A Single Nucleotide Mutation in Adenylate Cyclase Affects Vegetative Growth, Sclerotial Formation and Virulence of Botrytis cinerea
Abstract
1. Introduction
2. Results
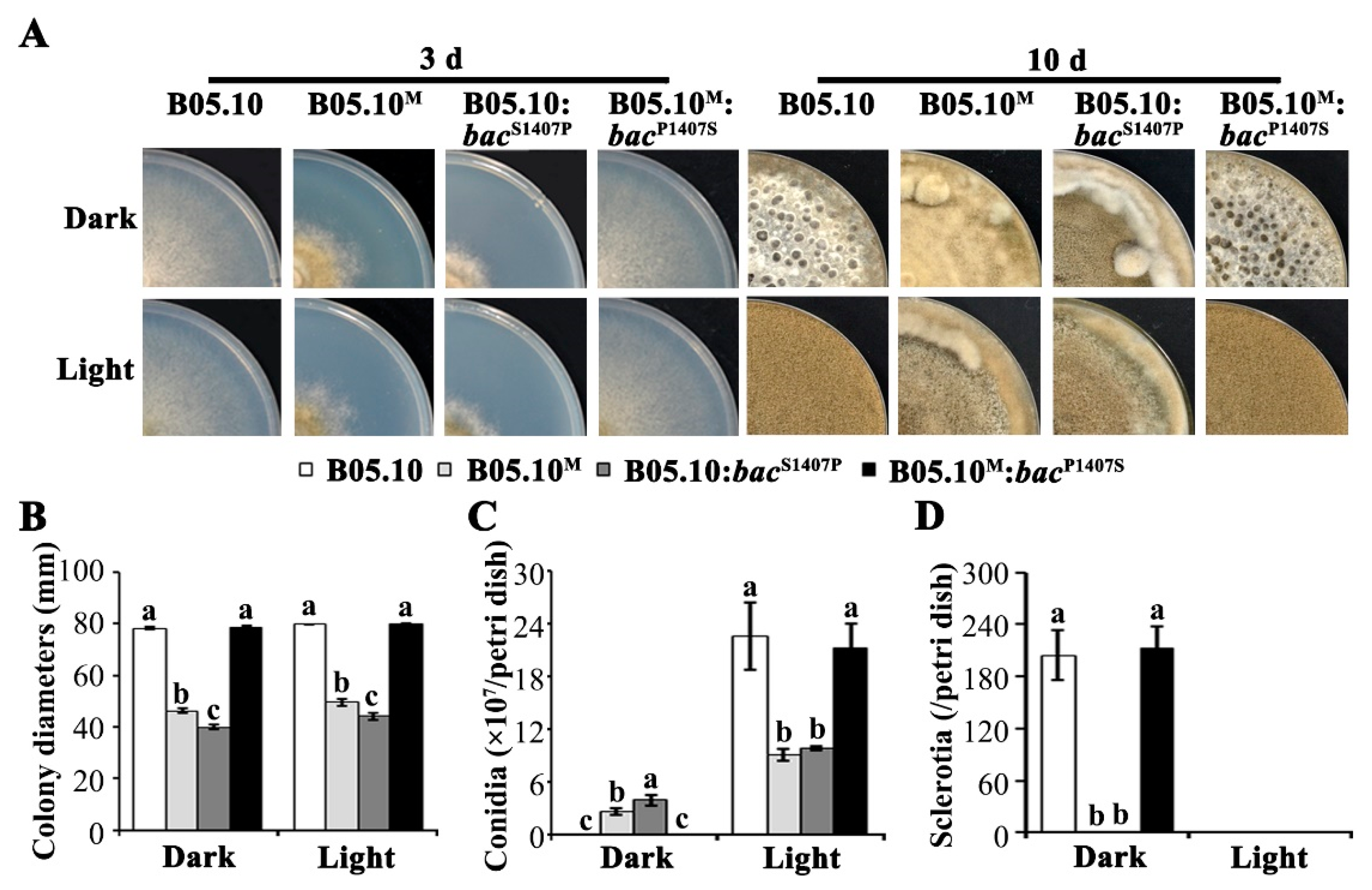
2.1. Phenotypes of B05.10 and B05.10M are Significantly Different in Vegetative Growth, Sclerotial Formation, Conidiation, and Virulence
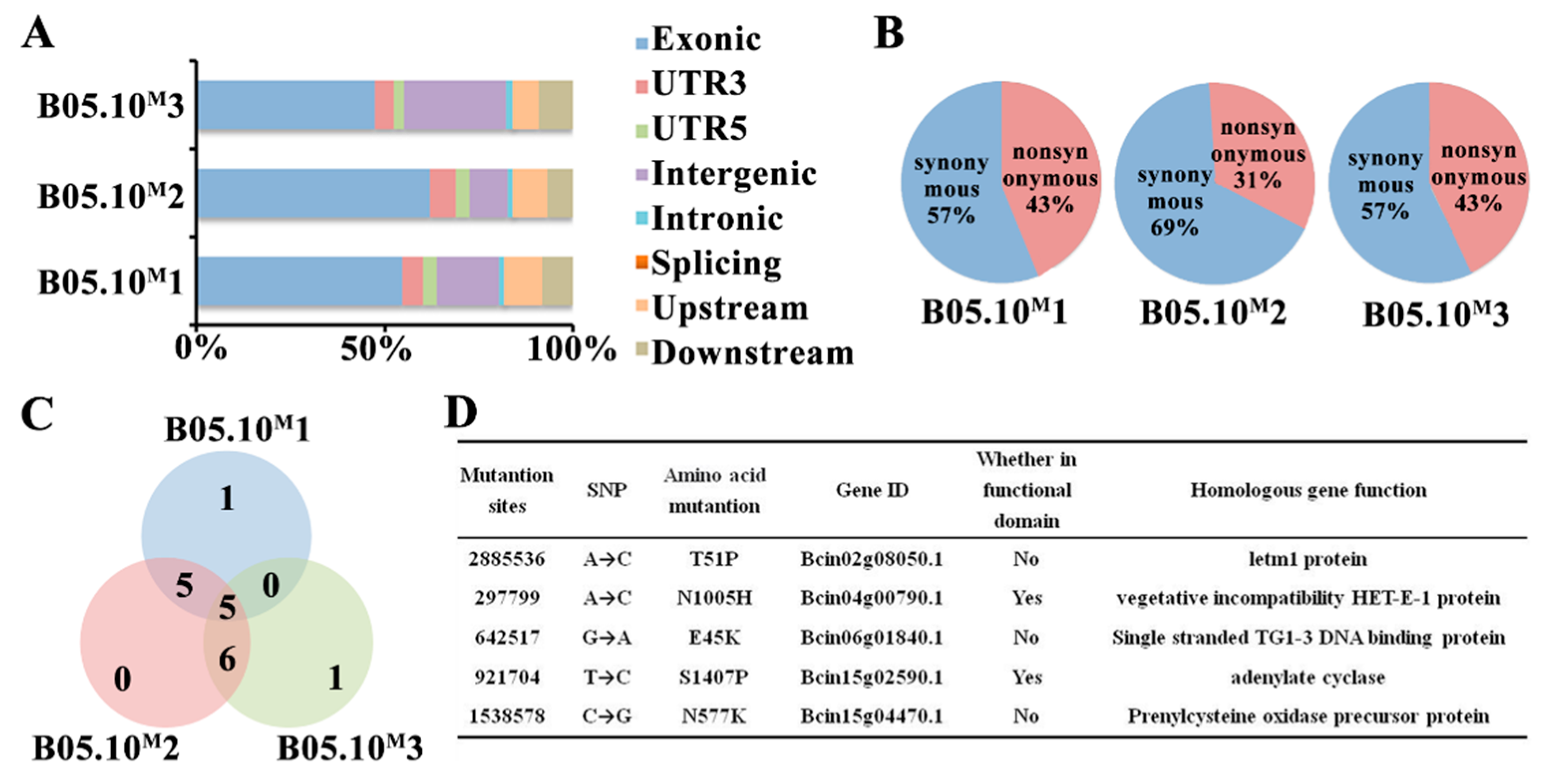
2.2. Whole-Genome Resequencing Analysis of B05.10M and B05.10
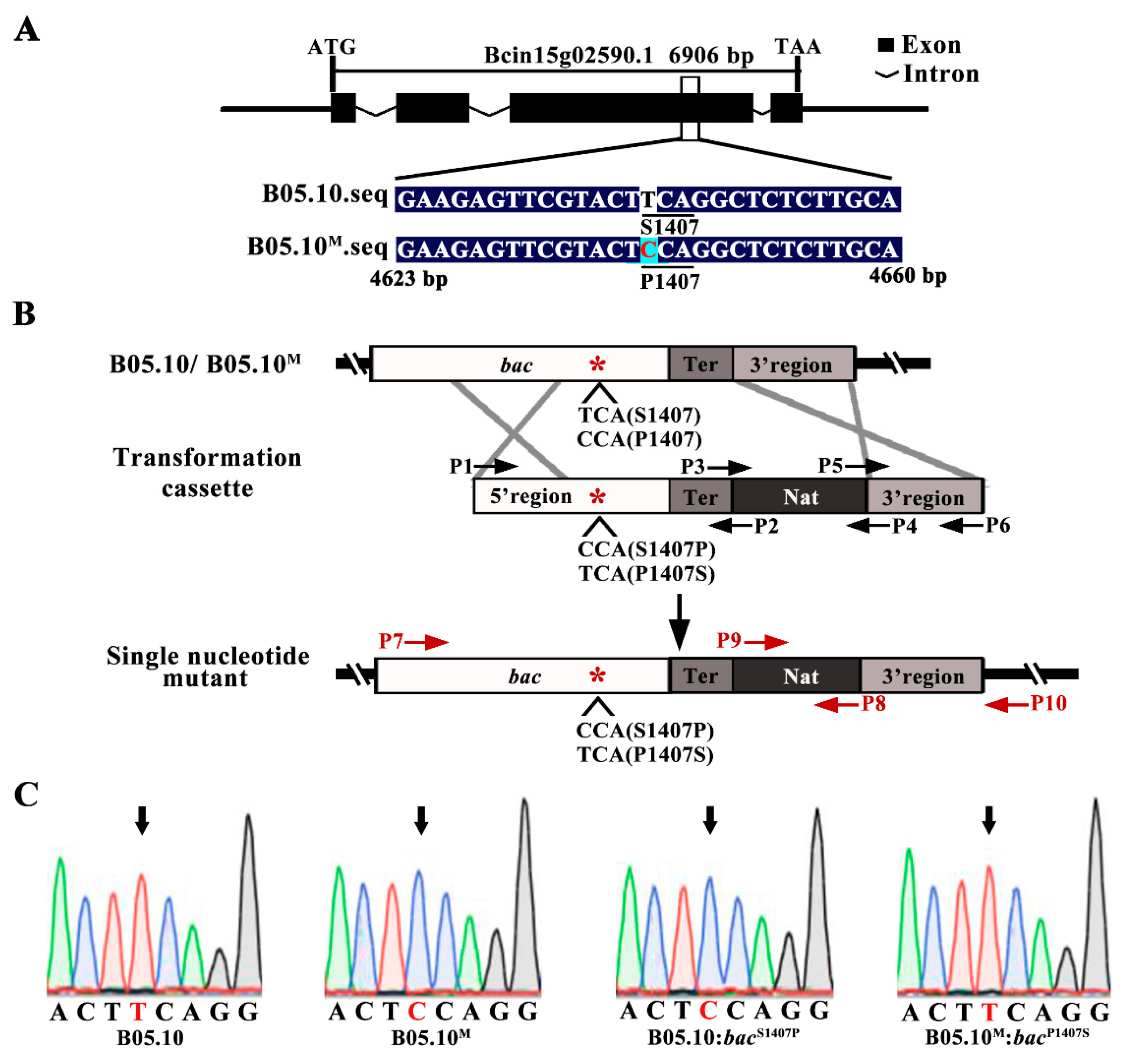
2.3. The S1407P Mutation in the Adenylate Cyclase Encoding Gene Bac Confers Serious Defects in Development and Virulence
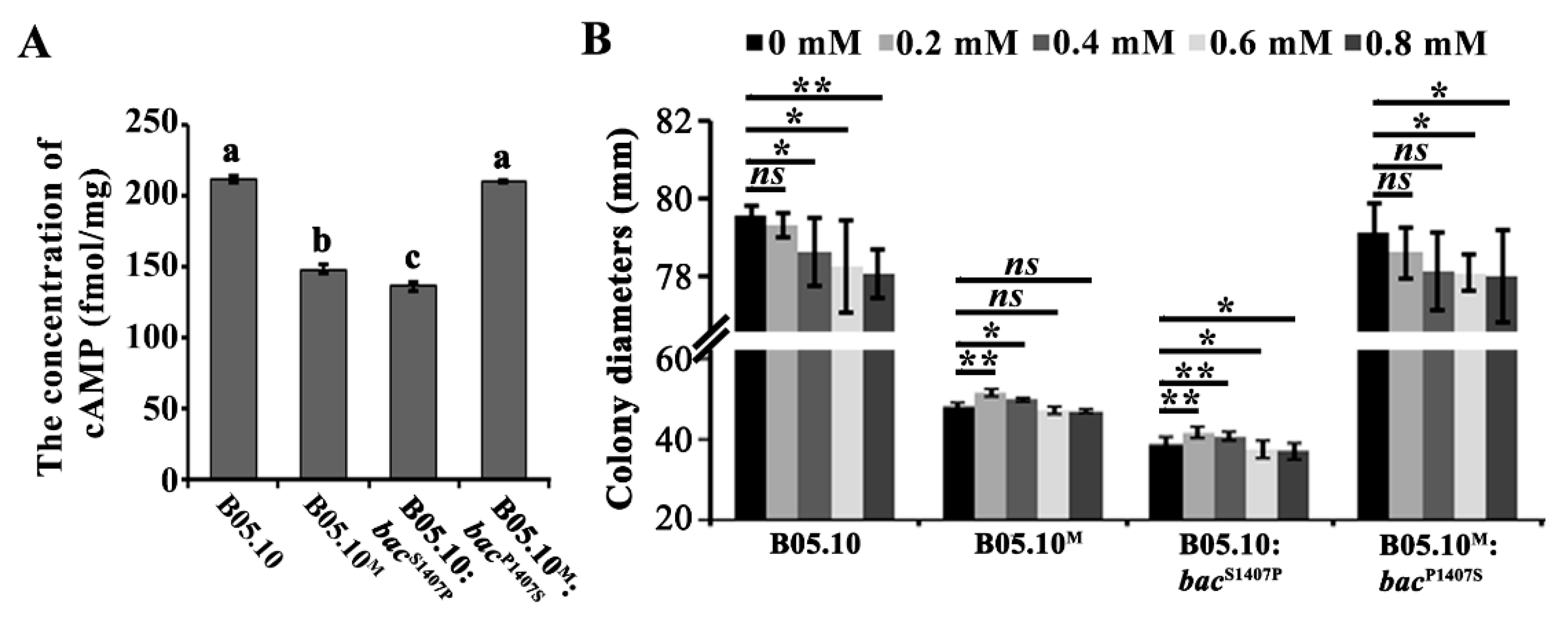
2.4. The S1407P Mutation in BAC Affects its Enzymatic Activity
2.5. The S1407P Mutation in Bac Alters the Fungal Cell Wall Integrity
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Culture Conditions
4.2. Generation of bac Site-Directed Mutants
4.3. Analysis of Growth and Development Phenotype
4.4. Virulence Assay
4.5. Genome Resequencing Analysis
4.6. Mycelia Lysis Assay
4.7. Measurement of Intracellular cAMP Content
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant Hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Faretra, F.; Antonacci, E.; Pollastro, S. Sexual behaviour and mating system of Botryotinia fuckeliana, teleomorph of Botrytis cinerea. J. Gen. Microbiol. 1988, 134, 2543–2550. [Google Scholar] [CrossRef]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Veloso, J.; van Kan, J.A.L. Many shades of grey in Botrytis–host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. Signal Transduction Cascades Regulating Differentiation and Virulence in Botrytis cinerea. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 247–267. [Google Scholar]
- Schumacher, J.; Kokkelink, L.; Huesmann, C.; Jimenez-Teja, D.; Collado, I.G.; Barakat, R.; Tudzynski, P.; Tudzynski, B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe Interact. 2008, 21, 1443–1459. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Schumacher, J.; Viaud, M.; Simon, A.; Tudzynski, B. The Galpha subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co-ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol. Microbiol. 2008, 67, 1027–1050. [Google Scholar] [CrossRef]
- Minz Dub, A.; Kokkelink, L.; Tudzynski, B.; Tudzynski, P.; Sharon, A. Involvement of Botrytis cinerea small GTPases BcRAS1 and BcRAC in differentiation, virulence, and the cell cycle. Eukaryot Cell 2013, 12, 1609–1618. [Google Scholar] [CrossRef]
- Segmuller, N.; Ellendorf, U.; Tudzynski, B.; Tudzynski, P. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 2007, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Schamber, A.; Leroch, M.; Diwo, J.; Mendgen, K.; Hahn, M. The role of mitogen-activated protein (MAP) kinase signalling components and the Ste12 transcription factor in germination and pathogenicity of Botrytis cinerea. Mol. Plant Pathol. 2010, 11, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, S.; Chui, C.; Ma, T.; Jiang, H.; Hahn, M.; Ma, Z. The MAPK kinase BcMkk1 suppresses oxalic acid biosynthesis via impeding phosphorylation of BcRim15 by BcSch9 in Botrytis cinerea. PLoS Pathog. 2018, 14, e1007285. [Google Scholar] [CrossRef]
- Heller, J.; Ruhnke, N.; Espino, J.J.; Massaroli, M.; Collado, I.G.; Tudzynski, P. The mitogen-activated protein kinase BcSak1 of Botrytis cinerea is required for pathogenic development and has broad regulatory functions beyond stress response. Mol. Plant Microbe Interact. 2012, 25, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Viaud, M.; Tudzynski, P. The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLoS Genet. 2014, 10, e1004040. [Google Scholar] [CrossRef]
- Cohrs, K.C.; Simon, A.; Viaud, M.; Schumacher, J. Light governs asexual differentiation in the grey mould fungus Botrytis cinerea via the putative transcription factor BcLTF2. Environ. Microbiol. 2016, 18, 4068–4086. [Google Scholar] [CrossRef]
- Schumacher, J.; Pradier, J.M.; Simon, A.; Traeger, S.; Moraga, J.; Collado, I.G.; Viaud, M.; Tudzynski, B. Natural variation in the VELVET gene bcvel1 affects virulence and light-dependent differentiation in Botrytis cinerea. PLoS ONE 2012, 7, e47840. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Traeger, S.; Porquier, A.; Dalmais, B.; Viaud, M.; Tudzynski, B. The VELVET complex in the gray mold fungus Botrytis cinerea: Impact of BcLAE1 on differentiation, secondary metabolism, and virulence. Mol. Plant Microbe Interact. 2015, 28, 659–674. [Google Scholar] [CrossRef]
- Kumari, S.; Tayal, P.; Sharma, E.; Kapoor, R. Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiol Res. 2014, 169, 862–872. [Google Scholar] [CrossRef]
- Kretschmer, M.; Hahn, M. Fungicide resistance and genetic diversity of Botrytis cinerea isolates from a vineyard in Germany. J. Plant Dis Protect. 2008, 115, 214–219. [Google Scholar] [CrossRef]
- Leroch, M.; Plesken, C.; Weber, R.W.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in german strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Kunkel, T.A. Fidelity of DNA replication. In DNA Replication in Eukaryotic Cells; DePamphilis, M.L., Ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1996; pp. 217–247. [Google Scholar]
- Choi, Y.E.; Xu, J.R. The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant Microbe Interact. 2010, 23, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, M.; Xiong, Z.; Peng, F.; Wei, W. The cAMP-PKA signaling pathway regulates pathogenicity, hyphal growth, appressorial formation, conidiation, and stress tolerance in Colletotrichum higginsianum. Front. Microbiol. 2017, 8, 1416. [Google Scholar] [CrossRef]
- Yang, K.; Qin, Q.; Liu, Y.; Zhang, L.; Liang, L.; Lan, H.; Chen, C.; You, Y.; Zhang, F.; Wang, S. Adenylate cyclase acyA regulates development, aflatoxin biosynthesis and fungal virulence in Aspergillus flavus. Front. Cell Infect. Microbiol. 2016, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, M.; Wang, J.; Zhu, C.; Chung, K.R.; Li, H. Adenylyl cyclase is required for cAMP production, growth, conidial germination, and virulence in the citrus green mold pathogen Penicillium digitatum. Microbiol. Res. 2016, 192, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Klimpel, A.; Gronover, C.S.; Williamson, B.; Stewart, J.A.; Tudzynski, B. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 2002, 3, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Moyano, C.; Alfonso, C.; Gallego, J.; Raposo, R.; Melgarejo, P. Comparison of RAPD and AFLP marker analysis as a means to study the genetic structure of Botrytis cinerea populations. Eur. J. Plant Pathol. 2003, 109, 515–522. [Google Scholar] [CrossRef]
- Valiuskaite, A.; Survilienė, E.; Baniulis, D. Genetic diversity and pathogenicity traits of Botrytis spp. isolated from horticultural hosts. Zemdirbyste 2010, 97, 85–90. [Google Scholar]
- Asadollahi, M.; Fekete, E.; Karaffa, L.; Flipphi, M.; Arnyasi, M.; Esmaeili, M.; Vaczy, K.Z.; Sandor, E. Comparison of Botrytis cinerea populations isolated from two open-field cultivated host plants. Microbiol. Res. 2013, 168, 379–388. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Hu, S.; Liu, H.; Xu, J.R. PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. PLoS Genet. 2017, 13, e1006954. [Google Scholar] [CrossRef]
- Koepfli, K.P.; Tamazian, G.; Wildt, D.; Dobrynin, P.; Kim, C.; Frandsen, P.B.; Godinho, R.; Yurchenko, A.A.; Komissarov, A.; Krasheninnikova, K.; et al. Whole genome sequencing and re-sequencing of the Sable Antelope (Hippotragus niger): A resource for monitoring diversity in ex Situ and in Situ Populations. G3 Genes Genomes Genet. 2019, 9, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland-Leclerc, F. Mechanisms of resitance of fungicides in field strains of Botrytis cinerea. Pest. Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.J.; Pradier, J.M.; Leroux, P.; De Waard, M.A.; et al. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Gautier, A.; Morgant, G.; Studt, L.; Ducrot, P.-H.; Le Pêcheur, P.; Azeddine, S.; Fillinger, S.; Leroux, P.; Tudzynski, B.; et al. A functional bikaverin biosynthesis gene cluster in rare strains of Botrytis cinerea is positively controlled by VELVET. PLoS ONE 2013, 8, e53729. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.; Cheng, D.; Chen, X.; Chen, Y.; Ma, Z. The ATP-binding protein FgArb1 is essential for penetration, infectious and normal growth of Fusarium graminearum. New Phytol. 2018, 219, 1447–1466. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Loros, J.J.; Dunlap, J.C. Alternative use of DNA binding domains by the Neurospora White Collar Complex dictates circadian regulation and light responses. Mol. Cell Biol. 2015, 36, 781–793. [Google Scholar] [CrossRef]
- Studt, L.; Humpf, H.U.; Tudzynski, B. Signaling governed by G proteins and cAMP is crucial for growth, secondary metabolism and sexual development in Fusarium fujikuroi. PLoS ONE 2013, 8, e58185. [Google Scholar] [CrossRef]
- Bormann, J.; Boenisch, M.J.; Bruckner, E.; Firat, D.; Schafer, W. The adenylyl cyclase plays a regulatory role in the morphogenetic switch from vegetative to pathogenic lifestyle of Fusarium graminearum on wheat. PLoS ONE 2014, 9, e91135. [Google Scholar] [CrossRef]
- Choi, W.; Dean, R.A. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 1997, 9, 1973–1983. [Google Scholar]
- Liu, S.; Peng, G.; Xia, Y. The adenylate cyclase gene MaAC is required for virulence and multi-stress tolerance of Metarhizium acridum. BMC Microbiol. 2012, 12, 163. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, G.; Ying, S.H.; Feng, M.G. Adenylate cyclase orthologues in two filamentous entomopathogens contribute differentially to growth, conidiation, pathogenicity, and multistress responses. Fungal Biol. 2014, 118, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Buttner, P.; Koch, F.; Voigt, K.; Quidde, T.; Risch, S.; Blaich, R.; Bruckner, B.; Tudzynski, P. Variations in ploidy among isolates of Botrytis cinerea: Implications for genetic and molecular analyses. Curr. Genet. 1994, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Catlett, N.; Lee, B.N.; Yoder, O.; Turgeon, G. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Patel, R.M.; Heneghan, M.N.; van Kan, J.A.; Bailey, A.M.; Foster, G.D. The pOT and pLOB vector systems: Improving ease of transgene expression in Botrytis cinerea. J. Gen. Appl. Microbiol. 2008, 54, 367–376. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, X.; Zhu, P.; Wang, Y.; Na, Y.; Guo, H.; Cai, Y.; Nie, H.; Jiang, Y.; Xu, L. A Single Nucleotide Mutation in Adenylate Cyclase Affects Vegetative Growth, Sclerotial Formation and Virulence of Botrytis cinerea. Int. J. Mol. Sci. 2020, 21, 2912. https://doi.org/10.3390/ijms21082912
Chen X, Zhang X, Zhu P, Wang Y, Na Y, Guo H, Cai Y, Nie H, Jiang Y, Xu L. A Single Nucleotide Mutation in Adenylate Cyclase Affects Vegetative Growth, Sclerotial Formation and Virulence of Botrytis cinerea. International Journal of Molecular Sciences. 2020; 21(8):2912. https://doi.org/10.3390/ijms21082912
Chicago/Turabian StyleChen, Xue, Xiaohong Zhang, Pinkuan Zhu, Yiwen Wang, Yantao Na, Han Guo, Yunfei Cai, Haozhen Nie, Yina Jiang, and Ling Xu. 2020. "A Single Nucleotide Mutation in Adenylate Cyclase Affects Vegetative Growth, Sclerotial Formation and Virulence of Botrytis cinerea" International Journal of Molecular Sciences 21, no. 8: 2912. https://doi.org/10.3390/ijms21082912
APA StyleChen, X., Zhang, X., Zhu, P., Wang, Y., Na, Y., Guo, H., Cai, Y., Nie, H., Jiang, Y., & Xu, L. (2020). A Single Nucleotide Mutation in Adenylate Cyclase Affects Vegetative Growth, Sclerotial Formation and Virulence of Botrytis cinerea. International Journal of Molecular Sciences, 21(8), 2912. https://doi.org/10.3390/ijms21082912





