ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance
Abstract
1. Introduction
2. Results
2.1. Loss of Doxorubicin Resistance Induces a Lower Cell Viability
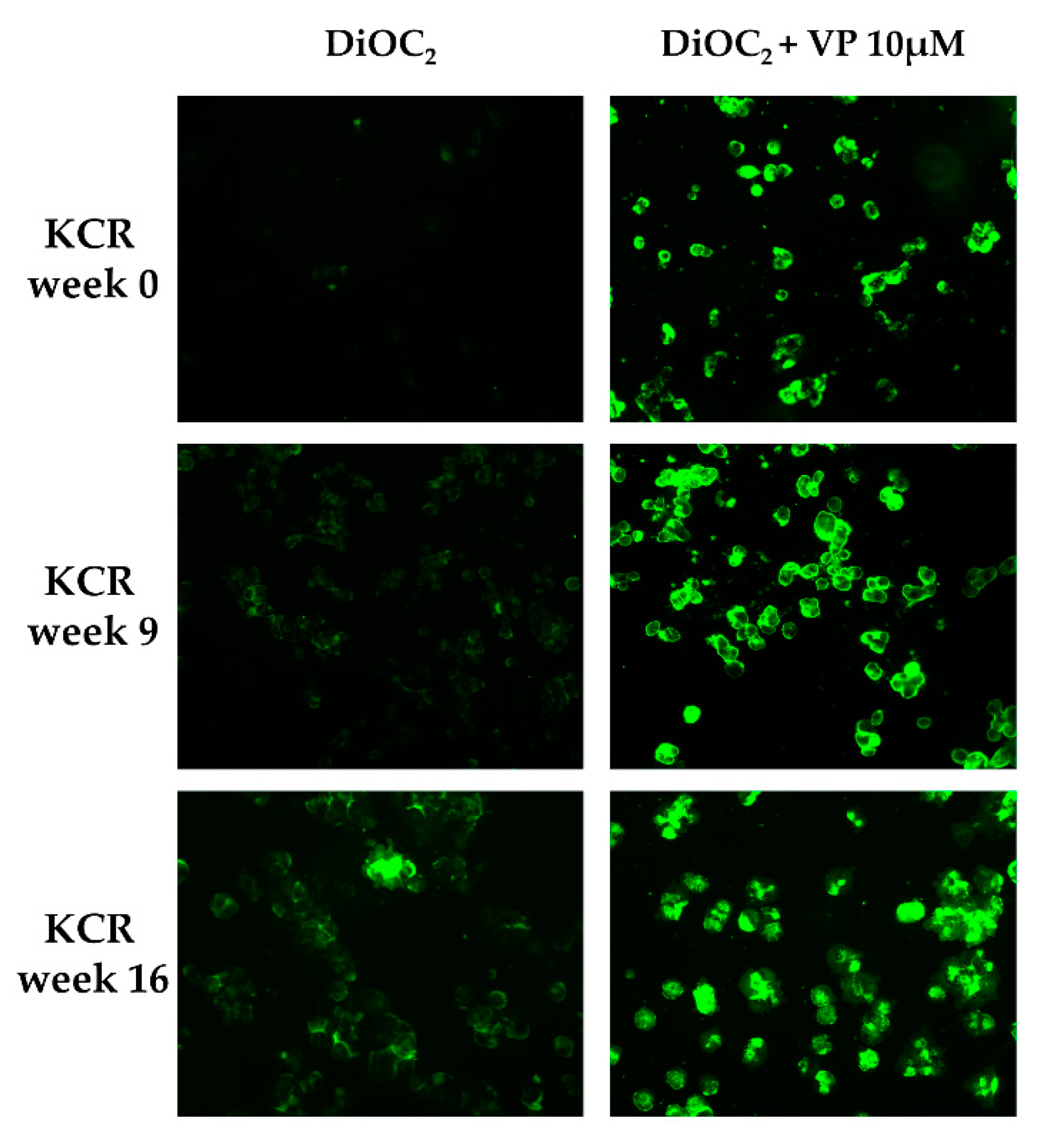
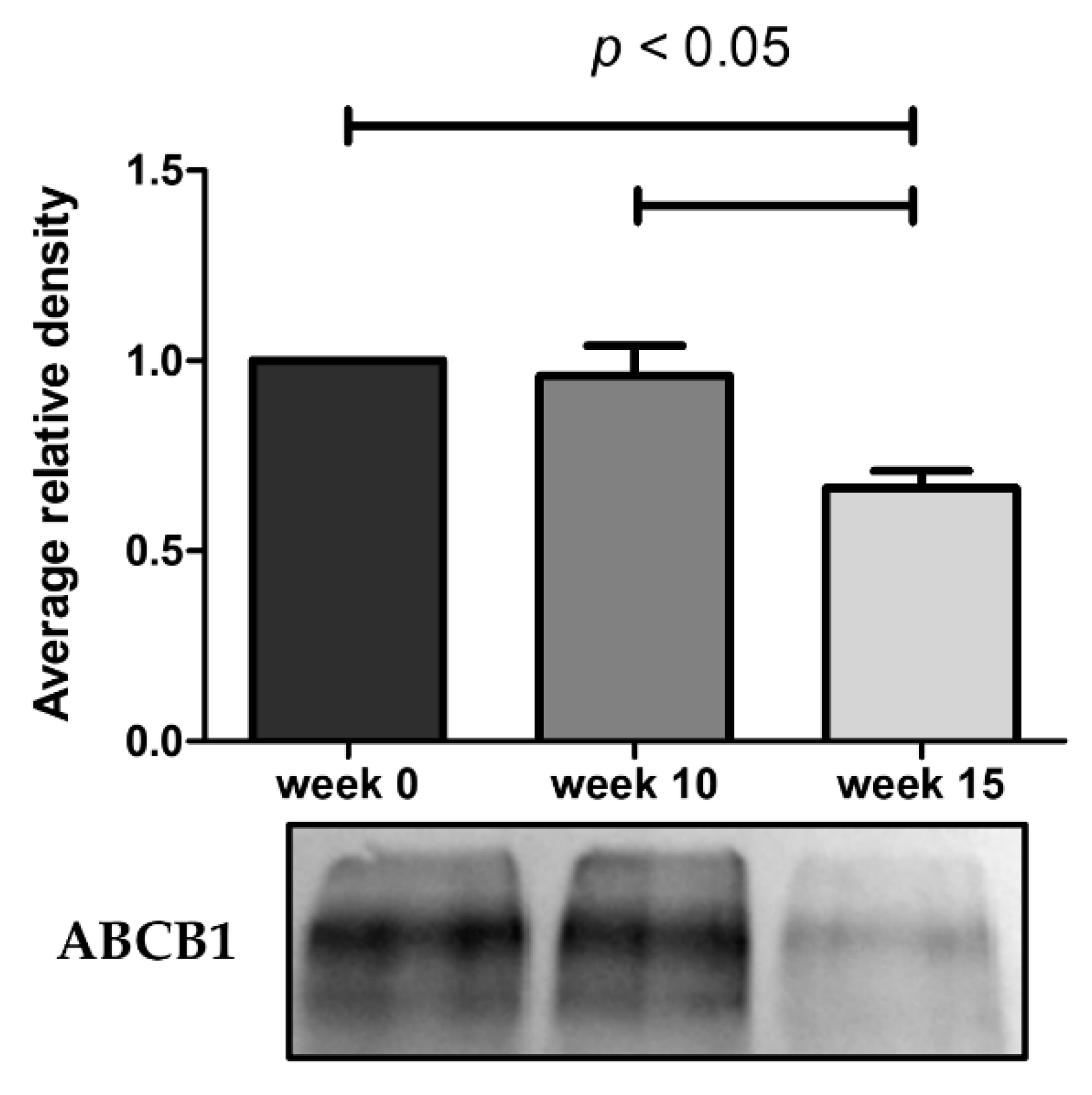
2.2. Loss of Doxorubicin Resistance is Accompanied by a Decrease in ABCB1 Activity
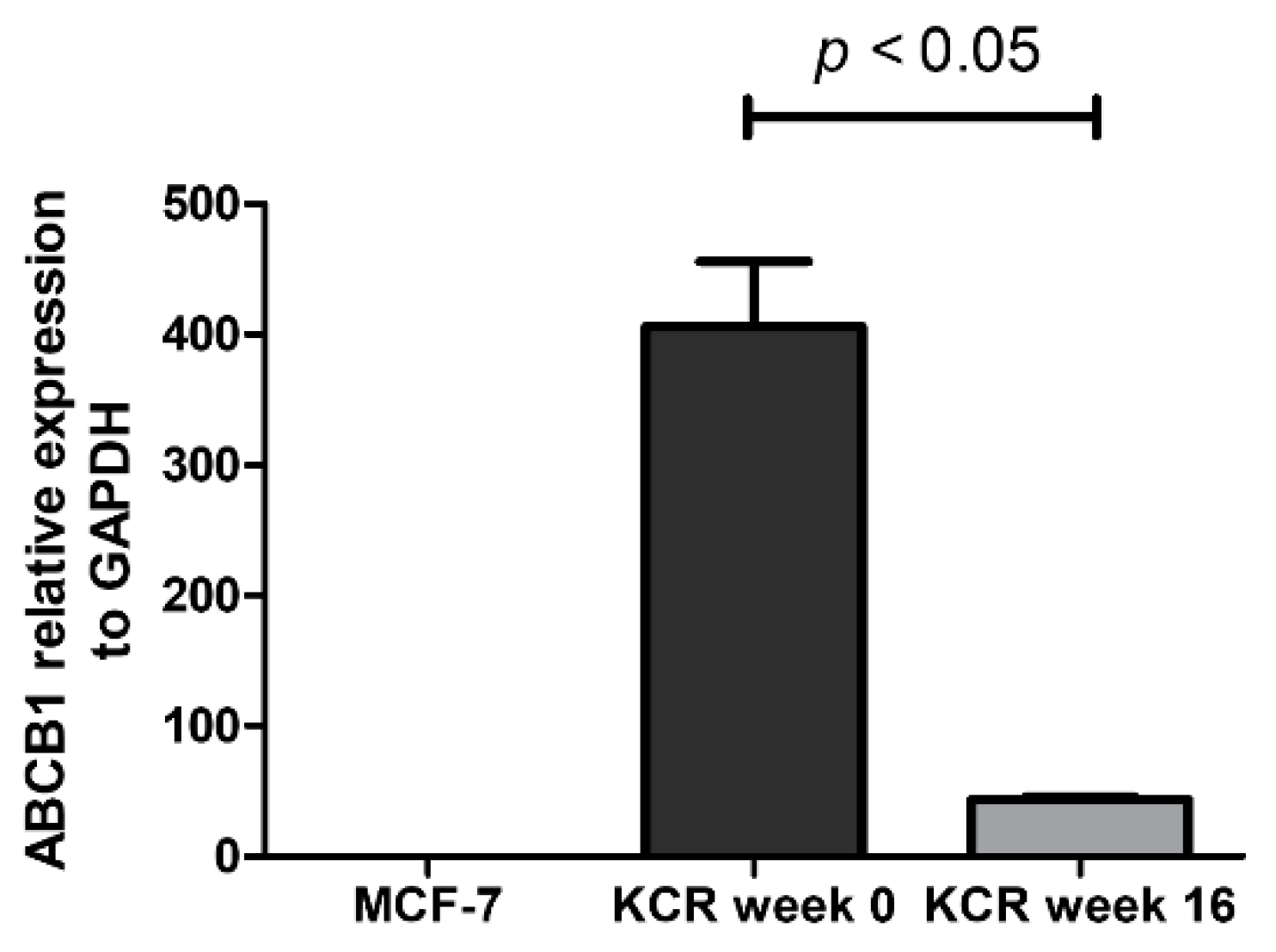
2.3. ABCB1 is Downregulated in KCR Cells without Doxorubicin for 16 Weeks
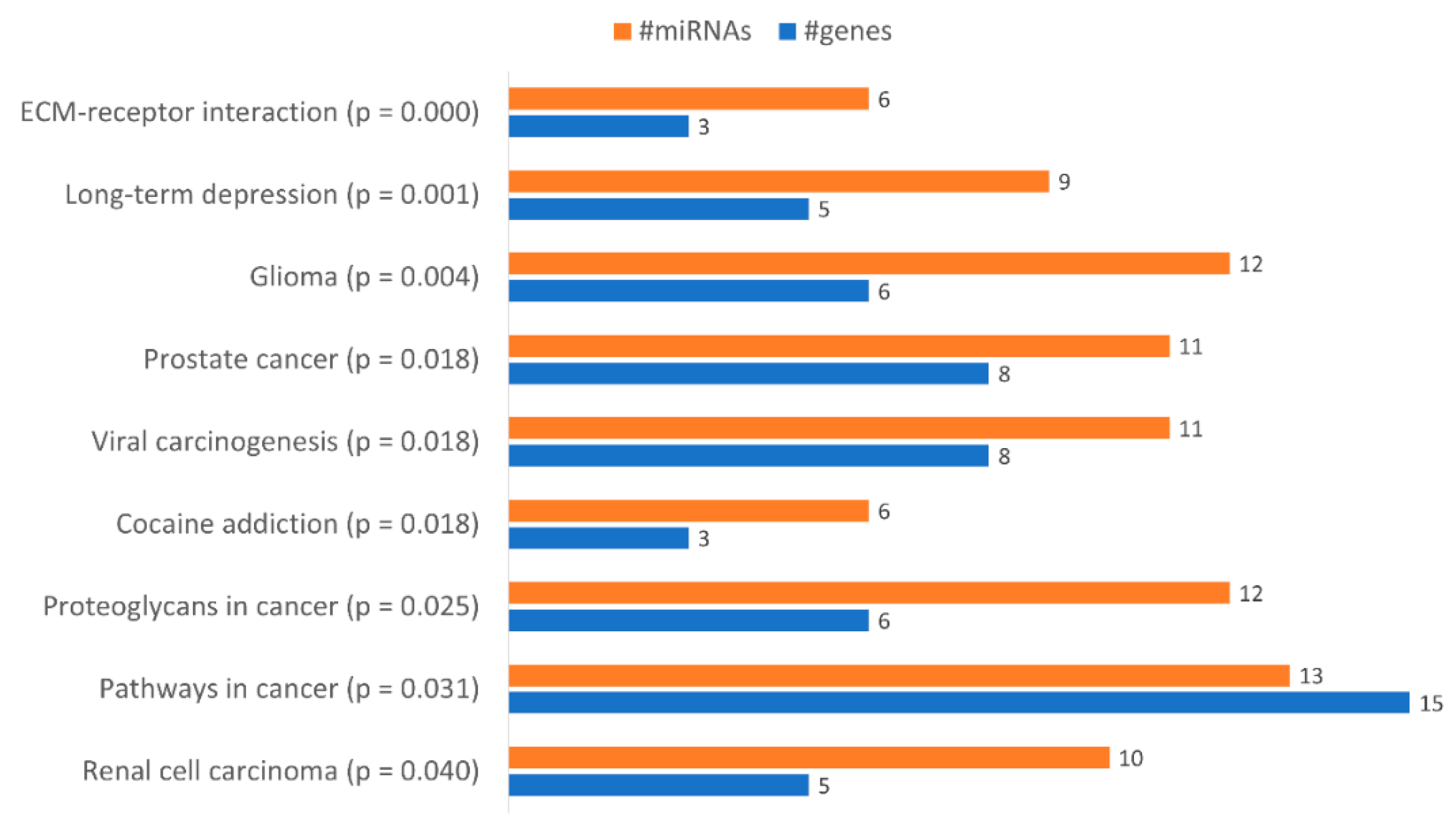
2.4. miRNAs are Differently Expressed in KCR Cell Line with Time
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability (MTT Assay)
4.3. Evaluation of ABCB1 Activity by Fluorescence Microscopy
4.4. Evaluation of ABCB1 Activity by Flow Cytometry
4.5. ABCB1 Protein Expression by Western BLOT
4.5.1. Purification of Membrane Proteins
4.5.2. Western Blot
4.6. Total RNA Purification
4.7. Real-time qPCR Quantification of ABCB1
4.8. Real-Time qPCR Quantification of miRNAs
4.9. Pathway Analysis of miRNA
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDR | Cancer drug resistance |
| DOX | Doxorubicin |
References
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol. Biol. 2016, 1395, 1–18. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Dinis, J.; Gromicho, M.; Martins, C.; Laires, A.; Rueff, J. Genomics and cancer drug resistance. Curr. Pharm. Biotechnol. 2012, 13, 651–673. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef]
- Gromicho, M.; Dinis, J.; Magalhães, M.; Fernandes, A.R.; Tavares, P.; Laires, A.; Rueff, J.; Rodrigues, A.S. Development of imatinib and dasatinib resistance: Dynamics of expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and SLC22A1. Leuk. Lymphoma 2011, 52, 1980–1990. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J. MicroRNAs are important regulators of drug resistance in colorectal cancer. Biol. Chem. 2017, 398, 929–938. [Google Scholar] [CrossRef]
- Hu, W.; Tan, C.; He, Y.; Zhang, G.; Xu, Y.; Tang, J. Functional miRNAs in breast cancer drug resistance. Onco Targets Ther. 2018, 11, 1529–1541. [Google Scholar] [CrossRef]
- An, X.; Sarmiento, C.; Tan, T.; Zhu, H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm. Sin. B 2017, 7, 38–51. [Google Scholar] [CrossRef]
- Xie, T.; Huang, M.; Wang, Y.; Wang, L.; Chen, C.; Chu, X. MicroRNAs as Regulators, Biomarkers and Therapeutic Targets in the Drug Resistance of Colorectal Cancer. Cell. Physiol. Biochem. 2016, 40, 62–76. [Google Scholar] [CrossRef]
- Gomes, B.C.; Rueff, J.; Rodrigues, A.S. MicroRNAs and Cancer Drug Resistance. Methods Mol. Biol. 2016, 1395, 137–162. [Google Scholar] [CrossRef]
- Melo, S.A.; Esteller, M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. 2011, 585, 2087–2099. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Haenisch, S.; Werk, A.N.; Cascorbi, I. MicroRNAs and their relevance to ABC transporters. Br. J. Clin. Pharmacol. 2014, 77, 587–596. [Google Scholar] [CrossRef]
- Ikemura, K.; Iwamoto, T.; Okuda, M. MicroRNAs as regulators of drug transporters, drug-metabolizing enzymes, and tight junctions: Implication for intestinal barrier function. Pharmacol. Ther. 2014, 143, 217–224. [Google Scholar] [CrossRef]
- Ikemura, K.; Yamamoto, M.; Miyazaki, S.; Mizutani, H.; Iwamoto, T.; Okuda, M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol. Pharmacol. 2013, 83, 399–405. [Google Scholar] [CrossRef]
- Armada, A.; Gomes, B.C.; Viveiros, M.; Rueff, J.; Rodrigues, A.S. Regulation of ABCB1 activity by microRNA-200c and microRNA-203a in breast cancer cells: The quest for microRNAs’ involvement in cancer drug resistance. Cancer Drug Resistance 2019, 2, 897–911. [Google Scholar] [CrossRef]
- Riquelme, I.; Letelier, P.; Riffo-Campos, A.L.; Brebi, P.; Roa, J.C. Emerging Role of miRNAs in the Drug Resistance of Gastric Cancer. Int. J. Mol. Sci. 2016, 17, 424. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, H.; Xia, J.; Li, Y.; Zhang, Y.; Huang, K.; Wagar, N.; Yoon, Y.; Cho, H.T.; Scala, S.; et al. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem. Pharmacol. 2010, 79, 817–824. [Google Scholar] [CrossRef]
- Katayama, K.; Noguchi, K.; Sugimoto, Y. Regulations of P-Glycoprotein/ABCB1/MDR1 in Human Cancer Cells. Available online: https://www.hindawi.com/journals/njos/2014/476974/ (accessed on 29 March 2020).
- Tomono, T.; Yano, K.; Ogihara, T. Snail-Induced Epithelial-to-Mesenchymal Transition Enhances P-gp-Mediated Multidrug Resistance in HCC827 Cells. J. Pharm. Sci. 2017, 106, 2642–2649. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Zhang, W.; Liu, G.; Yin, D.; Li, J.; Zhang, S.; Li, H. Establishment of paclitaxel-resistant cell line and the underlying mechanism on drug resistance. Int. J. Gynecol. Cancer 2012, 22, 1450–1456. [Google Scholar] [CrossRef]
- Kars, M.D.; Iseri, O.D.; Gündüz, U.; Ural, A.U.; Arpaci, F.; Molnár, J. Development of rational in vitro models for drug resistance in breast cancer and modulation of MDR by selected compounds. Anticancer Res. 2006, 26, 4559–4568. [Google Scholar]
- Reed, K.; Hembruff, S.L.; Sprowl, J.A.; Parissenti, A.M. The temporal relationship between ABCB1 promoter hypomethylation, ABCB1 expression and acquisition of drug resistance. Pharmacogenomics J. 2010, 10, 489–504. [Google Scholar] [CrossRef]
- Gromicho, M.; Magalhães, M.; Torres, F.; Dinis, J.; Fernandes, A.R.; Rendeiro, P.; Tavares, P.; Laires, A.; Rueff, J.; Sebastião Rodrigues, A. Instability of mRNA expression signatures of drug transporters in chronic myeloid leukemia patients resistant to imatinib. Oncol. Rep. 2013, 29, 741–750. [Google Scholar] [CrossRef]
- Armada, A.; Martins, C.; Spengler, G.; Molnar, J.; Amaral, L.; Rodrigues, A.S.; Viveiros, M. Fluorimetric Methods for Analysis of Permeability, Drug Transport Kinetics, and Inhibition of the ABCB1 Membrane Transporter. Methods Mol. Biol. 2016, 1395, 87–103. [Google Scholar] [CrossRef]
- Gomes, B.C.; Rueff, J.; Rodrigues, A.S. MicroRNAs and cancer drug resistance: Over two thousand characters in search of a role. Cancer Drug Resist. 2019, 2, 618–633. [Google Scholar] [CrossRef]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef]
- O’Driscoll, L.; Clynes, M. Biomarkers and multiple drug resistance in breast cancer. Curr. Cancer Drug Targets 2006, 6, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Kulkarni, P. The Genetic/Non-genetic Duality of Drug “Resistance” in Cancer. Trends Cancer 2018, 4, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Boros, I.M.; Balint, E. Elevated level of lysine 9-acetylated histone H3 at the MDR1 promoter in multidrug-resistant cells. Cancer Sci. 2012, 103, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA–gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef]
- Tang, R.; Qi, Q.; Wu, R.; Zhou, X.; Wu, D.; Zhou, H.; Mao, Y.; Li, R.; Liu, C.; Wang, L.; et al. The polymorphic terminal-loop of pre-miR-1307 binding with MBNL1 contributes to colorectal carcinogenesis via interference with Dicer1 recruitment. Carcinogenesis 2015, 36, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zou, H.; Lee, J.-W.; Han, J.; Kim, H.C.; Cheol, J.J.; Kim, L.-S.; Kim, H. miR-1307-3p Stimulates Breast Cancer Development and Progression by Targeting SMYD4. J. Cancer 2019, 10, 441–448. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, B.; Wang, Y.; Han, T.; Zheng, L.; Sun, C.; Liu, T.; Zhang, Y.; Qiu, X.; Fan, Q. miR-34a inhibits the metastasis of osteosarcoma cells by repressing the expression of CD44. Oncol. Rep. 2013, 29, 1027–1036. [Google Scholar] [CrossRef]
- Gao, J.; Li, N.; Dong, Y.; Li, S.; Xu, L.; Li, X.; Li, Y.; Li, Z.; Ng, S.S.; Sung, J.J.; et al. miR-34a-5p suppresses colorectal cancer metastasis and predicts recurrence in patients with stage II/III colorectal cancer. Oncogene 2015, 34, 4142–4152. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, Z.-M.; Li, X.-N.; Li, Z.-F.; Wang, Y.; Geng, Y.-F.; Bai, L.; Zhang, X.-B. MiR-223 modulates multidrug resistance via downregulation of ABCB1 in hepatocellular carcinoma cells. Exp. Biol. Med. (Maywood) 2013, 238, 1024–1032. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, T.; Yan, L.; Xu, H.; Wang, Y.; Li, Y.; Wang, H.; Chen, S.; Wang, W.; Chen, C.; et al. MiR-155-3p acts as a tumor suppressor and reverses paclitaxel resistance via negative regulation of MYD88 in human breast cancer. Gene 2019, 700, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhao, F.; Wang, H.; Cai, W.; Gao, J.; Li, Y.; Cai, S. MiR-34a-5p promotes the multi-drug resistance of osteosarcoma by targeting the CD117 gene. Oncotarget 2016, 7, 28420–28434. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [CrossRef]


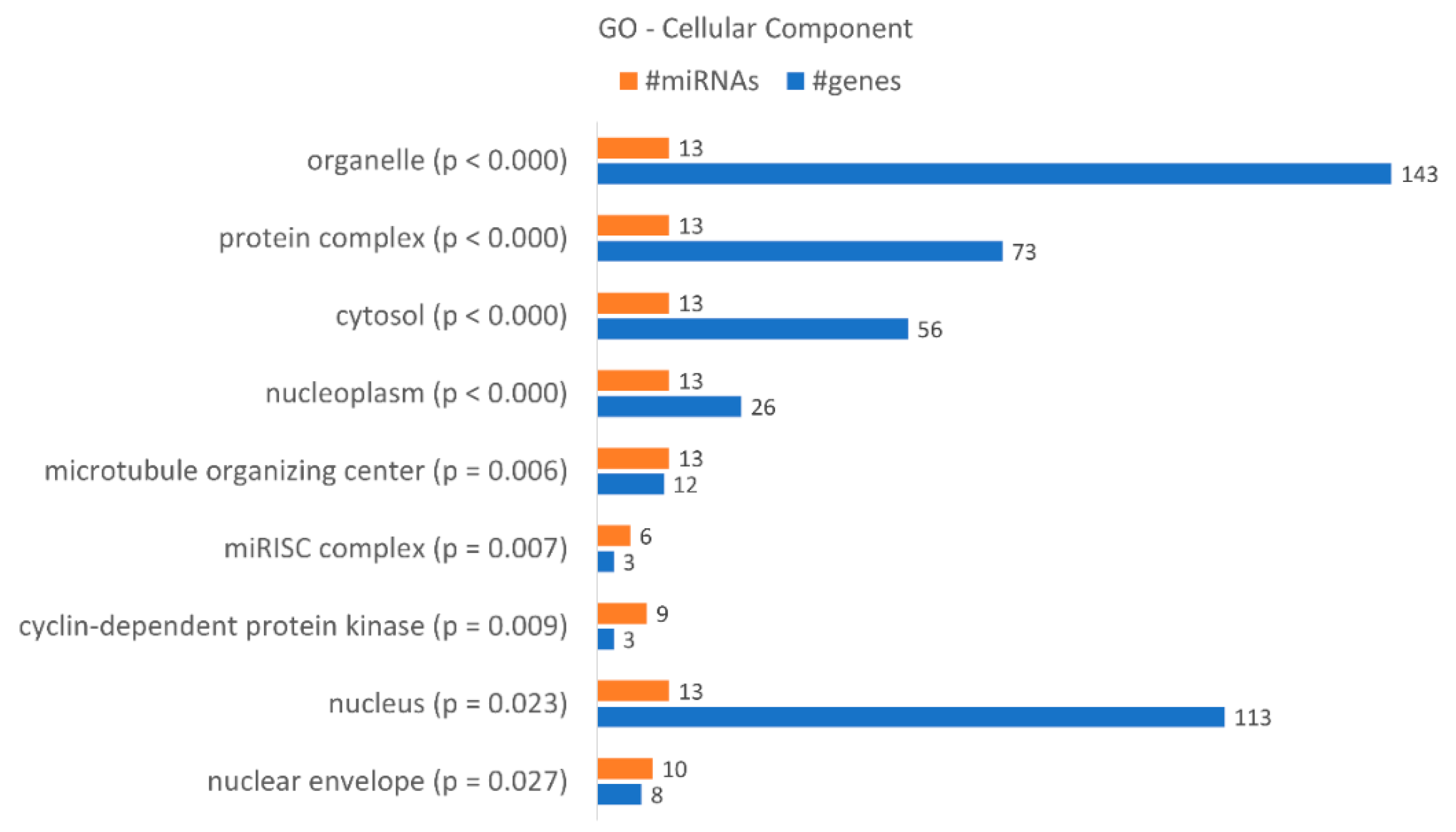
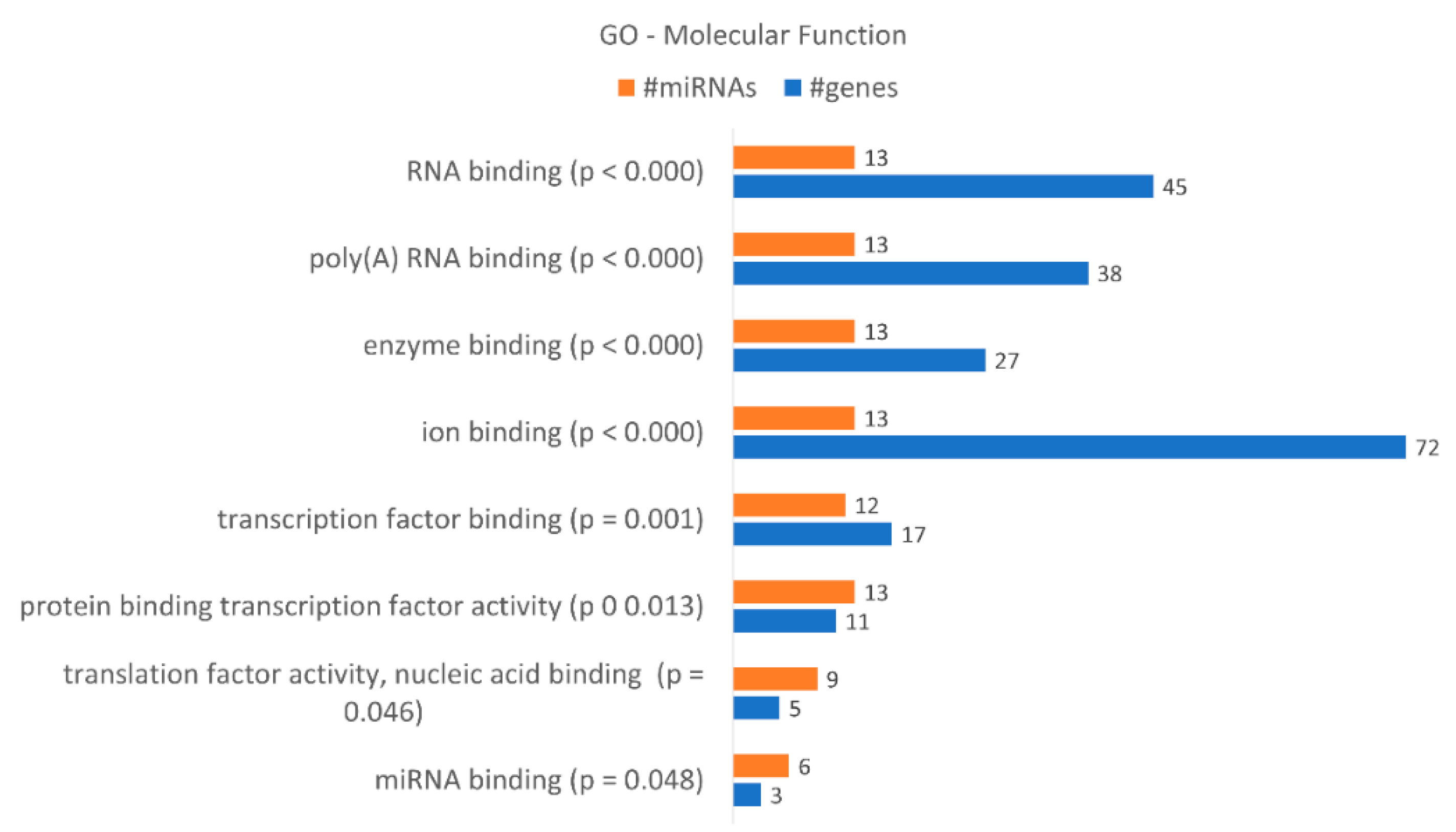
| miRNA Name | Accession Number | Fold-Change | ||
|---|---|---|---|---|
| KCR week 16 vs. KCR week 0 | Underexpressed | hsa-miR-585-3p | MIMAT0003250 | −2.8 |
| hsa-miR-34a-5p | MIMAT0000255 | −3.3 | ||
| hsa-miR-877-5p | MIMAT0004949 | −3.9 | ||
| hsa-miR-1287-5p | MIMAT0005878 | −7.0 | ||
| hsa-miR-1182 | MIMAT0005827 | −2.3 | ||
| hsa-miR-155-3p | MIMAT0004658 | Only expressed in KCR week 0 | ||
| hsa-miR-656-3p | MIMAT0003332 | −2.1 | ||
| hsa-miR-323b-5p | MIMAT0001630 | −3.0 | ||
| hsa-miR-4304 | MIMAT0016854 | Only expressed in KCR week 0 | ||
| hsa-miR-3691-5p | MIMAT0018120 | −4.6 | ||
| hsa-miR-676-5p | MIMAT0018203 | −2.4 | ||
| hsa-miR-4258 | MIMAT0016879 | Only expressed in KCR week 0 | ||
| hsa-miR-3177-3p | MIMAT0015054 | −5.3 | ||
| Overexpressed | hsa-miR-635 | MIMAT0003305 | Only expressed in KCR week 16 | |
| hsa-miR-502-5p | MIMAT0002873 | 4.1 | ||
| hsa-miR-342-3p | MIMAT0000753 | 3.1 | ||
| hsa-miR-767-5p | MIMAT0003882 | 2.3 | ||
| hsa-miR-1307-3p | MIMAT0005951 | 2.7 | ||
| hsa-miR-1207-5p | MIMAT0005871 | Only expressed in KCR week 16 | ||
| hsa-miR-548k | MIMAT0005882 | Only expressed in KCR week 16 | ||
| hsa-miR-183-3p | MIMAT0004560 | 4.7 | ||
| hsa-miR-1193 | MIMAT0015049 | Only expressed in KCR week 16 | ||
| hsa-miR-187-5p | MIMAT0004561 | Only expressed in KCR week 16 |
| Pathways in Cancer (hsa05200) | ||
|---|---|---|
| microRNA ID | Putative Targets | |
| Underexpressed | hsa-miR-34a-5p | CRKL | GNAS | ETS1 | BCL2 | CDK6 | CTNNB1 | E2F3 | LAMC1 | EP300| GNAI2 | CDKN1A |
| hsa-miR-877-5p | ITGB1 | CRKL | ETS1 | LAMC1 | |
| hsa-miR-3691-5p | ARNT | ETS1 | EP300 | |
| hsa-miR-155-3p | CDKN1A | |
| Overexpressed | hsa-miR-1307-3p | GNAS | BCL2 | CDK6 | CDKN1A |
| hsa-miR-1207-5p | BCL2 | IGF1R | CTNNB1 | EP 300 | GNAI2 | |
| hsa-miR-183-3p | NRAS | CDK6 | CTNNB1 | E2F3 | |
| hsa-miR-767-5p | NRAS | EP300 | |
| hsa-miR-187-5p | ETS1 | IGF1R | CTNNB1 | |
| hsa-miR-635 | ITGB1 | |
| hsa-miR-342-3p | ITGB1 | CRKL | GNAS | IGF1R | E2F3 | LAMC1 | EP 300 | |
| hsa-miR-548k | ARNT | CDK6 | CTNNB1 | LAMC1 | |
| hsa-miR-502-5p | NRAS | CRKL | ARNT | GNAI2 | CDKN1A | |
| Proteoglycans in Cancer (hsa05205) | ||
|---|---|---|
| microRNA ID | Putative Targets | |
| Underexpressed | hsa-miR-155-3p | CDKN1A |
| hsa-miR-877-5p | ITGB1 | |
| hsa-miR-34a-5p | THBS1 | CTNNB1 | CDKN1A | |
| Overexpressed | hsa-miR-342-3p | ITGB1 | THBS1 | IGF1R |
| hsa-miR-1207-5p | IGF1R | CTNNB1 | |
| hsa-miR-187-5p | IGF1R | CTNNB1 | |
| hsa-miR-1307-3p | THBS1 | CDKN1A | |
| hsa-miR-635 | ITGB1 | |
| hsa-miR-502-5p | NRAS | CDKN1A | |
| hsa-miR-767-5p | NRAS | |
| hsa-miR-183-3p | NRAS | CTNNB1 | |
| hsa-miR-548k | CTNNB1 | |
| ECM-Receptor Interaction (hsa04512) | ||
|---|---|---|
| microRNA ID | Putative Targets | |
| Underexpressed | hsa-miR-34a-5p | THBS1 | LAMC1 |
| hsa-miR-877-5p | ITGB1 | LAMC1 | |
| Overexpressed | hsa-miR-635 | ITGB1 |
| hsa-miR-548k | LAMC1 | |
| hsa-miR-342-3p | ITGB1 | THBS1 | LAMC1 | |
| hsa-miR-1307-3p | THBS1 | |
| GO Biological Process Terms | p-Value | Genes | miRNAs |
|---|---|---|---|
| cellular nitrogen compound metabolic process | 0.000 | 79 | 13 |
| biosynthetic process | 0.000 | 60 | 13 |
| response to stress | 0.000 | 46 | 13 |
| cellular protein modification process | 0.000 | 37 | 13 |
| gene expression | 0.000 | 24 | 11 |
| symbiosis, encompassing mutualism through parasitism | 0.000 | 16 | 12 |
| mitotic cell cycle | 0.000 | 13 | 13 |
| viral process | 0.000 | 13 | 12 |
| blood coagulation | 0.000 | 13 | 13 |
| immune system process | 0.001 | 29 | 13 |
| regulation of cell cycle | 0.001 | 11 | 11 |
| cell cycle arrest | 0.001 | 9 | 11 |
| Fc-epsilon receptor signaling pathway | 0.001 | 7 | 10 |
| cell cycle | 0.002 | 21 | 13 |
| platelet degranulation | 0.002 | 5 | 9 |
| intrinsic apoptotic signaling pathway | 0.004 | 5 | 7 |
| transcription, DNA-templated | 0.008 | 39 | 13 |
| Notch signaling pathway | 0.010 | 8 | 11 |
| platelet activation | 0.010 | 7 | 9 |
| neurotrophin TRK receptor signaling pathway | 0.010 | 7 | 10 |
| cellular response to hypoxia | 0.010 | 7 | 11 |
| fibroblast growth factor receptor signaling pathway | 0.010 | 7 | 12 |
| regulation of transcription from RNA polymerase II promoter in response to hypoxia | 0.010 | 3 | 7 |
| cellular protein metabolic process | 0.012 | 10 | 13 |
| negative regulation of translation involved in gene silencing by miRNA | 0.012 | 3 | 6 |
| cell death | 0.014 | 17 | 12 |
| nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay | 0.014 | 4 | 9 |
| positive regulation of protein insertion into mitochondrial membrane involved in apoptotic signaling pathway | 0.014 | 3 | 5 |
| 3′-UTR-mediated mRNA stabilization | 0.014 | 3 | 6 |
| mRNA processing | 0.015 | 13 | 11 |
| membrane organization | 0.015 | 12 | 11 |
| DNA methylation | 0.015 | 4 | 7 |
| positive regulation of nuclear-transcribed mRNA catabolic process, deadenylation-dependent decay | 0.015 | 3 | 7 |
| organ morphogenesis | 0.021 | 7 | 11 |
| mRNA splicing, via spliceosome | 0.022 | 8 | 11 |
| innate immune response | 0.026 | 14 | 12 |
| positive regulation of nuclear-transcribed mRNA poly(A) tail shortening | 0.026 | 3 | 7 |
| RNA splicing | 0.028 | 9 | 11 |
| mRNA metabolic process | 0.028 | 6 | 9 |
| regulation of translation | 0.028 | 6 | 9 |
| regulation of mRNA stability | 0.029 | 3 | 6 |
| chromatin organization | 0.031 | 5 | 10 |
| PML body organization | 0.031 | 2 | 5 |
| nucleobase-containing compound catabolic process | 0.032 | 15 | 12 |
| response to endoplasmic reticulum stress | 0.032 | 5 | 9 |
| nuclear-transcribed mRNA poly(A) tail shortening | 0.033 | 3 | 8 |
| epithelial cell differentiation involved in prostate gland development | 0.033 | 2 | 7 |
| catabolic process | 0.034 | 26 | 13 |
| epidermal growth factor receptor signaling pathway | 0.034 | 6 | 10 |
| phosphatidylinositol-mediated signaling | 0.034 | 5 | 9 |
| intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator | 0.034 | 4 | 9 |
| negative regulation of anoikis | 0.034 | 3 | 8 |
| regulation of viral genome replication | 0.034 | 2 | 6 |
| establishment or maintenance of microtubule cytoskeleton polarity | 0.042 | 2 | 4 |
| negative regulation of transcription from RNA polymerase II promoter | 0.043 | 19 | 13 |
| T cell differentiation in thymus | 0.043 | 4 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.C.; Honrado, M.; Armada, A.; Viveiros, M.; Rueff, J.; Rodrigues, A.S. ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance. Int. J. Mol. Sci. 2020, 21, 2985. https://doi.org/10.3390/ijms21082985
Gomes BC, Honrado M, Armada A, Viveiros M, Rueff J, Rodrigues AS. ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance. International Journal of Molecular Sciences. 2020; 21(8):2985. https://doi.org/10.3390/ijms21082985
Chicago/Turabian StyleGomes, Bruno C., Mónica Honrado, Ana Armada, Miguel Viveiros, José Rueff, and António S. Rodrigues. 2020. "ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance" International Journal of Molecular Sciences 21, no. 8: 2985. https://doi.org/10.3390/ijms21082985
APA StyleGomes, B. C., Honrado, M., Armada, A., Viveiros, M., Rueff, J., & Rodrigues, A. S. (2020). ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance. International Journal of Molecular Sciences, 21(8), 2985. https://doi.org/10.3390/ijms21082985







