Genes and Diet in the Prevention of Chronic Diseases in Future Generations
Abstract
1. Background
2. Dietary Habits and Human Reproduction
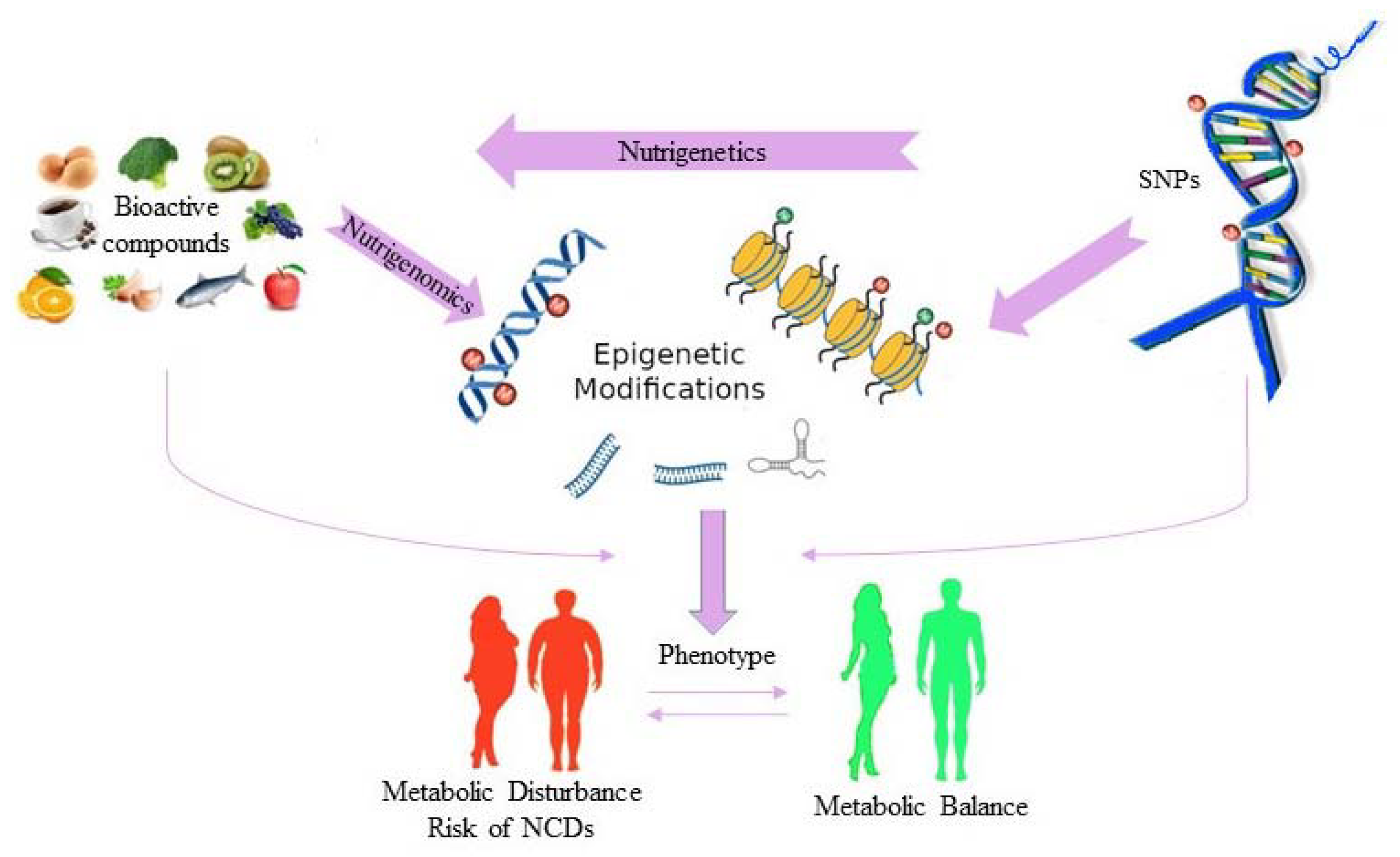
3. Nutrigenetics and Human Health
4. Epigenetics, Diet and Human Health
5. Other Viewpoints
6. Future Perspectives
Funding
Conflicts of Interest
References
- Di Daniele, N. The Role of Preventive Nutrition in Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, A.K.; Moley, K.H. Developmental and Transmittable Origins of Obesity-Associated Health Disorders. Trends Genet. 2017, 33, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Al-Beitawi, S.; Cipriani, S.; Alteri, A.; Chiaffarino, F.; Candiani, M.; Gerli, S.; Viganó, P.; Parazzini, F. Dietary habits and semen parameters: A systematic narrative review. Andrology 2018, 6, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2016, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Gavarkovs, A.; Tamez, M.; Mattei, J. The Influence of Diet on Fertility and the Implications for Public Health Nutrition in the United States. Front. Public Health 2018, 6, 211. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Hesketh, J.E.; Wybranska, I.; Dommels, Y.; King, M.; Elliott, R.; Pico, C.; Keijer, J. Nutrient–gene interactions in benefit–risk analysis. Br. J. Nutr. 2006, 95, 1232–1236. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Nutrigenetics/Nutrigenomics. Annu. Rev. Public Health 2010, 31, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; De Luis-Román, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1-Fields of Precision Nutrition. J. Nutr. Nutr. 2016, 9, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martínez, A. Future Perspectives of Personalized Weight Loss Interventions Based on Nutrigenetic, Epigenetic, and Metagenomic Data. J. Nutr. 2015, 146, 905S–912S. [Google Scholar] [CrossRef] [PubMed]
- Precone, V.; Beccari, T.; Stuppia, L.; Baglivo, M.; Paolacci, S.; Manara, E.; Miggiano, G.A.D.; Falsini, B.; Trifirò, A.; Zanlari, A.; et al. Taste, olfactory and texture related genes and food choices: Implications on health status. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1305–1321. [Google Scholar]
- Grimaldi, K. Nutrigenetics and personalized nutrition: Are we ready for DNA-based dietary advice? Pers. Med. 2014, 11, 297–307. [Google Scholar] [CrossRef]
- De Caterina, R.; El-Sohemy, A. Moving towards Specific Nutrigenetic Recommendation Algorithms: Caffeine, Genetic Variation and Cardiovascular Risk. J. Nutr. Nutr. 2016, 9, 106–115. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.; Zeggini, E.; Freathy, R.M.; Lin, D.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Hetherington, M.M.; Cecil, J.E. Gene-Environment Interactions in Obesity. Forum Nutr. 2009, 63, 195–203. [Google Scholar]
- Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; Martínez-González, M.Á.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc. Diabetol. 2012, 11, 137. [Google Scholar]
- Fisher, E.; Boeing, H.; Fritsche, A.; Doering, F.; Joost, H.-G.; Schulze, M.B. Whole-grain consumption and transcription factor-7-like 2 (TCF7L2) rs7903146: Gene–diet interaction in modulating type 2 diabetes risk. Br. J. Nutr. 2008, 101, 478–481. [Google Scholar] [CrossRef]
- Hindy, G.; Sonestedt, E.; Ericson, U.; Jing, X.-J.; Zhou, Y.; Hansson, O.; Renström, E.; Wirfält, E.; Orho-Melander, M. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 2012, 55, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Wirström, T.; Hilding, A.; Gu, H.F.; Ostenson, C.-G.; Björklund, A. Consumption of whole grain reduces risk of deteriorating glucose tolerance, including progression to prediabetes. Am. J. Clin. Nutr. 2012, 97, 179–187. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, M.M.; Garay-Sevilla, M.E.; Tejero, M.E.; Pérez-Luque, E. Analysis of the interaction between transcription factor 7-like 2 genetic variants with nopal and wholegrain fibre intake: Effects on anthropometric and metabolic characteristics in type 2 diabetes patients. Br. J. Nutr. 2016, 116, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Nicolucci, A.; Celentano, C.; Liberati, M.; Stuppia, L.; Vitacolonna, E. Molecular Analysis of a Genetic Variants Panel Related to Nutrients and Metabolism: Association with Susceptibility to Gestational Diabetes and Cardiometabolic Risk in Affected Women. J. Diabetes Res. 2017, 2017, 4612623. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Marchetti, D.; Celentano, C.; Liberati, M.; Stuppia, L.; Vitacolonna, E. Nutrigenetic variants and cardio-metabolic risk in women with or without gestational diabetes. Diabetes Res. Clin. Pract. 2018, 137, 64–71. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Di Nicola, M.; Bianco, F.; Marchetti, D.; Celentano, C.; Liberati, M.; De Caterina, R.; Stuppia, L.; Vitacolonna, E. Early Subclinical Atherosclerosis in Gestational Diabetes: The Predictive Role of Routine Biomarkers and Nutrigenetic Variants. J. Diabetes Res. 2018, 2018, 9242579. [Google Scholar] [CrossRef]
- Dhurandhar, N.V.; Schoeller, D.; Brown, A.W.; Heymsfield, S.B.; Thomas, D.; Sørensen, T.I.; Speakman, J.R.; Jeansonne, M.; Allison, D.B.; The Energy Balance Measurement Working Group. Energy balance measurement: When something is not better than nothing. Int. J. Obes. 2014, 39, 1109–1113. [Google Scholar] [CrossRef]
- Archer, E.; Marlow, M.L.; Lavie, C.J. Controversy and Debate: Memory Based Methods Paper 3: Nutrition’s ‘Black Swans’: Our reply. J. Clin. Epidemiol. 2018, 104, 130–135. [Google Scholar] [CrossRef]
- Saukko, P.; Reed, M.; Britten, N.; Hogarth, S. Negotiating the boundary between medicine and consumer culture: Online marketing of nutrigenetic tests. Soc. Sci. Med. 2009, 70, 744–753. [Google Scholar] [CrossRef]
- Hurlimann, T.; Robitaille, J.; Vohl, M.-C.; Godard, B. Ethical considerations in the implementation of nutrigenetics/nutrigenomics. Pers. Med. 2017, 14, 75–83. [Google Scholar] [CrossRef]
- Burke, L.M.; Trinidad, S.B. The Deceptive Appeal of Direct-to-Consumer Genetics. Ann. Intern. Med. 2016, 164, 675. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.-X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Gabbianelli, R.; Laura, B.; Gabbianelli, R. Primers on nutrigenetics and nutri(epi)genomics: Origins and development of precision nutrition. Biochimie 2019, 160, 156–171. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef]
- Drake, A.J.; McPherson, R.C.; Godfrey, K.M.; Cooper, C.; Lillycrop, K.A.; Hanson, M.; Meehan, R.R.; Seckl, J.R.; Reynolds, R.M. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin. Endocrinol. 2012, 77, 808–815. [Google Scholar] [CrossRef]
- Dominguez-Salas, P.; Moore, S.E.; Baker, M.S.; Bergen, A.W.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Guan, Y.; Laritsky, E.; Silver, M.J.; et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat. Commun. 2014, 5, 3746. [Google Scholar] [CrossRef]
- Hjort, L.; Martino, D.; Grunnet, L.G.; Naeem, H.; Maksimovic, J.; Olsson, A.H.; Zhang, C.; Ling, C.; Olsen, S.F.; Saffery, R.; et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 2018, 3, 122572. [Google Scholar] [CrossRef]
- Martin, C.; Jima, D.; Sharp, G.C.; McCullough, L.E.; Park, S.S.; Gowdy, K.M.; Skaar, D.; Cowley, M.; Maguire, R.L.; Fuemmeler, B.; et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: An epigenome-wide association study. Epigenetics 2019, 14, 325–340. [Google Scholar] [CrossRef]
- Lane, M.; Robker, R.L.; Robertson, S.A. Parenting from before conception. Scicnce 2014, 345, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Lee, H.-J.; Muhlhauser, A.; Tilly, J.L. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 2011, 108, 12319–12324. [Google Scholar] [CrossRef] [PubMed]
- Nehra, D.; Le, H.D.; Fallon, E.M.; Carlson, S.J.; Woods, D.; White, Y.A.; Pan, A.H.; Guo, L.; Rodig, S.J.; Tilly, J.L.; et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell 2012, 11, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and male reproduction: The consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin. Epigenetics 2015, 7, 120. [Google Scholar] [CrossRef]
- Morgan, C.P.; Chan, J.C.; Bale, T.L. Driving the Next Generation: Paternal Lifetime Experiences Transmitted via Extracellular Vesicles and Their Small RNA Cargo. Boil. Psychiatry 2019, 85, 164–171. [Google Scholar] [CrossRef]
- Franzago, M.; La Rovere, M.; Franchi, P.G.; Vitacolonna, E.; Stuppia, L. Epigenetics and human reproduction: The primary prevention of the noncommunicable diseases. Epigenomics 2019, 11, 1441–1460. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.R.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Soubry, A.; Guo, S.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin. Epigenetics 2016, 8, 51. [Google Scholar] [CrossRef]
- Dupont, C.; Kappeler, L.; Saget, S.; Grandjean, V.; Lévy, R. Role of miRNA in the Transmission of Metabolic Diseases Associated With Paternal Diet-Induced Obesity. Front. Genet. 2019, 10, 337. [Google Scholar] [CrossRef]
- Bygren, L.; Kaati, G.; Edvinsson, S. Longevity determined by paternal ancestors’ nutrition during their slow growth period. Acta Biotheor. 2001, 49, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kaati, G.; Bygren, L.; Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002, 10, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kaati, G.; Bygren, L.O.; Pembrey, M.; Sjöström, M. Transgenerational response to nutrition, early life circumstances and longevity. Eur. J. Hum. Genet. 2007, 15, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.-C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2010, 32, 159–224. [Google Scholar] [CrossRef]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef]
- Schagdarsurengin, U.; Steger, K. Epigenetics in male reproduction: Effect of paternal diet on sperm quality and offspring health. Nat. Rev. Urol. 2016, 13, 584–595. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Li, Y.; Tollefsbol, T.O. Prenatal epigenetics diets play protective roles against environmental pollution. Clin. Epigenetics 2019, 11, 82. [Google Scholar] [CrossRef]
- Archer, E. In reply—Maternal, paternal, and societal efforts are needed to “cure” childhood obesity. Mayo Clin. Proc. 2015, 90, 555–557. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Day, T. Nongenetic Inheritance and Its Evolutionary Implications. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 103–125. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Crean, A.J.; Day, T. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 2011, 5, 192–201. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Day, T. Extended Heredity: A New Understanding of Inheritance and Evolution; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Archer, E. The childhood obesity epidemic as a result of nongenetic evolution: The maternal resources hypothesis. Mayo Clin. Proc. 2014, 90, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Lavie, C.J.; Hill, J.O. The Contributions of ‘Diet’, ‘Genes’, and Physical Activity to the Etiology of Obesity: Contrary Evidence and Consilience. Prog. Cardiovasc. Dis. 2018, 61, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Pavela, G.; McDonald, S.; Lavie, C.J.; Hill, J.O. Cell-Specific “Competition for Calories” Drives Asymmetric Nutrient-Energy Partitioning, Obesity, and Metabolic Diseases in Human and Non-human Animals. Front. Physiol. 2018, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Archer, E. In Defense of Sugar: A Critique of Diet-Centrism. Prog. Cardiovasc. Dis. 2018, 61, 10–19. [Google Scholar] [CrossRef]
- Archer, E. The Demonization of ’Diet’ Is Nothing New. Prog. Cardiovasc. Dis. 2018, 61, 386–387. [Google Scholar] [CrossRef]
- Archer, E.; Lavie, C.J.; Hill, J.O. The Failure to Measure Dietary Intake Engendered a Fictional Discourse on Diet-Disease Relations. Front. Nutr. 2018, 5, 105. [Google Scholar] [CrossRef]
- Doaei, S.; Kalantari, N.; Izadi, P.; Salonurmi, T.; Jarrahi, A.M.; Rafieifar, S.; Tabesh, G.A.; Rahimzadeh, G.; Gholamalizadeh, M.; Goodarzi, M.O. Changes in FTO and IRX3 gene expression in obese and overweight male adolescents undergoing an intensive lifestyle intervention and the role of FTO genotype in this interaction. J. Transl. Med. 2019, 17, 176. [Google Scholar] [CrossRef]
- Doaei, S.; Kalantari, N.; Izadi, P.; Salonurmi, T.; Jarrahi, A.M.; Rafieifar, S.; Tabesh, G.A.; Rahimzadeh, G.; Gholamalizadeh, M.; Goodarzi, M.O. Interactions between macro-nutrients’ intake, FTO and IRX3 gene expression, and FTO genotype in obese and overweight male adolescents. Adipocyte 2019, 8, 386–391. [Google Scholar] [CrossRef]
- Sandovici, I.; Smith, N.H.; Nitert, M.D.; Ackers-Johnson, M.; Uribe-Lewis, S.; Ito, Y.; Jones, R.H.; Marquez, V.E.; Cairns, W.; Tadayyon, M.; et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc. Natl. Acad. Sci. USA 2011, 108, 5449–5454. [Google Scholar] [CrossRef]
- Borengasser, S.J.; Zhong, Y.; Kang, P.; Lindsey, F.; Ronis, M.J.J.; Badger, T.M.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013, 154, 4113–4125. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhou, D.; Moody, L.; Lezmi, S.; Chen, H.; Pan, Y.-X. High-fat diet caused widespread epigenomic differences on hepatic methylome in rat. Physiol. Genom. 2015, 47, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [PubMed]
- Nazari, Z.; Shahryari, A.; Ghafari, S.; Nabiuni, M.; Golalipour, M.J. In Utero Exposure to Gestational Diabetes Alters DNA Methylation and Gene Expression of CDKN2A/B in Langerhans Islets of Rat Offspring. Cell J. 2019, 22, 203–211. [Google Scholar] [PubMed]
- Côté, S.; Gagné-Ouellet, V.; Guay, S.-P.; Allard, C.; Houde, A.-A.; Perron, P.; Baillargeon, J.-P.; Gaudet, D.; Guérin, R.; Brisson, D.; et al. PPARGC1α gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin. Epigenetics 2016, 8, 72. [Google Scholar] [CrossRef]
- Chen, P.; Piaggi, P.; Traurig, M.; Bogardus, C.; Knowler, W.C.; Baier, L.J.; Hanson, R.L. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia 2017, 60, 645–655. [Google Scholar] [CrossRef]
- Gagné-Ouellet, V.; Houde, A.-A.; Guay, S.-P.; Perron, P.; Gaudet, D.; Guérin, R.; Jean-Patrice, B.; Hivert, M.-F.; Brisson, D.; Bouchard, L. Placental lipoprotein lipase DNA methylation alterations are associated with gestational diabetes and body composition at 5 years of age. Epigenetics 2017, 12, 616–625. [Google Scholar] [CrossRef]
- Cardenas, A.; Gagné-Ouellet, V.; Allard, C.; Brisson, D.; Perron, P.; Bouchard, L.; Hivert, M.-F. Placental DNA Methylation Adaptation to Maternal Glycemic Response in Pregnancy. Diabetes 2018, 67, 1673–1683. [Google Scholar] [CrossRef]
- Howe, C.G.; Cox, B.; Fore, R.; Jungius, J.; Kvist, T.; Lent, S.; Miles, H.E.; Salas, L.A.; Rifas-Shiman, S.; Starling, A.P.; et al. Maternal Gestational Diabetes Mellitus and Newborn DNA Methylation: Findings From the Pregnancy and Childhood Epigenetics Consortium. Diabetes Care 2019, 43, 98–105. [Google Scholar] [CrossRef]
- Fernandez-Twinn, D.S.; Alfaradhi, M.Z.; Martin-Gronert, M.S.; Duque-Guimaraes, D.E.; Piekarz, A.; Ferland-McCollough, D.; Bushell, M.; Ozanne, S.E. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol. Metab. 2014, 3, 325–333. [Google Scholar] [CrossRef]
- Benatti, R.O.; Melo, A.M.; Borges, F.O.; Ignacio-Souza, L.M.; Simino, L.A.P.; Milanski, M.; Velloso, L.A.; Torsoni, M.A.; Torsoni, A.S. Maternal high-fat diet consumption modulates hepatic lipid metabolism and microRNA-122 (miR-122) and microRNA-370 (miR-370) expression in offspring. Br. J. Nutr. 2014, 111, 2112–2122. [Google Scholar] [CrossRef]
- Soubry, A.; Schildkraut, J.M.; Murtha, A.P.; Wang, F.; Huang, Z.; Bernal, A.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K.; Hoyo, C. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.D.C.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2015, 5, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.; Peng, H.; Zhang, X.; Qian, J.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2015, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Dias, I.H.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef] [PubMed]
- Bodden, C.; Hannan, A.J.; Reichelt, A.C. Diet-Induced Modification of the Sperm Epigenome Programs Metabolism and Behavior. Trends Endocrinol. Metab. 2020, 31, 131–149. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barrès, R.; Owens, J.; Morris, M.J. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef]
- Mitchell, M.; Bakos, H.W.; Lane, M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil. Steril. 2011, 95, 1349–1353. [Google Scholar] [CrossRef]
- Fullston, T.; Teague, E.M.C.O.; Palmer, N.O.; De Blasio, M.; Mitchell, M.; Corbett, M.; Print, C.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F 2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef]
- Govic, A.; Penman, J.; Tammer, A.H.; Paolini, A.G.; Information, P.E.K.F.C. Paternal calorie restriction prior to conception alters anxiety-like behavior of the adult rat progeny. Psychoneuroendocrinology 2016, 64, 1–11. [Google Scholar] [CrossRef]
- Anderson, L.M.; Riffle, L.; Wilson, R.; Travlos, G.S.; Lubomirski, M.S.; Alvord, W.G. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition 2006, 22, 327–331. [Google Scholar] [CrossRef]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Sinclair, K.D. Paternal low protein diet affects adult offspring cardiovascular and metabolic function in mice. Am. J. Physiol. Circ. Physiol. 2014, 306, H1444–H1452. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.H.; Maekawa, T.; Yoshida, K.; Liu, Y.; Muratani, M.; Ishii, S. RNA-Sequencing Analysis of Paternal Low-Protein Diet-Induced Gene Expression Change in Mouse Offspring Adipocytes. G3 (Bethesda) 2019, 9, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Reproductive Disease. Boil. Reprod. 2015, 93, 145. [Google Scholar] [CrossRef]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M.S. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef]
- Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sadri-Vakili, G.; Pierce, R.C. Epigenetic inheritance of a cocaine-resistance phenotype. Nat. Neurosci. 2012, 16, 42–47. [Google Scholar] [CrossRef]
- Hawkey, A.B.; White, H.; Pippen, E.; Greengrove, E.; Rezvani, A.H.; Murphy, S.K.; Levin, E.D. Paternal nicotine exposure in rats produces long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 2019, 74, 106808. [Google Scholar] [CrossRef]
- Levin, E.D.; Hawkey, A.B.; Hall, B.J.; Cauley, M.; Slade, S.; Yazdani, E.; Kenou, B.; White, H.; Wells, C.; Rezvani, A.H.; et al. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol. Teratol. 2019, 74, 106806. [Google Scholar] [CrossRef]
- Rissman, E.F.; Adli, M. Minireview: Transgenerational epigenetic inheritance: Focus on endocrine disrupting compounds. Endocrinology 2014, 155, 2770–2780. [Google Scholar] [CrossRef]
- Latchney, S.E.; Fields, A.M.; Susiarjo, M. Linking inter-individual variability to endocrine disruptors: Insights for epigenetic inheritance. Mamm. Genome 2017, 29, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Flaws, J.A. The epigenetic impacts of endocrine disruptors on female reproduction across generations dagger. Biol. Reprod. 2019, 101, 635. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.; Owens, J.; Fullston, T.; Lane, M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am. J. Physiol. Metab. 2015, 308, E805–E821. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.F.; Derieppe, M.-A.; Rémy, J.-J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef] [PubMed]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rando, O.J. Metabolic Inputs into the Epigenome. Cell Metab. 2017, 25, 544–558. [Google Scholar] [CrossRef]
- Archer, E. In reply—Epigenetics and Childhood Obesity. Mayo Clin. Proc. 2015, 90, 693–695. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; James, E.R.; Carrell, D.T. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Boil. Reprod. Med. 2017, 63, 69–76. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzago, M.; Santurbano, D.; Vitacolonna, E.; Stuppia, L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. Int. J. Mol. Sci. 2020, 21, 2633. https://doi.org/10.3390/ijms21072633
Franzago M, Santurbano D, Vitacolonna E, Stuppia L. Genes and Diet in the Prevention of Chronic Diseases in Future Generations. International Journal of Molecular Sciences. 2020; 21(7):2633. https://doi.org/10.3390/ijms21072633
Chicago/Turabian StyleFranzago, Marica, Daniele Santurbano, Ester Vitacolonna, and Liborio Stuppia. 2020. "Genes and Diet in the Prevention of Chronic Diseases in Future Generations" International Journal of Molecular Sciences 21, no. 7: 2633. https://doi.org/10.3390/ijms21072633
APA StyleFranzago, M., Santurbano, D., Vitacolonna, E., & Stuppia, L. (2020). Genes and Diet in the Prevention of Chronic Diseases in Future Generations. International Journal of Molecular Sciences, 21(7), 2633. https://doi.org/10.3390/ijms21072633




