rats. J. Nutr. 2001, 131, 46–52. [Google Scholar] [CrossRef]
- Lopez, V.; Kelleher, S.L. Zinc transporter-2 (znt2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem. J. 2009, 422, 43–52. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Findley, S.D. Znt-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996, 15, 1784–1791. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef]
- Salazar, G.; Love, R.; Styers, M.L.; Werner, E.; Peden, A.; Rodriguez, S.; Gearing, M.; Wainer, B.H.; Faundez, V. AP-3-dependent mechanisms control the targeting of a chloride channel (ClC-3) in neuronal and non-neuronal cells. J. Biol. Chem. 2004, 279, 25430–25439. [Google Scholar] [CrossRef]
- Smidt, K.; Jessen, N.; Petersen, A.B.; Larsen, A.; Magnusson, N.; Jeppesen, J.B.; Stoltenberg, M.; Culvenor, J.G.; Tsatsanis, A.; Brock, B.; et al. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS ONE 2009, 4, e5684. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef] [PubMed]
- McCormick, N.H.; Kelleher, S.L. Znt4 provides zinc to zinc-dependent proteins in the trans-golgi network critical for cell function and zn export in mammary epithelial cells. Am. J. Physiol. Cell Physiol. 2012, 303, C291–C297. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, A.A.; Allen, J.; Blomeley, R.C.; Ackland, M.L. Constitutive expression of hznt4 zinc transporter in human breast epithelial cells. Biochem. J. 2002, 364, 105–113. [Google Scholar] [CrossRef]
- Murgia, C.; Vespignani, I.; Cerase, J.; Nobili, F.; Perozzi, G. Cloning, expression and vesicular localization of zinc transporter dri 27/znt4 in intestinal tissue and cells. Am. J. Physiol. 1999, 277, G1231–G1239. [Google Scholar] [CrossRef]
- Inoue, K.; Matsuda, K.; Itoh, M.; Kawaguchi, H.; Tomoike, H.; Aoyagi, T.; Nagai, R.; Hori, M.; Nakamura, Y.; Tanaka, T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, znt5. Human Mol. Genet. 2002, 11, 1775–1784. [Google Scholar] [CrossRef]
- Jackson, K.A.; Helston, R.M.; McKay, J.A.; O’Neill, E.D.; Mathers, J.C.; Ford, D. Splice variants of the human zinc transporter znt5 (slc30a5) are differentially localized and regulated by zinc through transcription and mrna stability. J. Biol. Chem. 2007, 282, 10423–10431. [Google Scholar] [CrossRef]
- Kambe, T.; Narita, H.; Yamaguchi-Iwai, Y.; Hirose, J.; Amano, T.; Sugiura, N.; Sasaki, R.; Mori, K.; Iwanaga, T.; Nagao, M. Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 2002, 277, 19049–19055. [Google Scholar] [CrossRef]
- Huang, L.; Kirschke, C.P.; Gitschier, J. Functional characterization of a novel mammalian zinc transporter, znt6. J. Biol. Chem. 2002, 277, 26389–26395. [Google Scholar] [CrossRef]
- Smith, J.L.; Xiong, S.; Markesbery, W.R.; Lovell, M.A. Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late alzheimer’s disease brain. Neuroscience 2006, 140, 879–888. [Google Scholar] [CrossRef]
- Asano, N.; Kondoh, M.; Ebihara, C.; Fujii, M.; Nakanishi, T.; Soares, M.J.; Nakashima, E.; Tanaka, K.; Sato, M.; Watanabe, Y. Expression profiles of zinc transporters in rodent placental models. Toxicol. Lett. 2004, 154, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. Znt7, a novel mammalian zinc transporter, accumulates zinc in the golgi apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ishihara, K.; Migaki, H.; Matsuura, W.; Kohda, A.; Okumura, K.; Nagao, M.; Yamaguchi-Iwai, Y.; Kambe, T. Zinc transporters, znt5 and znt7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J. Biol. Chem. 2005, 280, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, F.; Devergnas, S.; Favier, A.; Seve, M. Identification and cloning of a beta-cell-specific zinc transporter, znt-8, localized into insulin secretory granules. Diabetes 2004, 53, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Devirgiliis, C.; Mancini, E.; Donadel, G.; Zalewski, P.; Perozzi, G. Diabetes-linked zinc transporter znt8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc. Natl. Acad. Sci. USA 2004, 101, 4918–4923. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Perez, Y.; Shorer, Z.; Liani-Leibson, K.; Chabosseau, P.; Kadir, R.; Volodarsky, M.; Halperin, D.; Barber-Zucker, S.; Shalev, H.; Schreiber, R.; et al. Slc30a9 mutation affecting intracellular zinc homeostasis causes a novel cerebro-renal syndrome. Brain J. Neurol. 2017, 140, 928–939. [Google Scholar] [CrossRef]
- Bosomworth, H.J.; Thornton, J.K.; Coneyworth, L.J.; Ford, D.; Valentine, R.A. Efflux function, tissue-specific expression and intracellular trafficking of the zn transporter znt10 indicate roles in adult zn homeostasis. Met. Integr. Biometal Sci. 2012, 4, 771–779. [Google Scholar] [CrossRef]
- Patrushev, N.; Seidel-Rogol, B.; Salazar, G. Angiotensin ii requires zinc and downregulation of the zinc transporters znt3 and znt10 to induce senescence of vascular smooth muscle cells. PLoS ONE 2012, 7, e33211. [Google Scholar] [CrossRef]
- Seve, M.; Chimienti, F.; Devergnas, S.; Favier, A. In silico identification and expression of slc30 family genes: An expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genom. 2004, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Mawson, C.A.; Fischer, M.I. The occurrence of zinc in the human prostate gland. Can. J. Med. Sci. 1952, 30, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Gyorkey, F.; Min, K.W.; Huff, J.A.; Gyorkey, P. Zinc and magnesium in human prostate gland: Normal, hyperplastic and neoplastic. Cancer Res. 1967, 27, 1348–1353. [Google Scholar] [PubMed]
- Zaichick, V.; Sviridova, T.V.; Zaichick, S.V. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer 2006, 5, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christudoss, P.; Selvakumar, R.; Fleming, J.J.; Gopalakrishnan, G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J. Urol. 2011, 27, 14–18. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Hu, X.; Dong, X.; Wang, L.; Liu, Q.; Long, Z.; Li, L. Comparative study of serum zinc concentrations in benign and malignant prostate disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 25778. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Yuce, K. Zinc transporter proteins. Neurochem. Res. 2018, 43, 517–530. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The functions of metallothionein and zip and znt transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Johnson, L.A.; Kanak, M.A.; Kajdacsy-Balla, A.; Pestaner, J.P.; Bagasra, O. Differential zinc accumulation and expression of human zinc transporter 1 (hzip1) in prostate glands. Methods 2010, 52, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C.P.; Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: A possible role in prostate cancer progression. Cancer Cell Int. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B.; Zou, J.; Feng, P.; Bok, R.; Swanson, M.G.; Kurhanewicz, J. Human prostate cancer zip1/zinc/citrate genetic/metabolic relationship in the tramp prostate cancer animal model. Cancer Biol. Ther. 2011, 12, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. Hzip1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Gaither, L.A.; Eide, D.J. Eukaryotic zinc transporters and their regulation. Biometals 2001, 14, 251–270. [Google Scholar] [CrossRef]
- Desouki, M.M.; Geradts, J.; Milon, B.; Franklin, R.B.; Costello, L.C. Hzip2 and hzip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol. Cancer 2007, 6, 37. [Google Scholar] [CrossRef]
- Chen, Q.G.; Zhang, Z.; Yang, Q.; Shan, G.Y.; Yu, X.Y.; Kong, C.Z. The role of zinc transporter zip4 in prostate carcinoma. Urol. Oncol. 2012, 30, 906–911. [Google Scholar] [CrossRef]
- Hasumi, M.; Suzuki, K.; Matsui, H.; Koike, H.; Ito, K.; Yamanaka, H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003, 200, 187–195. [Google Scholar] [CrossRef]
- Henshall, S.M.; Afar, D.E.; Rasiah, K.K.; Horvath, L.G.; Gish, K.; Caras, I.; Ramakrishnan, V.; Wong, M.; Jeffry, U.; Kench, J.G.; et al. Expression of the zinc transporter znt4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 2003, 22, 6005–6012. [Google Scholar] [CrossRef]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of zinc-exporters expression in prostate cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Huang, L.; Kirschke, C.P. A null-mutation in the znt7 gene accelerates prostate tumor formation in a transgenic adenocarcinoma mouse prostate model. Cancer Lett. 2011, 308, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.M.; Waghray, A.; Bello, D. Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin. Cancer Res. 1995, 1, 1089–1094. [Google Scholar] [PubMed]
- Ishii, K.; Otsuka, T.; Iguchi, K.; Usui, S.; Yamamoto, H.; Sugimura, Y.; Yoshikawa, K.; Hayward, S.W.; Hirano, K. Evidence that the prostate-specific antigen (psa)/zn2+ axis may play a role in human prostate cancer cell invasion. Cancer Lett. 2004, 207, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Uzzo, R.G.; Leavis, P.; Hatch, W.; Gabai, V.L.; Dulin, N.; Zvartau, N.; Kolenko, V.M. Zinc inhibits nuclear factor-kappa b activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 2002, 8, 3579–3583. [Google Scholar]
- Uzzo, R.G.; Crispen, P.L.; Golovine, K.; Makhov, P.; Horwitz, E.M.; Kolenko, V.M. Diverse effects of zinc on nf-kappab and ap-1 transcription factors: Implications for prostate cancer progression. Carcinogenesis 2006, 27, 1980–1990. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, I.J.; Kang, T.W.; Choi, C.; Kim, K.K.; Kim, M.S.; Nam, K.I.; Jung, C. Hoxb13 downregulates intracellular zinc and increases nf-kappab signaling to promote prostate cancer metastasis. Oncogene 2014, 33, 4558–4567. [Google Scholar] [CrossRef]
- Ishii, K.; Usui, S.; Sugimura, Y.; Yamamoto, H.; Yoshikawa, K.; Hirano, K. Inhibition of aminopeptidase n (ap-n) and urokinase-type plasminogen activator (upa) by zinc suppresses the invasion activity in human urological cancer cells. Biol. Pharm. Bull. 2001, 24, 226–230. [Google Scholar] [CrossRef]
- Adzersen, K.H.; Jess, P.; Freivogel, K.W.; Gerhard, I.; Bastert, G. Raw and cooked vegetables, fruits, selected micronutrients and breast cancer risk: A case-control study in germany. Nutr. Cancer 2003, 46, 131–137. [Google Scholar] [CrossRef]
- Schlag, P.; Seeling, W.; Merkle, P.; Betzler, M. [changes of serum-zinc in breast cancer (author’s transl)]. Langenbecks Arch. Chir. 1978, 346, 129–133. [Google Scholar] [CrossRef]
- Geraki, K.; Farquharson, M.J.; Bradley, D.A. Concentrations of fe, cu and zn in breast tissue: A synchrotron xrf study. Phys. Med. Biol. 2002, 47, 2327–2339. [Google Scholar] [CrossRef]
- Margalioth, E.J.; Schenker, J.G.; Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 1983, 52, 868–872. [Google Scholar] [CrossRef]
- Santoliquido, P.M.; Southwick, H.W.; Olwin, J.H. Trace metal levels in cancer of the breast. Surg. Gynecol. Obstet. 1976, 142, 65–70. [Google Scholar] [PubMed]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef] [PubMed]
- Kasper, G.; Weiser, A.A.; Rump, A.; Sparbier, K.; Dahl, E.; Hartmann, A.; Wild, P.; Schwidetzky, U.; Castanos-Velez, E.; Lehmann, K. Expression levels of the putative zinc transporter liv-1 are associated with a better outcome of breast cancer patients. Int. J. Cancer 2005, 117, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Takatani-Nakase, T.; Hatano, Y.; Kawahara, S.; Nakase, I.; Takahashi, K. Zinc and its transporter zip6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 2017, 591, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Takatani-Nakase, T. Zinc transporters and the progression of breast cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Ackland, M.L.; Hiscox, S.; Taylor, K.M. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel zip6 and stat3 (signal transducer and activator of transcription 3). Biochem. J. 2013, 455, 229–237. [Google Scholar] [CrossRef]
- Shen, H.; Qin, H.; Guo, J. Concordant correlation of liv-1 and e-cadherin expression in human breast cancer cell mcf-7. Mol. Biol. Rep. 2009, 36, 653–659. [Google Scholar] [CrossRef]
- Gumulec, J.; Masarik, M.; Krizkova, S.; Adam, V.; Hubalek, J.; Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Kizek, R. Insight to physiology and pathology of zinc(ii) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011, 18, 5041–5051. [Google Scholar] [CrossRef]
- Manning, D.L.; Robertson, J.F.; Ellis, I.O.; Elston, C.W.; McClelland, R.A.; Gee, J.M.; Jones, R.J.; Green, C.D.; Cannon, P.; Blamey, R.W.; et al. Oestrogen-regulated genes in breast cancer: Association of pliv1 with lymph node involvement. Eur. J. Cancer 1994, 30A, 675–678. [Google Scholar] [CrossRef]
- Schneider, J.; Ruschhaupt, M.; Buness, A.; Asslaber, M.; Regitnig, P.; Zatloukal, K.; Schippinger, W.; Ploner, F.; Poustka, A.; Sultmann, H. Identification and meta-analysis of a small gene expression signature for the diagnosis of estrogen receptor status in invasive ductal breast cancer. Int. J. Cancer 2006, 119, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R. New strategies in estrogen receptor-positive breast cancer. Clin. Cancer Res. 2010, 16, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Tamoxifen: A most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003, 2, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Kelleher, S.L. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (t47d) cells. Exp. Cell Res. 2010, 316, 366–375. [Google Scholar] [CrossRef]
- Taylor, K.M.; Vichova, P.; Jordan, N.; Hiscox, S.; Hendley, R.; Nicholson, R.I. Zip7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology 2008, 149, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Levy, B.A.; Desouki, M.M.; Zou, J.; Bagasra, O.; Johnson, L.A.; Hanna, N.; Franklin, R.B. Decreased zinc and downregulation of zip3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 2011, 12, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.M.; Cousins, R.J.; et al. Aberrant expression of zinc transporter zip4 (slc39a4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef]
- Lipman, T.O.; Diamond, A.; Mellow, M.H.; Patterson, K.Y. Esophageal zinc content in human squamous esophageal cancer. J. Am. Coll. Nutr. 1987, 6, 41–46. [Google Scholar] [CrossRef]
- Jin, J.; Li, Z.; Liu, J.; Wu, Y.; Gao, X.; He, Y. Knockdown of zinc transporter zip5 (slc39a5) expression significantly inhibits human esophageal cancer progression. Oncol. Rep. 2015, 34, 1431–1439. [Google Scholar] [CrossRef]
- Huang, C.; Cui, X.; Sun, X.; Yang, J.; Li, M. Zinc transporters are differentially expressed in human non-small cell lung cancer. Oncotarget 2016, 7, 66935–66943. [Google Scholar] [CrossRef]
- Jing, H.W.; Kong, C.Z.; Liu, T.; Zeng, Y.; Zhang, Z. Zinc transporter 1 (znt1) is overexpressed in bladder cancer and promotes the proliferation and invasion of bladder cancer biu87 cells. Int. J. Clin. Exp. Med. 2018, 11, 5323–5331. [Google Scholar]
- Tashiro, H.; Kawamoto, T.; Okubo, T.; Koide, O. Variation in the distribution of trace elements in hepatoma. Biol. Trace Elem. Res. 2003, 95, 49–63. [Google Scholar] [CrossRef]
- Franz, M.C.; Anderle, P.; Burzle, M.; Suzuki, Y.; Freeman, M.R.; Hediger, M.A.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Aspects Med. 2013, 34, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Fenselau, C.C.; Franklin, R.B. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 2011, 105, 589–599. [Google Scholar] [CrossRef]
- Costello, L.C.; Zou, J.; Franklin, R.B. In situ clinical evidence that zinc levels are decreased in breast invasive ductal carcinoma. Cancer Causes Control. 2016, 27, 729–735. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B.; Feng, P.; Tan, M.; Bagasra, O. Zinc and prostate cancer: A critical scientific, medical and public interest issue (united states). Cancer Causes Control. 2005, 16, 901–915. [Google Scholar] [CrossRef]
- Thiers, R.E.; Vallee, B.L. Distribution of metals in subcellular fractions of rat liver. J. Biol. Chem. 1957, 226, 911–920. [Google Scholar]
- Smeyers-Verbeke, J.; May, C.; Drochmans, P.; Massart, D.L. The determination of cu, zn and mn in subcellular rat liver fractions. Anal. Biochem. 1977, 83, 746–753. [Google Scholar] [CrossRef]
- Franklin, R.B.; Milon, B.; Feng, P.; Costello, L.C. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front. Biosci 2005, 10, 2230–2239. [Google Scholar] [CrossRef]
- Marshall, K.M.; Laval, M.; Estacio, O.; Hudson, D.F.; Kalitsis, P.; Shulkes, A.; Baldwin, G.S.; Patel, O. Activation by zinc of the human gastrin gene promoter in colon cancer cells in vitro and in vivo. Metallomics 2015, 7, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Liu, Y.Y.; Zou, J.; Franklin, R.B.; Costello, L.C.; Feng, P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate 1999, 40, 200–207. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate 2002, 52, 311–318. [Google Scholar] [CrossRef]
- Feng, P.; Liang, J.Y.; Li, T.L.; Guan, Z.X.; Zou, J.; Franklin, R.; Costello, L.C. Zinc induces mitochondria apoptogenesis in prostate cells. Mol. Urol. 2000, 4, 31–36. [Google Scholar] [PubMed]
- Kriedt, C.L.; Baldassare, J.; Shah, M.; Klein, C. Zinc functions as a cytotoxic agent for prostate cancer cells independent of culture and growth conditions. J. Exp. Ther. Oncol. 2010, 8, 287–295. [Google Scholar] [PubMed]
- Shah, M.R.; Kriedt, C.L.; Lents, N.H.; Hoyer, M.K.; Jamaluddin, N.; Klein, C.; Baldassare, J. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J. Exp. Clin. Cancer Res. 2009, 28, 84. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Pantisano, V.; Puca, R.; Porru, M.; Aiello, A.; Grasselli, A.; Leonetti, C.; Safran, M.; Rechavi, G.; Givol, D.; et al. Zinc downregulates hif-1alpha and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 2010, 5, e15048. [Google Scholar] [CrossRef]
- Ishii, K.; Usui, S.; Sugimura, Y.; Yoshida, S.; Hioki, T.; Tatematsu, M.; Yamamoto, H.; Hirano, K. Aminopeptidase n regulated by zinc in human prostate participates in tumor cell invasion. Int. J. Cancer 2001, 92, 49–54. [Google Scholar] [CrossRef]
- Golovine, K.; Makhov, P.; Uzzo, R.G.; Shaw, T.; Kunkle, D.; Kolenko, V.M. Overexpression of the zinc uptake transporter hzip1 inhibits nuclear factor-kappab and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008, 14, 5376–5384. [Google Scholar] [CrossRef]
- Wetherell, D.; Baldwin, G.S.; Shulkes, A.; Bolton, D.; Ischia, J.; Patel, O. Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of hif1alpha. Oncotarget 2018, 9, 8463–8477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Effect of zinc on prostatic tumorigenicity in nude mice. Ann. N Y Acad. Sci. 2003, 1010, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Mukhtar, H.; Beck, F.W.; Adhami, V.M.; Siddiqui, I.A.; Din, M.; Hafeez, B.B.; Kucuk, O. Dietary zinc and prostate cancer in the tramp mouse model. J. Med. Food 2010, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- To, P.K.; Do, M.H.; Cho, Y.S.; Kwon, S.Y.; Kim, M.S.; Jung, C. Zinc inhibits expression of androgen receptor to suppress growth of prostate cancer cells. Int. J. Mol. Sci. 2018, 19, 3062. [Google Scholar] [CrossRef]
- Banudevi, S.; Elumalai, P.; Sharmila, G.; Arunkumar, R.; Senthilkumar, K.; Arunakaran, J. Protective effect of zinc on n-methyl-n-nitrosourea and testosterone-induced prostatic intraepithelial neoplasia in the dorsolateral prostate of sprague dawley rats. Exp. Biol. Med. 2011, 236, 1012–1021. [Google Scholar] [CrossRef]
- Kristal, A.R.; Stanford, J.L.; Cohen, J.H.; Wicklund, K.; Patterson, R.E. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomark. Prev. 1999, 8, 887–892. [Google Scholar]
- Epstein, M.M.; Kasperzyk, J.L.; Andren, O.; Giovannucci, E.L.; Wolk, A.; Hakansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Aconitase activity, citrate oxidation and zinc inhibition in rat ventral prostate. Enzyme 1981, 26, 281–287. [Google Scholar] [CrossRef]
- Gallus, S.; Foschi, R.; Negri, E.; Talamini, R.; Franceschi, S.; Montella, M.; Ramazzotti, V.; Tavani, A.; Dal Maso, L.; La Vecchia, C. Dietary zinc and prostate cancer risk: A case-control study from italy. Eur. Urol. 2007, 52, 1052–1056. [Google Scholar] [CrossRef]
- Lagiou, P.; Wuu, J.; Trichopoulou, A.; Hsieh, C.C.; Adami, H.O.; Trichopoulos, D. Diet and benign prostatic hyperplasia: A study in greece. Urology 1999, 54, 284–290. [Google Scholar] [CrossRef]
- Gonzalez, A.; Peters, U.; Lampe, J.W.; White, E. Zinc intake from supplements and diet and prostate cancer. Nutr. Cancer 2009, 61, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
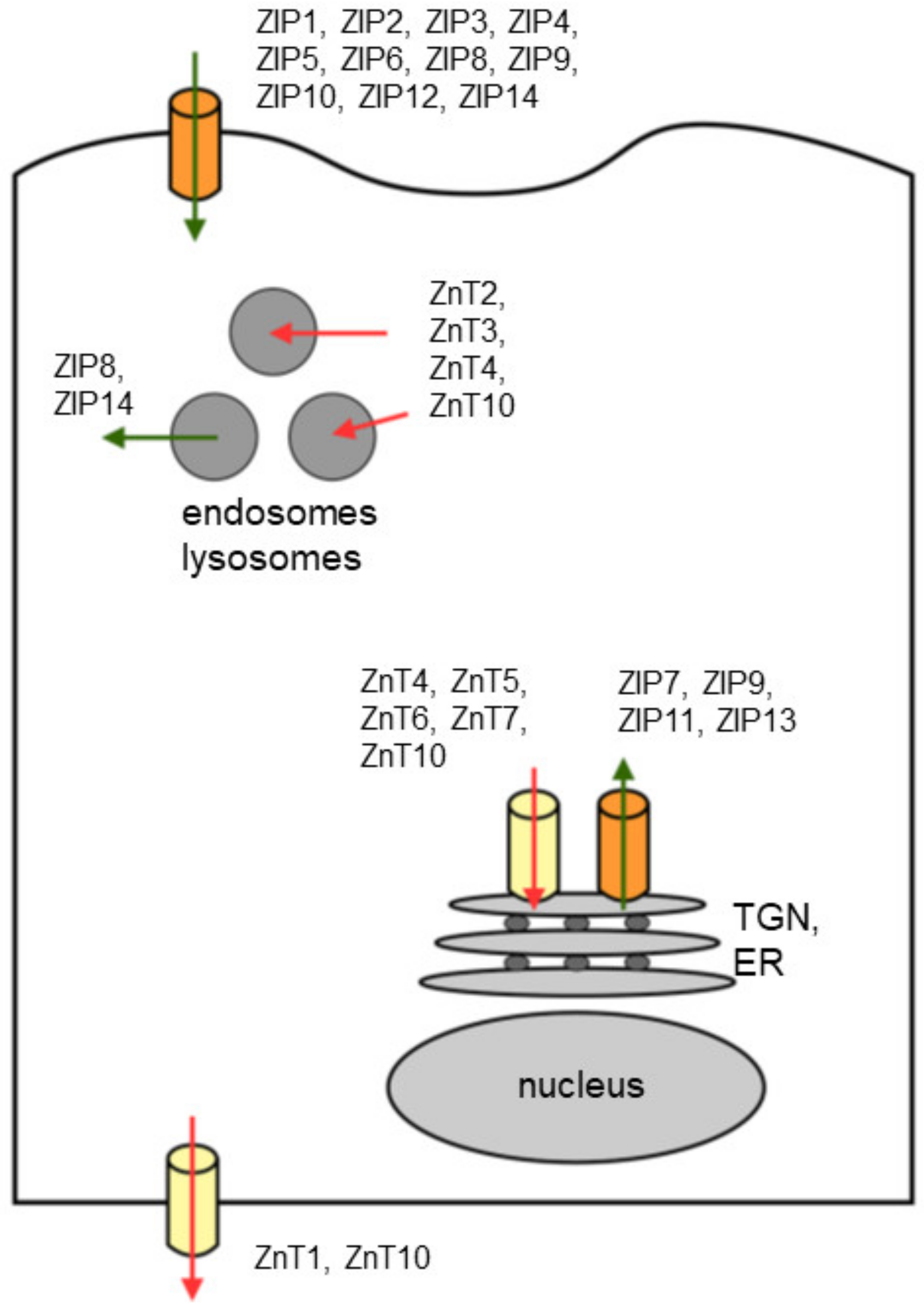
| Protein | Gene Locus | Tissue/Cell Distribution | Subcellular Localization | References |
|---|---|---|---|---|
| ZIP1/ZIRTL | 1q21 | wide spread | plasma membrane, | [26,27,28] |
| ZIP2/Eti-1/6A1 | 14q11.1 | wide spread | plasma membrane | [29,30,31] |
| ZIP3 | 19p13.3 | wide spread, predominant in testis | plasma membrane | [32,33] |
| ZIP4 | 8q24.3 | gastrointestinal tract, kidney, hippocampal neurons | plasma membrane | [34,35,36] |
| ZIP5/LZT-Hs7 | 12q13.13 | pancreas, kidney, liver, stomach, intestine | plasma membrane | [37,38,39] |
| ZIP6/LIV1 | 18q12.1 | widespread | plasma membrane | [40,41] |
| ZIP7/HKE4 | 6p21.3 | widespread | ER, Golgi, intracellular vesicles | [42,43,44] |
| ZIP8/BIGM103/LZT-Hs6 | 4q22-q24 | widespread, predominant in pancreas | plasma membrane, lysosomes, endosomes, mitochondria | [45,46] |
| ZIP9 | 14q24.1 | widespread | plasma membrane, trans-Golgi (TGN) | [47,48] |
| ZIP10/LZT-Hs2 | 2q33.1 | brain, liver, erythroid, kidney | plasma membrane | [40,49,50,51] |
| ZIP11 | 17q25.1 | testis, digestive system | TGN, cytoplasm and nuclei | [52,53] |
| ZIP12 | 10p12.33 | brain, lung, testis, retina | plasma membrane, | [54,55] |
| ZIP13 | 11p11.12 | widespread | intracellular vesicles, Golgi | [56,57] |
| ZIP14 | 8p21.2 | widespread | plasma membrane, endosomes | [58,59,60,61,62] |
| Protein | Gene Locus | Tissue/Cell Distribution | Subcellular Localization | References |
|---|---|---|---|---|
| ZnT1 | 1q32.3 | widespread | plasma membrane | [25,63,64] |
| ZnT2 | 1p35.3 | mammary gland, prostate, retina, pancreas, small intestine, kidney | plasma membrane, endosomes, lysosomes, secretory vesicles and mitochondria | [65,66,67,68] |
| ZnT3 | 2p23.3 | brain, testes, pancreas | synaptic vesicles | [69,70,71,72] |
| ZnT4/ Dri27 | 15q21.1 | widespread, predominant in mammary gland, placenta, prostate, brain and kidney | plasma membrane, endosomes, secretory vesicles | [73,74,75] |
| ZnT5/ ZTL1 | 5q13.1 | widespread, predominant in pancreas, liver. kidney | TGN, plasma membrane | [76,77,78] |
| ZnT6 | 2p22.3 | widespread | TGN, unknown vesicles | [79,80] |
| ZnT7 | 1p21.2 | widespread, enriched in stomach, prostate, retina, pancreas, testis and muscle | Golgi, unknown vesicles | [81,82,83] |
| ZnT8 | 1q41 | pancreas, thyroid, adrenal gland, testis | secretory vesicles | [84,85,86] |
| ZnT9/ C4orf1 | 4p13 | widespread | cytoplasm, nucleus | [87,88] |
| ZnT10 | 1q41 | brain, retina, liver | endosomes, endosomes, plasma membrane | [89,90,91] |
| Cells | Animals | Zinc Dosages | Delivery | Effects | References |
|---|---|---|---|---|---|
| PC3, LNCaP | in vitro | up to 1 µg/mL ZnSO4 | culture media | Inhibition of cell growth: induction of apoptosis by G2/M arrest and increase of p21Waf/Cip1/Sdi1 expression | [153,154,155] |
| PC3, LNCaP | in vitro | 50–150 μM zinc acetate | matrigel | Inhibition of cell invasion: Suppression of PSA and uPA activities | [113,159] |
| PC-3, DU145 | in vitro | 0.06–0.55 μg/mL ZnSO4 | culture media | Inhibition of cell metastasis by regulation NF-κB and c-IAP2 activities; stimulation of AP-1; suppressed expression of VEGF, IL-6, IL-8 and MMP-9 | [114,115] |
| PC3ZIP1 | in vitro; C.B.17 SCID mice | 1.5 μg/mL ZnSO4; 2000 ppm ZnSO4 | culture media; drinking water | Overexpression of ZIP1 reduced cell growth and invasion by Inhibition of NF-κB activity | [114,115,160] |
| PC3 | NOD/SCID mice | 200 µL of 3 mM zinc acetate | intratumoral injection | Inhibition of tumor growth enhancement of animal survival | [157] |
| PC3 | NOD/SCID mice | 3–20 mg/kg ZnCl2 | intraperitoneal injection | No effects on xenograft tumor cell growth | [161] |
| PC3 | nude mice | ZnSO4 (30–45 μg/day) for 28 days | osmotic pumps | Inhibition of tumor growth by increased Bax/Bcl-2 protein expression | [162] |
| Transgenic prostate cancer | TRAMP mice | 0.85, 30, or 150 ppm zinc carbonate (52.1% Zn) for 22 weeks | pellet | Increased tumor weights upon deficient or high zinc uptake | [163] |
| TRAMP-C2 | C57BL/6 mice | 10 mg/kg ZnCl2 for 2 weeks | intraperitoneal injection | Repressed tumor growth and androgen receptor expression | [164] |
| MNU and testosterone-induced PIN | Sprague Dawley rat | 100 ppm ZnCl2 for 20 weeks | drinking water | Reverse effects on MNU and testosterone-mediated PIN | [165] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. https://doi.org/10.3390/ijms21082991
To PK, Do MH, Cho J-H, Jung C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. International Journal of Molecular Sciences. 2020; 21(8):2991. https://doi.org/10.3390/ijms21082991
Chicago/Turabian StyleTo, Phuong Kim, Manh Hung Do, Jin-Hyoung Cho, and Chaeyong Jung. 2020. "Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics" International Journal of Molecular Sciences 21, no. 8: 2991. https://doi.org/10.3390/ijms21082991
APA StyleTo, P. K., Do, M. H., Cho, J.-H., & Jung, C. (2020). Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. International Journal of Molecular Sciences, 21(8), 2991. https://doi.org/10.3390/ijms21082991




