Design, Synthesis, and Anticancer Evaluation of Novel Indole Derivatives of Ursolic Acid as Potential Topoisomerase II Inhibitors
Abstract
1. Introduction
2. Results
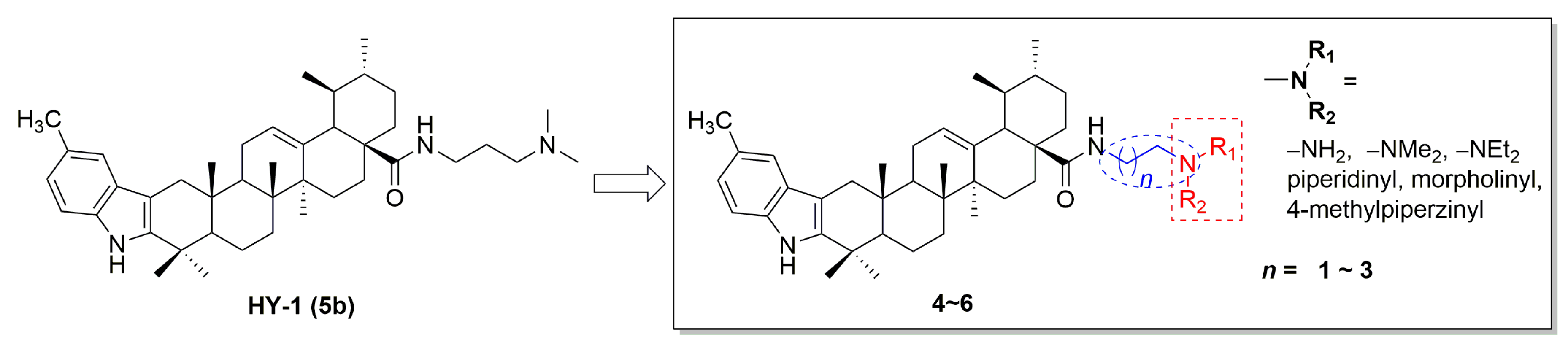
2.1. Chemistry
2.2. Cytotoxic Assay
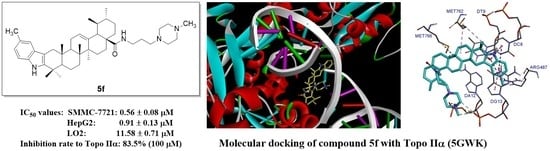
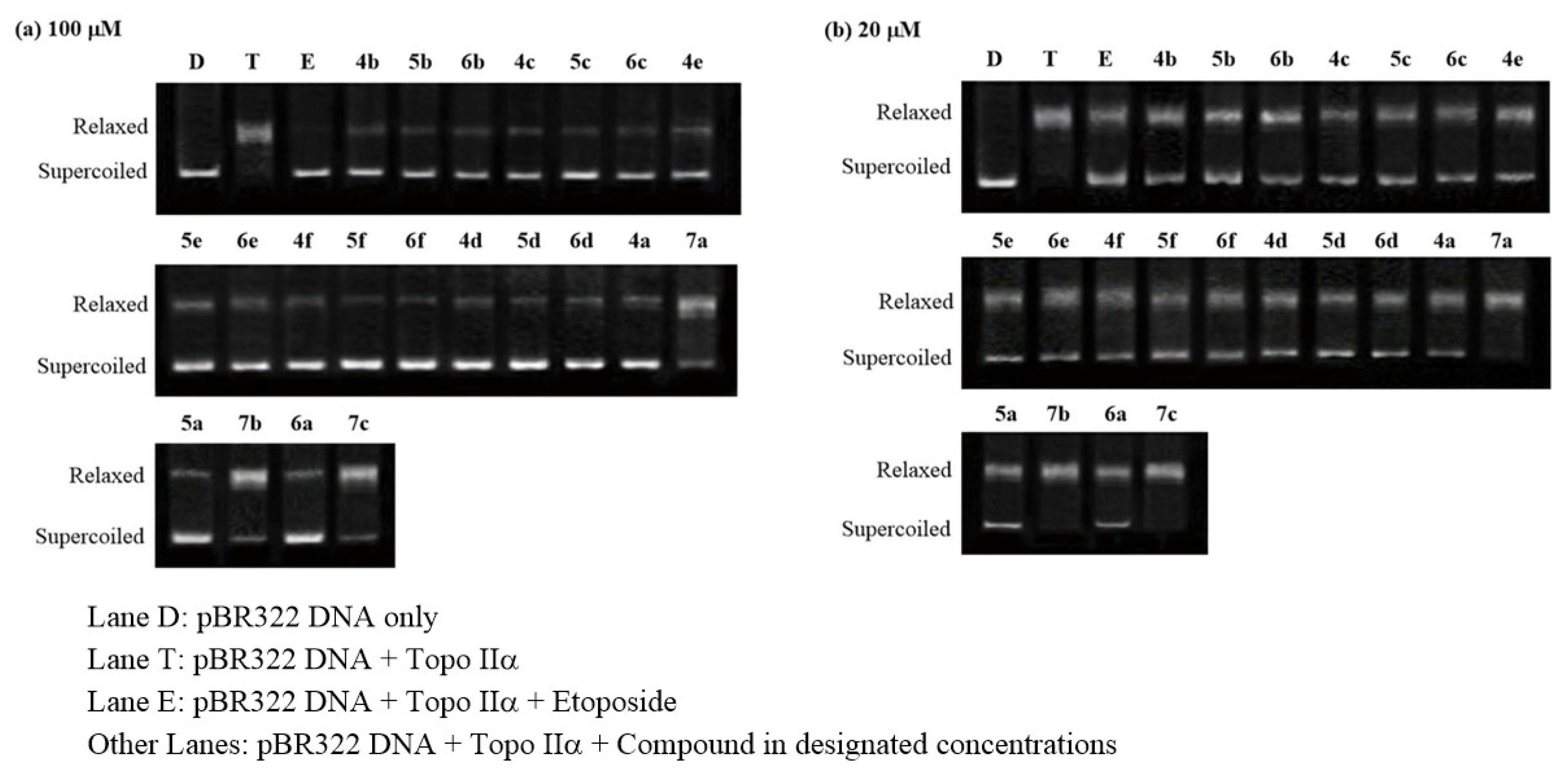
2.3. Topoisomerase Inhibition Assay
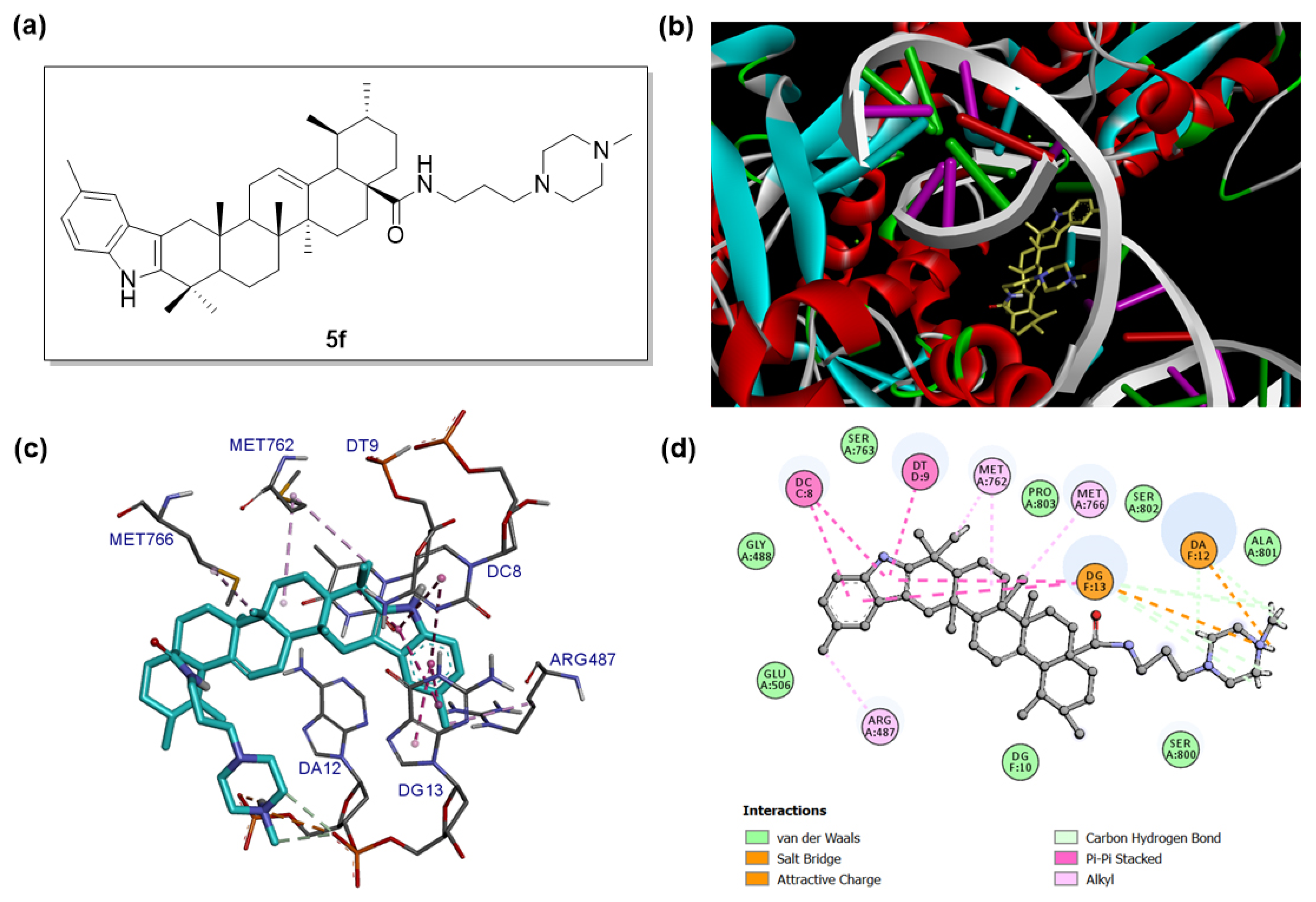
2.4. Molecular Docking
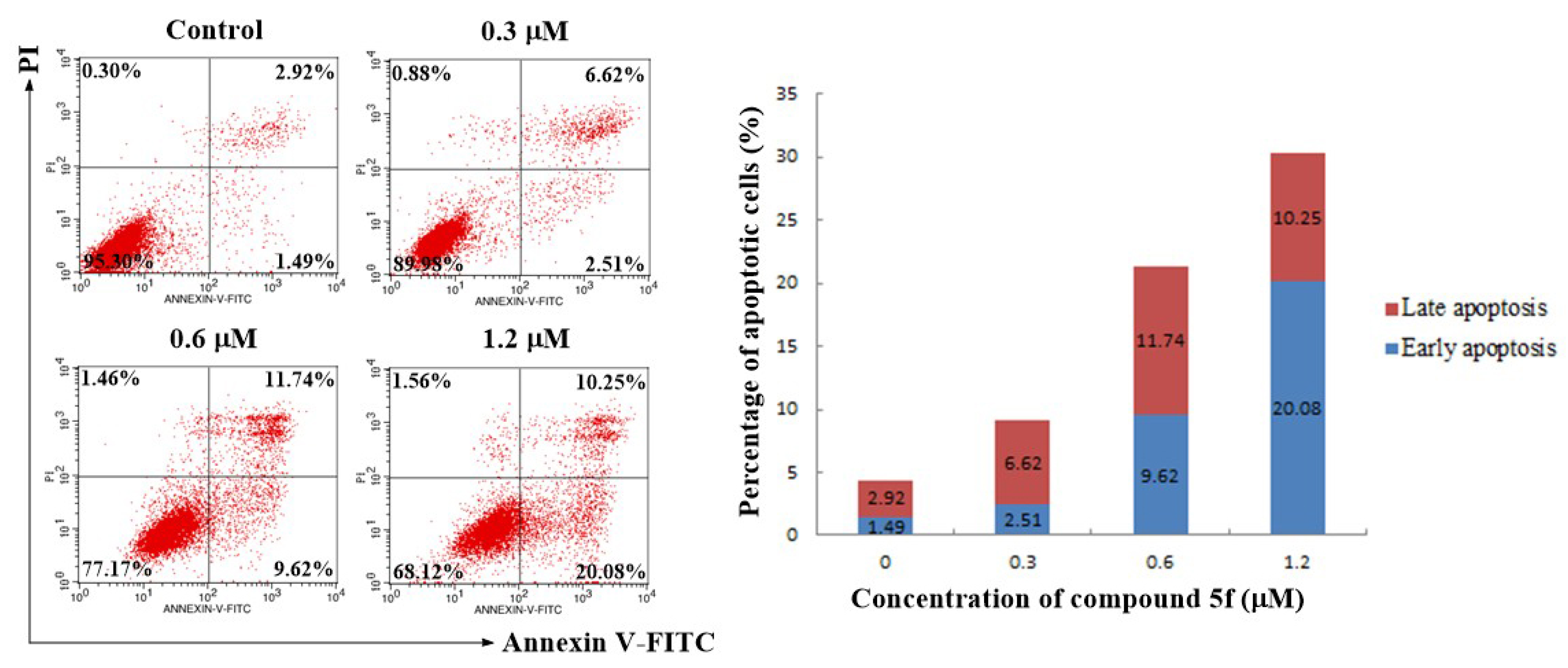
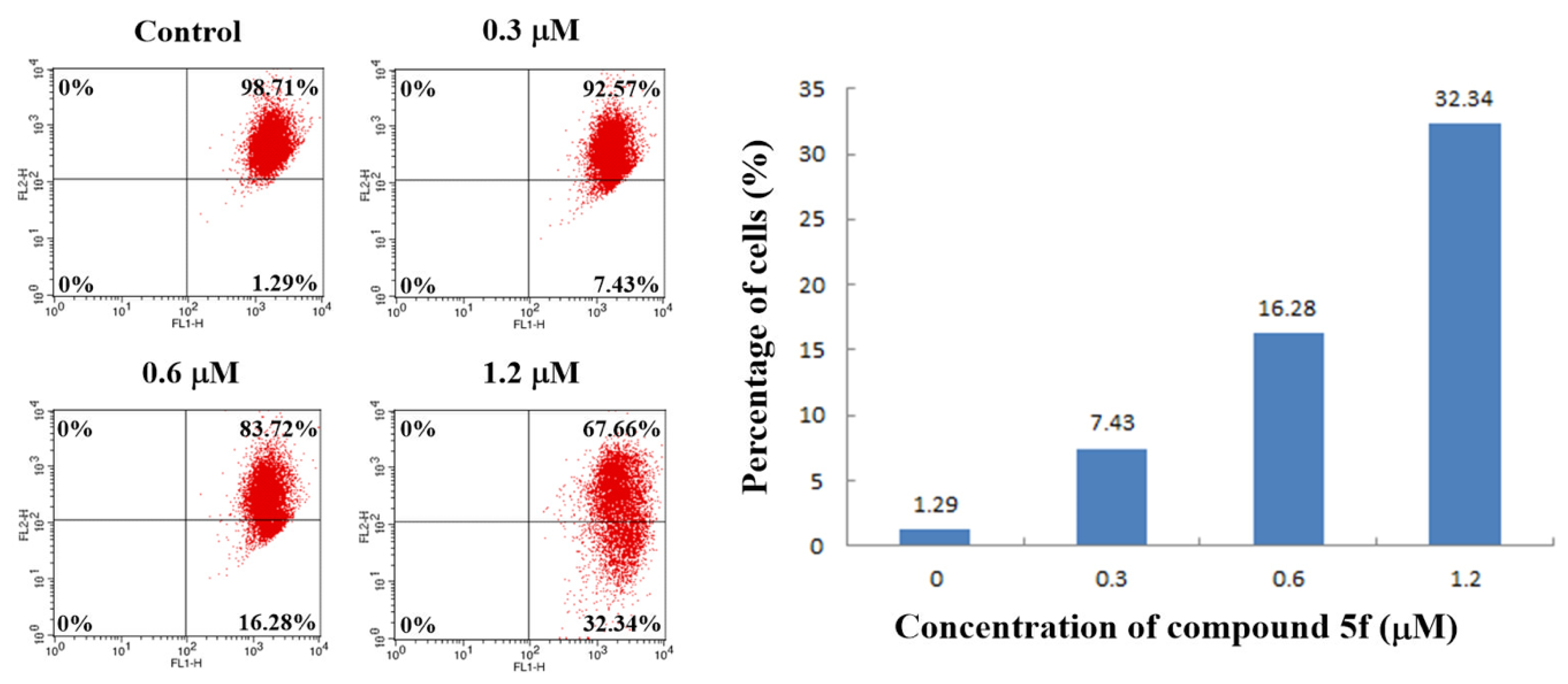
2.5. Annexin V-FITC/PI Staining Assay
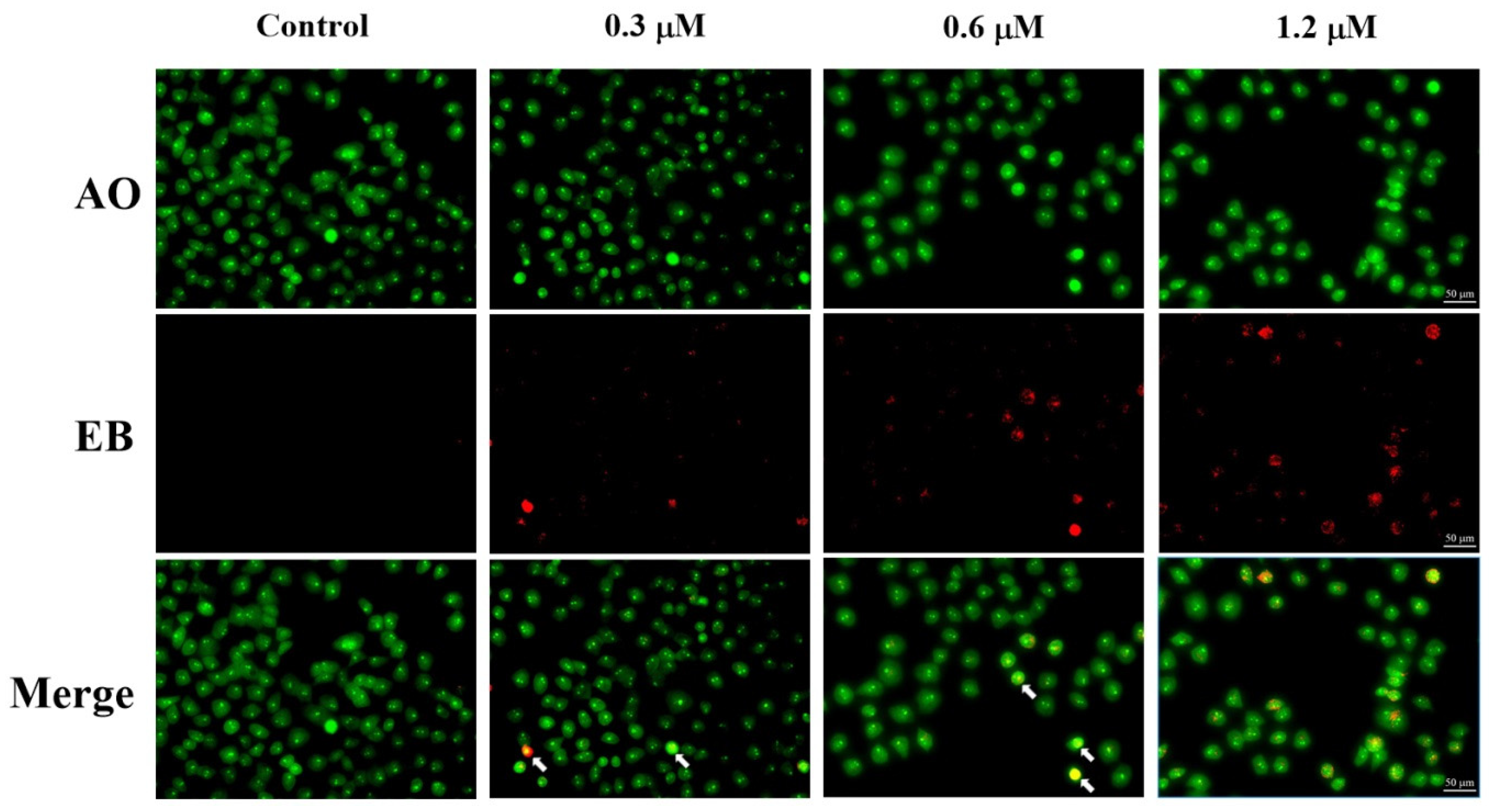
2.6. AO/EB Staining Assay
2.7. ROS Generation Assay
2.8. Mitochondrial Membrane Potential Assay
3. Discussion
4. Materials and Methods
4.1. General
4.2. Procedure for the Synthesis of Compound 3
4.3. General Procedure for the Synthesis of Compounds 4a–f, 5a–f, and 6a–f
4.3.1. N-(2-Aminoethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4a)
4.3.2. N-(2-(Dimethylamino)ethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4b)
4.3.3. N-(2-(Diethylamino)ethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4c)
4.3.4. N-(2-(Piperidin-1-yl)ethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4d)
4.3.5. N-(2-Morpholinoethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4e)
4.3.6. N-(2-(4-Methylpiperazin-1-yl)ethyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (4f)
4.3.7. N-(3-Aminopropyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5a)
4.3.8. N-(3-(Dimethylamino)propyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5b)
4.3.9. N-(3-(Diethylamino)propyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5c)
4.3.10. N-(3-(Piperidin-1-yl)propyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5d)
4.3.11. N-(3-Morpholinopropyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5e)
4.3.12. N-(3-(4-Methylpiperazin-1-yl)propyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (5f)
4.3.13. N-(4-Aminobutyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6a)
4.3.14. N-(4-(Dimethylamino)butyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6b)
4.3.15. N-(4-(Diethylamino)butyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6c)
4.3.16. N-(4-(Piperidin-1-yl)butyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6d)
4.3.17. N-(4-Morpholinobutyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6e)
4.3.18. N-(4-(4-Methylpiperazin-1-yl)butyl)-5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide (6f)
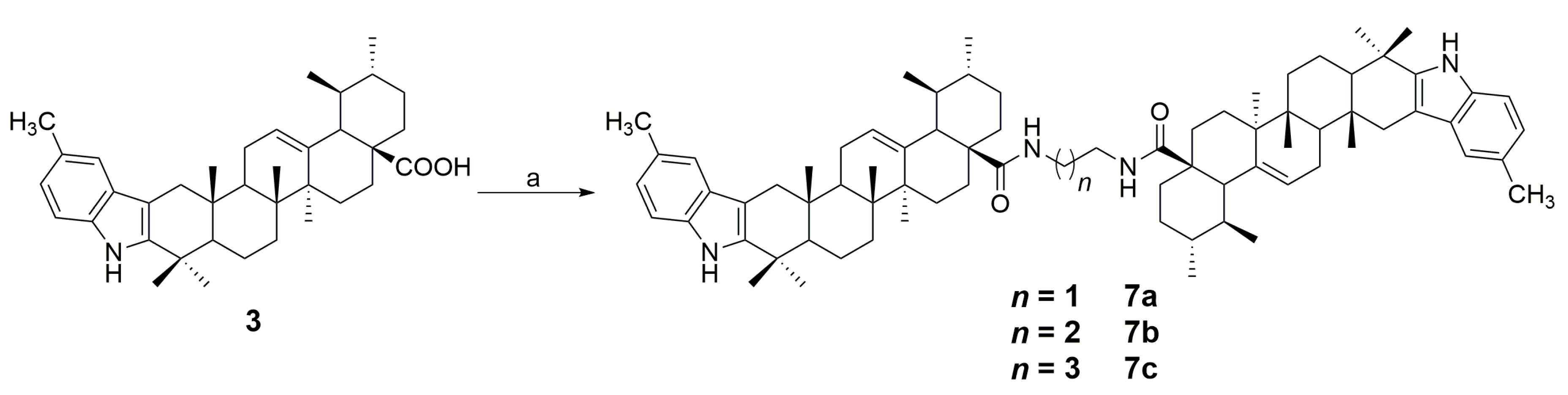
4.4. General Procedure for the Synthesis of Compounds 7a–c
4.4.1. N,N′-(ethane-1,2-diyl)-bis(5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide) (7a)
4.4.2. N,N′-(propane-1,3-diyl)-bis(5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide) (7b)
4.4.3. N,N′-(butane-1,4-diyl)-bis(5′-methyl-1′H-ursa-2,12-dieno[3,2-b]indol-28-carboxamide) (7c)
4.5. Biological Evaluation
4.5.1. In Vitro Anti-Proliferation Assay
4.5.2. Topoisomerase I Inhibition Assay
4.5.3. Topoisomerase II Inhibition Assay
4.5.4. Molecular Docking
4.5.5. Cell Apoptosis Analysis
4.5.6. Acridine Orange/Ethidium Bromide (AO/EB) Staining
4.5.7. ROS Generation Assay
4.5.8. JC-1 Mitochondrial Membrane Potential Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Topo | Topoisomerase |
| UA | Ursolic acid |
| HOBt | 1-Hydroxybenzotriazole |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| MTT | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| PI | Propidium Iodide |
| AO | Acridine orange |
| EB | Ethidium bromide |
| ROS | Reactive oxygen species |
| EDTA | Ethylene diamine tetraacetic acid |
| PBS | Phosphate buffer saline |
| SDS | Sodium dodecyl sulfate |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Wang, L.-J.; Jiang, B.; Wu, N.; Li, X.-Q.; Luo, J.; Wang, B.-C.; Zhang, R.-S.; Xu, Q.; Shi, D.-Y. Synthesis and biological evaluation of novel fluorinated anticancer agents incorporating the indolin-2-one moiety. RSC Adv. 2015, 5, 91795–91801. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA Topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Jun, K.Y.; Lee, E.Y.; Jung, M.J.; Lee, O.H.; Lee, E.S.; Park Choo, H.Y.; Na, Y.; Kwon, Y. Synthesis, biological evaluation, and molecular docking study of 3-(3′-heteroatom substituted-2′-hydroxy-1′-propyloxy) xanthone analogues as novel topoisomerase IIα catalytic inhibitor. Eur. J. Med. Chem. 2011, 46, 1964–1971. [Google Scholar] [CrossRef]
- Park, S.-E.; Chang, I.-H.; Jun, K.-Y.; Lee, E.; Lee, E.-S.; Na, Y.; Kwon, Y. 3-(3-Butylamino-2-hydroxy-propoxy)-1-hydroxy-xanthen-9-one acts as a topoisomerase IIα catalytic inhibitor with low DNA damage. Eur. J. Med. Chem. 2013, 69, 139–145. [Google Scholar] [CrossRef]
- Byl, J.A.W.; Cline, S.D.; Utsugi, T.; Kobunai, T.; Yamada, Y.; Osheroff, N. DNA topoisomerase II as the target for the anticancer drug TOP-53: Mechanistic basis for drug action. Biochemistry 2001, 40, 712–718. [Google Scholar] [CrossRef]
- Chikamori, K.; Grozav, A.G.; Kozuki, T.; Grabowski, D.; Ganapathi, R.; Ganapathi, M.K. DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr. Cancer Drug Targets 2010, 10, 758–771. [Google Scholar] [CrossRef]
- Azarova, A.M.; Lyu, Y.L.; Lin, C.-P.; Tsai, Y.-C.; Lau, J.Y.-N.; Wang, J.C.; Liu, L. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl. Acad. Sci. USA 2007, 104, 11014–11019. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Safe, S.; Kasiappan, R. Natural products as mechanism-based anticancer agents: Sp transcription factors as targets. Phytother. Res. 2016, 30, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, Z.; Kang, O.-H.; Mun, S.-H.; Seo, Y.-S.; Kong, R.; Shin, D.-W.; Liu, X.-Q.; Kwon, D.-Y. Antimicrobial activity and synergism of ursolic acid 3-O-α-L-arabinopyranoside with oxacillin against methicillin-resistant Staphylococcus aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.; Zhang, Z.-H.; Zhang, L.-H.; Jin, X.; Ma, J.; Piao, H.-R. Design, synthesis, and screening of novel ursolic acid derivatives as potential anti-cancer agents that target the HIF-1α pathway. Bioorg. Med. Chem. Lett. 2019, 29, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Tohmé, M.; Gimenez, M.; Peralta, A.; Colombo, M.; Delgui, L.R. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox. Res. 2019, 36, 452–462. [Google Scholar] [CrossRef]
- Wu, P.; Huang, T.; Li, D.; Hu, Q.; Cheng, A.; Jiang, Z.; Jiao, L.; Zhao, S.; Zhang, K. Synthesis and evaluation of novel triterpene analogues of ursolic acid as potential antidiabetic agent. PLoS ONE 2015, 10, e0138767. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 2018, 9, 569. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ali, R.N.I.; Khan, I.; Ali, Z.; Al-Sadi, A.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef]
- Fu, H.-J.; Zhou, Y.-R.; Bao, B.-H.; Jia, M.-X.; Zhao, Y.; Zhang, L.; Li, J.-X.; He, H.; Zhou, X. Tryptophan hydroxylase 1 (Tph-1)-targeted bone anabolic agents for osteoporosis. J. Med. Chem. 2014, 57, 4692–4709. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ahn, Y.J.; Medina, E.A.; Asmis, R. Dietary 23–hydroxy ursolic acid protects against atherosclerosis and obesity by preventing dyslipidemia-induced monocyte priming and dysfunction. Atherosclerosis 2018, 275, 333–341. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 2017, 31, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Ghosh, S.; Bothra, A.K.; Nanda, A.K.; Ghosh, P. Synthesis of friedelan triterpenoid analogs with DNA topoisomerase IIα inhibitory activity and their molecular docking studies. Eur. J. Med. Chem. 2012, 54, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Baek, J.H.; Kang, C.M.; Yoo, M.A.; Sung, J.W.; Chung, H.Y.; Kim, N.D.; Choi, Y.H.; Lee, S.H.; Kim, K.W. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int. J. Cancer 2000, 87, 629–636. [Google Scholar] [CrossRef]
- Mizushina, Y.; Iida, A.; Ohta, K.; Sugawara, F.; Sakaguchi, K. Novel triterpenoids inhibit both DNA polymerase and DNA topoisomerase. Biochem. J. 2000, 3, 757–763. [Google Scholar] [CrossRef]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.; Miao, T.-T.; Zhang, K.-P. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef]
- Wang, R.; Yang, W.; Fan, Y.; Dehaen, W.; Li, Y.; Li, H.-J.; Wang, W.; Zheng, Q.; Huai, Q.-Y. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid ester derivatives with cytotoxic properties. Bioorg. Chem. 2019, 88, 102951. [Google Scholar] [CrossRef]
- Huang, X.; Huang, R.; Liao, Z.; Pan, Y.; Gou, S.; Wang, H.-S. Synthesis and pharmacological evaluation of dehydroabietic acid thiourea derivatives containing bisphosphonate moiety as an inducer of apoptosis. Eur. J. Med. Chem. 2016, 108, 381–391. [Google Scholar] [CrossRef]
- Kumar, N.P.; Vanjari, Y.; Thatikonda, S.; Pooladanda, V.; Sharma, P.; Sridhar, B.; Godugu, C.; Kamal, A.; Shankaraiah, N.; Sridhar, B. Synthesis of enamino-2-oxindoles via conjugate addition between α-azido ketones and 3-alkenyl oxindoles: Cytotoxicity evaluation and apoptosis inducing studies. Bioorg. Med. Chem. Lett. 2018, 28, 3564–3573. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Boil. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Gnoatto, S.B.; Dassonville-Klimpt, A.; Da Nascimento, S.; Galéra, P.; Boumediene, K.; Gosmann, G.; Sonnet, P.; Moslemi, S. Evaluation of ursolic acid isolated from Ilex paraguariensis and derivatives on aromatase inhibition. Eur. J. Med. Chem. 2008, 43, 1865–1877. [Google Scholar] [CrossRef]
- Wang, C.; Delcros, J.-G.; Biggerstaff, J.; Phanstiel, O., IV. Synthesis and biological evaluation ofN1-(Anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J. Med. Chem. 2003, 46, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-B.; Ou, J.-B.; Huang, Z.; Chen, S.-B.; Ou, T.-M.; Tan, J.-H.; Li, D.; Shen, L.-L.; Huang, S.-L.; Gu, L.Q.; et al. Synthesis and evaluation of mansonone F derivatives as topoisomerase inhibitors. Eur. J. Med. Chem. 2011, 46, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, J.Y.; Din, Z.H.; Su, J.H.; Yang, Z.Y.; Chen, Y.J.; Wang, R.Y.; Wu, Y.J. 13-acetoxysarcocrassolide induces apoptosis on human gastric carcinoma cells through mitochondria- related apoptotic pathways: p38/JNK activation and PI3K/AKT suppression. Mar. Drugs 2014, 12, 5295–5315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, Z.; Zhang, C.; Peng, Y.; Zhang, L.; Zhou, G.; Wang, S.; Zhang, J. Synthesis, characterization and ROS-mediated antitumor effects of palladium(II) complexes of curcuminoids. Eur. J. Med. Chem. 2018, 144, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Donthiboina, K.; Anchi, P.; Ramya, P.S.; Karri, S.; Srinivasulu, G.; Godugu, C.; Shankaraiah, N.; Kamal, A. Synthesis of substituted biphenyl methylene indolinones as apoptosis inducers and tubulin polymerization inhibitors. Bioorg. Chem. 2019, 86, 210–223. [Google Scholar] [CrossRef]



| IC50 Values (μM) | Topo I Inhibition (%) | Topo II Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|
| SMMC-7721 | HepG2 | LO2 | 100 μM | 20 μM | 100 μM | 20 μM | |
| 4a | 3.50 ± 0.61 | 3.31 ± 0.35 | 29.59 ± 2.12 | 15.1 | 5.6 | 69.7 | 25.9 |
| 4b | 4.42 ± 0.71 | 4.11 ± 0.55 | 31.06 ± 2.63 | 3.7 | -1 | 68.0 | 36.9 |
| 4c | 2.10 ± 0.21 | 2.24 ± 0.32 | 26.39 ± 1.32 | 0 | - | 68.7 | 38.3 |
| 4d | 1.75 ± 0.11 | 1.47 ± 0.12 | 16.03 ± 1.07 | 13.0 | 0 | 71.7 | 32.7 |
| 4e | 7.82 ± 0.78 | 10.72 ± 2.09 | >50 | 0 | - | 63.3 | 26.8 |
| 4f | 1.24 ± 0.16 | 1.35 ± 0.17 | 18.27 ± 0.78 | 0 | - | 73.7 | 31.3 |
| 5a | 2.02 ± 0.21 | 2.45 ± 0.38 | 21.32 ± 1.59 | 24.5 | 12.4 | 72.3 | 31.2 |
| 5b | 1.08 ± 0.22 | 1.26 ± 0.17 | 20.65 ± 1.73 | 34.2 | 9.0 | 72.5 | 38.2 |
| 5c | 1.15 ± 0.14 | 1.20 ± 0.24 | 15.85 ± 1.06 | 0 | - | 75.8 | 38.9 |
| 5d | 0.89 ± 0.11 | 1.04 ± 0.09 | 12.82 ± 0.83 | 0 | - | 81.1 | 41.3 |
| 5e | 3.75 ± 0.37 | 5.27 ± 0.67 | 28.62 ± 0.76 | 0 | - | 70.8 | 31.5 |
| 5f | 0.56 ± 0.08 | 0.91 ± 0.13 | 10.58 ± 0.52 | 0 | - | 83.5 | 43.2 |
| 6a | 1.21 ± 0.19 | 1.32 ± 0.21 | 15.23 ± 1.15 | 28.8 | 13.7 | 71.1 | 27.0 |
| 6b | 2.43 ± 0.21 | 2.91 ± 0.18 | 18.19 ± 1.64 | 15.1 | 0 | 69.0 | 31.7 |
| 6c | 3.62 ± 0.23 | 3.45 ± 0.25 | 28.42 ± 2.98 | 16.8 | 0 | 70.0 | 35.2 |
| 6d | 2.72 ± 0.31 | 2.31 ± 0.21 | 18.65 ± 1.36 | 0 | - | 72.5 | 32.8 |
| 6e | 4.55 ± 1.56 | 7.84 ± 2.31 | 35.39 ± 3.13 | 0 | - | 65.6 | 28.2 |
| 6f | 0.65 ± 0.07 | 1.01 ± 0.12 | 13.72 ± 1.28 | 0 | - | 81.8 | 39.8 |
| 7a | >50 | >50 | >50 | 0 | - | 23.8 | 9.7 |
| 7b | >50 | >50 | >50 | 2.0 | - | 17.8 | 9.8 |
| 7c | >50 | >50 | >50 | 0 | - | 16.9 | 8.1 |
| CPT 2 | 0.56 ± 0.18 | 1.12 ± 0.26 | 8.93 ± 0.52 | 75.1 | 38.2 | - | - |
| VP-16 | 0.36 ± 0.10 | 0.57 ± 0.16 | 5.92 ± 0.29 | - | - | 88.5 | 47.8 |
| DOX | 0.68 ± 0.11 | 0.81 ± 0.19 | 5.32 ± 0.67 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.-L.; Hao, Y.; Wang, W.-Y.; Liu, Q.-S.; Sun, Y.; Gu, W. Design, Synthesis, and Anticancer Evaluation of Novel Indole Derivatives of Ursolic Acid as Potential Topoisomerase II Inhibitors. Int. J. Mol. Sci. 2020, 21, 2876. https://doi.org/10.3390/ijms21082876
Li A-L, Hao Y, Wang W-Y, Liu Q-S, Sun Y, Gu W. Design, Synthesis, and Anticancer Evaluation of Novel Indole Derivatives of Ursolic Acid as Potential Topoisomerase II Inhibitors. International Journal of Molecular Sciences. 2020; 21(8):2876. https://doi.org/10.3390/ijms21082876
Chicago/Turabian StyleLi, A-Liang, Yun Hao, Wen-Yan Wang, Qing-Song Liu, Yue Sun, and Wen Gu. 2020. "Design, Synthesis, and Anticancer Evaluation of Novel Indole Derivatives of Ursolic Acid as Potential Topoisomerase II Inhibitors" International Journal of Molecular Sciences 21, no. 8: 2876. https://doi.org/10.3390/ijms21082876
APA StyleLi, A.-L., Hao, Y., Wang, W.-Y., Liu, Q.-S., Sun, Y., & Gu, W. (2020). Design, Synthesis, and Anticancer Evaluation of Novel Indole Derivatives of Ursolic Acid as Potential Topoisomerase II Inhibitors. International Journal of Molecular Sciences, 21(8), 2876. https://doi.org/10.3390/ijms21082876