The Aquaporin-3-Inhibiting Potential of Polyoxotungstates
Abstract
1. Introduction
2. Results
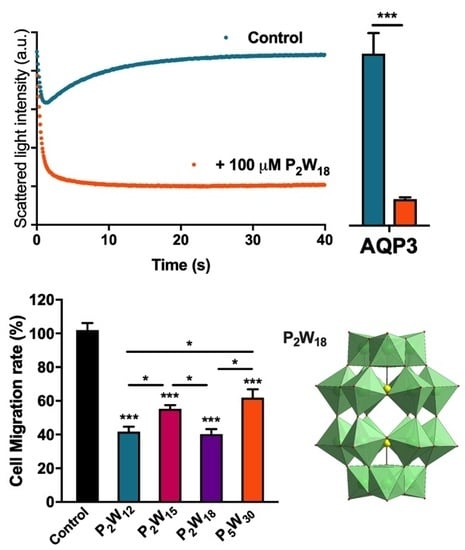
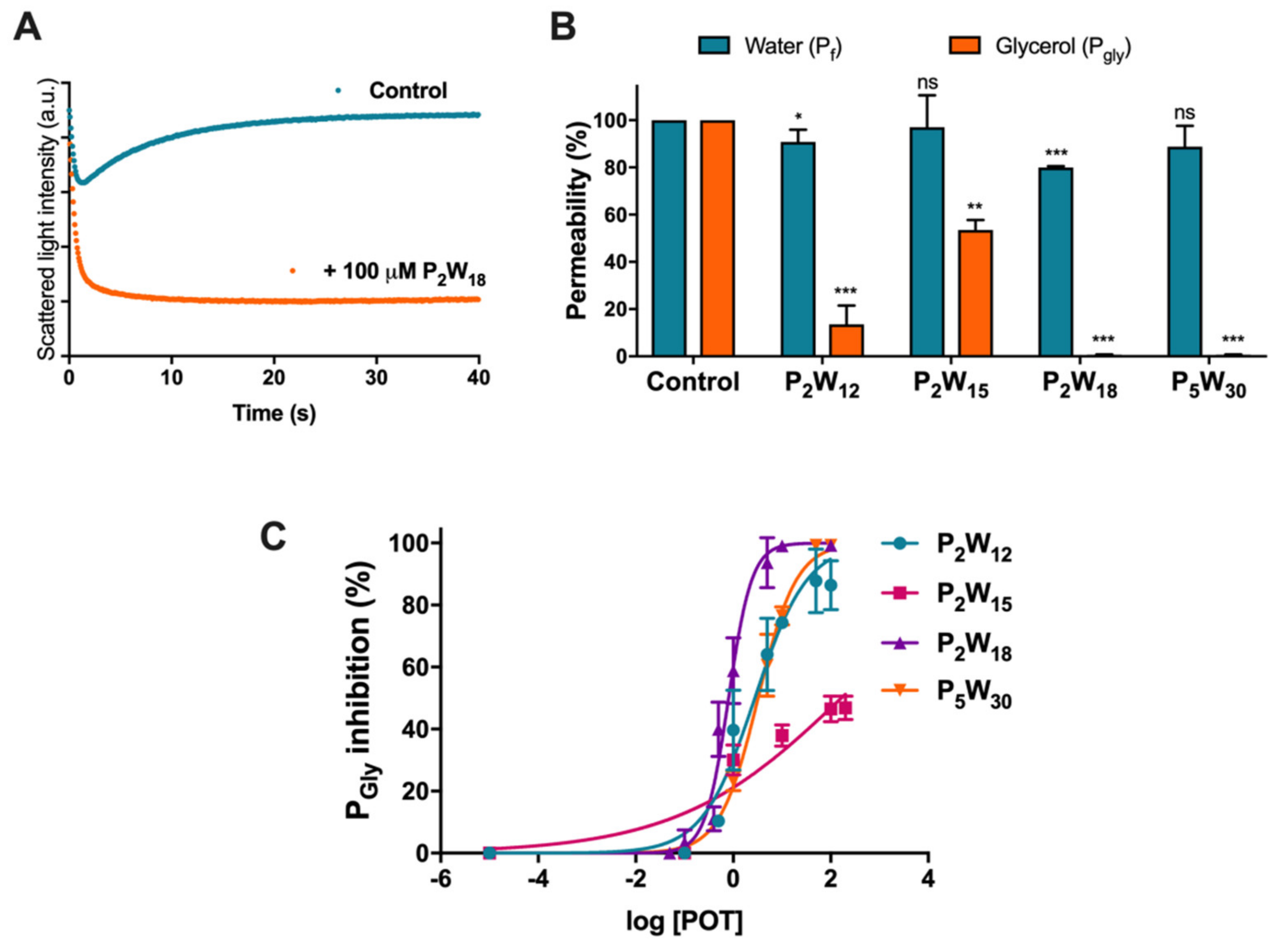
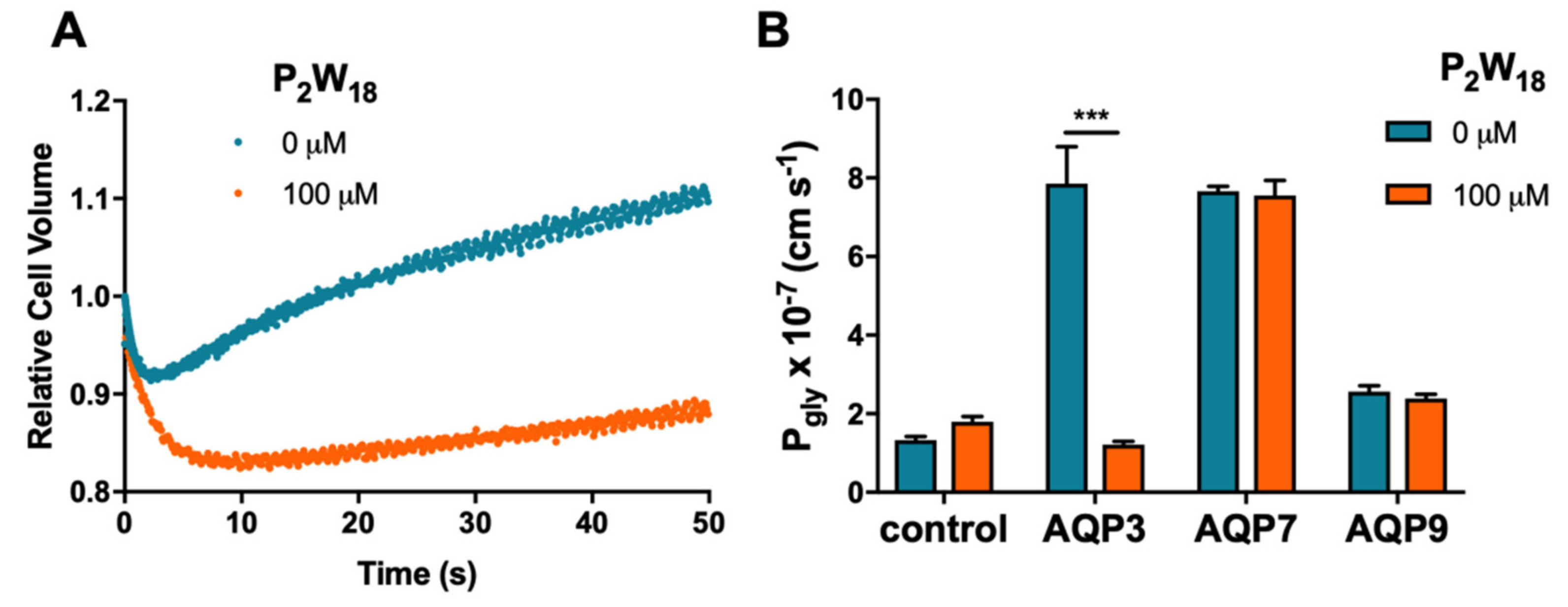
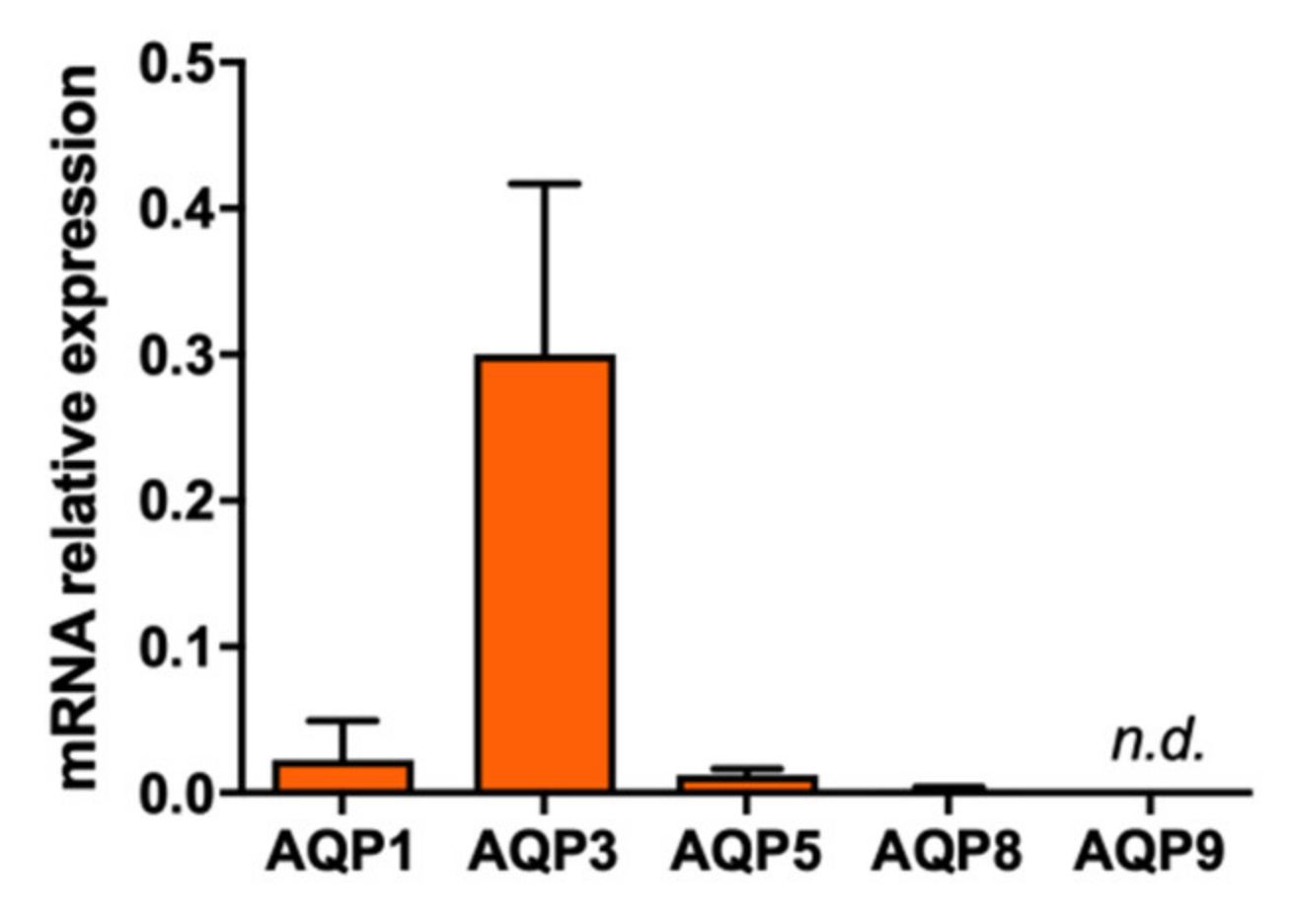
2.1. Effect of Polyoxotungstates on the Permeability of Water and Glycerol
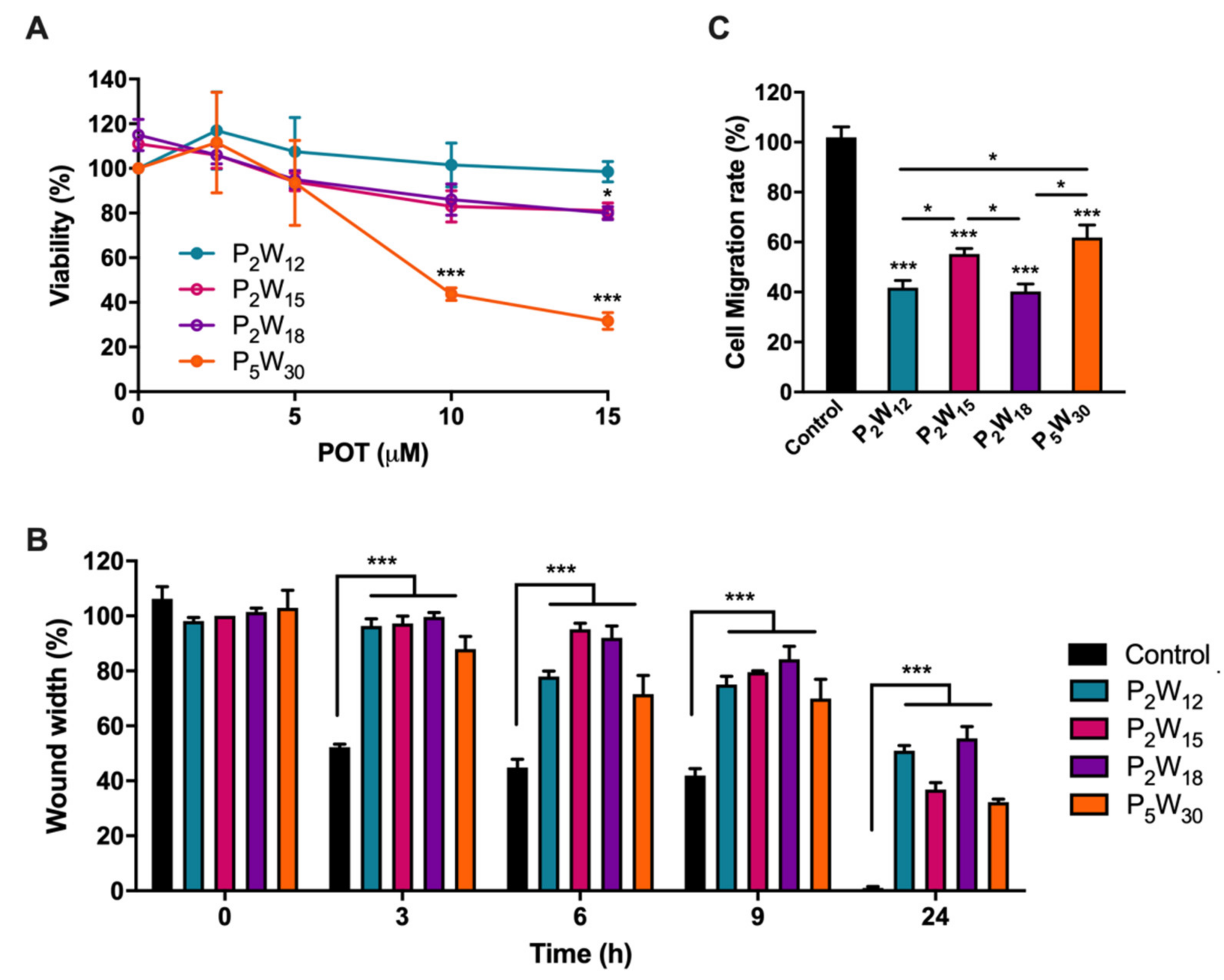
2.2. Effect of Polyoxotungstates on Melanoma Cell Migration
3. Discussion
4. Materials and Methods
4.1. Polyoxometalates
4.2. Ethics Statement
4.3. Erythrocyte Sampling and Preparation
4.4. Strains, Plasmids, and Growth Conditions
4.5. Permeability Assays
4.6. Cell Culture
4.7. RNA Isolation, cDNA Synthesis, and Quantitative PCR
4.8. Viability Assay
4.9. Migration Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| hRBC | Human red blood cells |
| Pf | Water permeability coefficient |
| Pgly | Glycerol permeability coefficient |
| POMs | Polyoxometalates |
| POTs | Polyoxotungstates |
References
- Sánchez-Lara, E.; Treviño, S.; Sánchez-Gaytán, B.L.; Sánchez-Mora, E.; Castro, M.E.; Meléndez-Bustamante, F.J.; Méndez-Rojas, M.A.; González-Vergara, E. Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs Against Diabetes and Cancer. Front. Chem. 2018, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Stuckart, M.; Monakhov, K. Polyoxometalates as components of supramolecular assemblies. Chem. Sci. 2019, 10, 4364–4376. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef]
- Chi, G.; Wang, L.; Chen, B.; Li, J.; Hu, J.; Zhang, S.; Zhao, M.; Ding, X.; Li, Y. Polyoxometalates: Study of inhibitory kinetics and mechanism against α-glucosidase. J. Inorg. Biochem. 2019, 199, 110784. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Krivosudský, L.; Fraqueza, G.; Breibeck, J.; Al-Sayed, E.; Tanuhadi, E.; Bijelic, A.; Fuentes, J.; Aureliano, M.; Rompel, A. The P-type ATPase inhibiting potential of polyoxotungstates. Metallomics 2018, 10, 287–295. [Google Scholar] [CrossRef]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between polyoxometalates and biological systems: From drug design to artificial enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef]
- Zhang, B.; Qiu, J.; Wu, C.; Li, Y.; Liu, Z. Anti-tumor and immunomodulatory activity of iron hepta-tungsten phosphate oxygen clusters complex. Int. Immunopharmacol. 2015, 29, 293–301. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-Lopez, A.; Arredondo, M.; Lopez, P.; Diéguez, A.R.; Montes-Bayon, M.; Aranda, P.; Llopis, J.; Sánchez-González, C. In vitro study of the protective effect of manganese against vanadium-mediated nuclear and mitochondrial DNA damage. Food Chem. Toxicol. 2020, 135, 110900. [Google Scholar] [CrossRef]
- Bernold, H. Polyoxometalates: Introduction to a class of inorganic compounds and their biomedical applications. Front. Biosci. 2005, 10, 275–287. [Google Scholar]
- Shah, H.S.; Al-Oweini, R.; Haider, A.; Kortz, U.; Iqbal, J. Cytotoxicity and enzyme inhibition studies of polyoxometalates and their chitosan nanoassemblies. Toxicol. Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Sánchez-González, C.; Vera-Ramirez, L.; Giampieri, F.; Navarro-Hortal, M.D.; Xiao, J.; Llopis, J.; Battino, M.; Varela-Lopez, A. Reductive Stress, Bioactive Compounds, Redox-Active Metals, and Dormant Tumor Cell Biology to Develop Redox-Based Tools for the Treatment of Cancer. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Direito, I.; Paulino, J.; Vigia, E.; Brito, M.A.; Soveral, G. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2017, 115, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, J.M.; Agre, P. Discovery of the Aquaporins and Development of the Field; Springer: Berlin/Heidelberg, Germany, 2009; Volume 190, pp. 3–28. [Google Scholar]
- Benga, G. On the definition, nomenclature and classification of water channel proteins (aquaporins and relatives). Mol. Asp. Med. 2012, 33, 514–517. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1507–1512. [Google Scholar] [CrossRef]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; De Moura, T.M.F.; Mallafré, V.C.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Bertolotti, M.; Bestetti, S.; Garcia-Manteiga, J.M.; Medraño-Fernandez, I.; Mas, A.D.; Malosio, M.L.; Sitia, R. Tyrosine Kinase Signal Modulation: A Matter of H2O2 Membrane Permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Gašparović, A.Č.; Soveral, G. Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 2090. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; Da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Nielsen, S.; Casini, A. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery, 1st ed.; Soveral, G., Nielsen, S., Eds.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-971-4987-0783-1. [Google Scholar]
- Soveral, G.; Casini, A. Aquaporin modulators: A patent review (2010–2015). Expert Opin. Ther. Patents 2016, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Timar, J.; Gyorffy, B.; Raso, E. Gene signature of the metastatic potential of cutaneous melanoma: Too much for too little? Clin. Exp. Metastasis 2010, 27, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Osorio, G.; Zulueta-Dorado, T.; González-Rodríguez, P.; Bernabéu-Wittel, J.; Conejo-Mir, J.; Ramírez-Lorca, R.; Echevarría, M. Expression Pattern of Aquaporin 1 and Aquaporin 3 in Melanocytic and Nonmelanocytic Skin Tumors. Am. J. Clin. Pathol. 2019, 152, 446–457. [Google Scholar] [CrossRef]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Cobo, A.G.; Echevarría, M.; De Moura, T.M.F.; Re, N.; Casini, A.; Soveral, G. Targeting Aquaporin Function: Potent Inhibition of Aquaglyceroporin-3 by a Gold-Based Compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Boil. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef]
- Madeira, A.; De Moura, T.M.F.; Soveral, G. Detecting Aquaporin Function and Regulation. Front. Chem. 2016, 4, 1468. [Google Scholar] [CrossRef]
- Campos, E.; De Moura, T.M.F.; Oliva, A.; Leandro, P.; Soveral, G. Lack of Aquaporin 3 in bovine erythrocyte membranes correlates with low glycerol permeation. Biochem. Biophys. Res. Commun. 2011, 408, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Madeira, A.; Loureiro-Dias, M.; De Moura, T.M.F. Water Transport in Intact Yeast Cells as Assessed by Fluorescence Self-Quenching. Appl. Environ. Microbiol. 2007, 73, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.; Martins, A.P.; Mósca, A.F.; Wijma, H.J.; Prista, C.; Soveral, G.; Casini, A. Exploring the gating mechanisms of aquaporin-3: New clues for the design of inhibitors? Mol. BioSyst. 2016, 12, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Mósca, A.F.; De Almeida, A.; Wragg, D.; Martins, A.P.; Sabir, F.; Leoni, S.; Moura, T.F.; Prista, C.; Casini, A.; Soveral, G. Molecular Basis of Aquaporin-7 Permeability Regulation by pH. Cells 2018, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Dianat, S.; Bordbar, A.; Tangestaninejad, S.; Yadollahi, B.; Esfahani, S.H.Z.; Habibi, P. ctDNA binding affinity and in vitro antitumor activity of three Keggin type polyoxotungestates. J. Photochem. Photobiol. B Boil. 2013, 124, 27–33. [Google Scholar] [CrossRef]
- Fu, L.; Gao, H.; Yan, M.; Li, S.; Li, X.; Dai, Z.; Liu, S. Polyoxometalate-Based Organic-Inorganic Hybrids as Antitumor Drugs. Small 2015, 11, 2938–2945. [Google Scholar] [CrossRef]
- Sun, T.; Cui, W.; Yan, M.; Qin, G.; Guo, W.; Gu, H.; Liu, S.; Wu, Q. Target Delivery of a Novel Antitumor Organoplatinum(IV)-Substituted Polyoxometalate Complex for Safer and More Effective Colorectal Cancer Therapy In Vivo. Adv. Mater. 2016, 28, 7397–7404. [Google Scholar] [CrossRef]
- Wang, L.; Yu, K.; Zhou, B.-B.; Su, Z.-H.; Gao, S.; Chu, L.-L.; Liu, J.-R. The inhibitory effects of a new cobalt-based polyoxometalate on the growth of human cancer cells. Dalton Trans. 2014, 43, 6070–6078. [Google Scholar] [CrossRef]
- León, I.E.; Porro, V.; Astrada, S.; Egusquiza, M.; Cabello, C.; Bollati-Fogolin, M.; Etcheverry, S.B. Polyoxometalates as antitumor agents: Bioactivity of a new polyoxometalate with copper on a human osteosarcoma model. Chem. Interactions 2014, 222, 87–96. [Google Scholar] [CrossRef]
- Ogata, A.; Mitsui, S.; Yanagie, H.; Kasano, H.; Hisa, T.; Yamase, T.; Eriguchi, M. A novel anti-tumor agent, polyoxomolybdate induces apoptotic cell death in AsPC-1 human pancreatic cancer cells. Biomed. Pharmacother. 2005, 59, 240–244. [Google Scholar] [CrossRef]
- Mitsui, S.; Ogata, A.; Yanagie, H.; Kasano, H.; Hisa, T.; Yamase, T.; Eriguchi, M. Antitumor activity of polyoxomolybdate, [NH3Pri]6[Mo7O24]·3H2O, against, human gastric cancer model. Biomed. Pharmacother. 2006, 60, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Fujita, T.; Sakurai, T.; Yamase, T.; Seto, Y. Antitumor Activity of New Antitumor Substance, Polyoxomolybdate, against Several human Cancers in Athymic Nude Mice. Tohoku J. Exp. Med. 1992, 168, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell. Boil. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- De Moura, T.M.F.; Macey, R.I.; Chien, D.Y.; Karan, D.; Santos, H. Thermodynamics of all-or-none water channel closure in red cells. J. Membr. Boil. 1984, 81, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Ciancetta, A.; De Almeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin Inhibition by Gold(III) Compounds: New Insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef]
- De Almeida, A.; Mósca, A.F.; Wragg, D.; Wenzel, M.N.; Kavanagh, P.; Barone, G.; Leoni, S.; Soveral, G.; Casini, A. The mechanism of aquaporin inhibition by gold compounds elucidated by biophysical and computational methods. Chem. Commun. 2017, 53, 3830–3833. [Google Scholar] [CrossRef]
- Madeira, A.; Camps, M.; Zorzano, A.; De Moura, T.M.F.; Soveral, G. Biophysical Assessment of Human Aquaporin-7 as a Water and Glycerol Channel in 3T3-L1 Adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef]
- Liu, J.-F.; Chen, Y.-G.; Meng, L.; Guo, J.; Liu, Y.; Pope, M.T. Synthesis and characterization of novel heteropoly-tungstoarsenates containing lanthanides [LnAs4W40O140]25− and their biological activity. Polyhedron 1998, 17, 1541–1546. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, S.; Liu, S.; Wang, E. In vitro inhibitory effect of polyoxometalates on human tumor cells. Transit. Met. Chem. 2005, 30, 113–117. [Google Scholar] [CrossRef]
- Xing, R.; Wang, F.; Dong, L.; Zheng, A.-P.; Wang, L.; Su, W.-J.; Lin, T. Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem. 2016, 197, 205–211. [Google Scholar] [CrossRef]
- Gabas, I.M.; Stepien, G.; Moros, M.; Mitchell, S.G.; De La Fuente, J.M. In vitro cell cytotoxicity profile and morphological response to polyoxometalate-stabilised gold nanoparticles. New J. Chem. 2016, 40, 1039–1047. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Inorganic Chemistry; Willey & Sons: New York, NY, USA, 1990; Volume 27. [Google Scholar]
- Jeannin, Y.; Martin-Frère, J.; Choi, D.J.; Pope, M.T. The Sodium Pentaphosphato(V)-Triacontatungstate Anion Isolated as the Ammonium Salt. Inorg. Synth. 2007, 27, 115–118. [Google Scholar]
- Dawson, B. The structure of the 9(18)-heteropoly anion in potassium 9(18)-tungstophosphate, K6(P2W18O62).14H2O. Acta Crystallogr. 1953, 6, 113–126. [Google Scholar] [CrossRef]
- Mal, S.S.; Kortz, U. The Wheel-Shaped Cu20 Tungstophosphate [Cu20Cl(OH)24(H2O)12(P8W48O184)]25− Ion. Angew. Chem. Int. Ed. 2005, 44, 3777–3780. [Google Scholar] [CrossRef] [PubMed]
- Finke, R.G.; Lyon, D.K.; Nomiya, K.; Weakley, T.J.R. Structure of nonasodium α-triniobatopentadecawolframatodiphosphate–acetonitrile–water (1/2/23), Na9[P2W15Nb3O62]2CH3CN.23H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 1592–1596. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Harmalker, S.P.; Jeannin, Y.; Martin-Frere, J.; Pope, M.T. A heteropolyanion with fivefold molecular symmetry that contains a nonlabile encapsulated sodium ion. The structure and chemistry of [NaP5W30O110]14−. J. Am. Chem. Soc. 1985, 107, 2662–2669. [Google Scholar] [CrossRef]
- Güldener, U. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996, 24, 2519–2524. [Google Scholar] [CrossRef]
- Pronk, J.T. Auxotrophic Yeast Strains in Fundamental and Applied Research. Appl. Environ. Microbiol. 2002, 68, 2095–2100. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Da Silva, I.V.; Barroso, M.; Moura, T.F.; E Castro, M.R.A.; Soveral, G. Endothelial Aquaporins and Hypomethylation: Potential Implications for Atherosclerosis and Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 130. [Google Scholar] [CrossRef]
| POTs | Glycerol Permeability | |
|---|---|---|
| Max Inhibition (%) | IC50 (µM) | |
| P2W12 | 86.40 ± 7.91 | 2.78 ± 0.09 |
| P2W15 | 46.47 ± 4.18 | >100 |
| P2W18 | 99.24 ± 0.03 | 0.80 ± 0.04 |
| P5W30 | 99.31 ± 0.14 | 3.24 ± 0.03 |
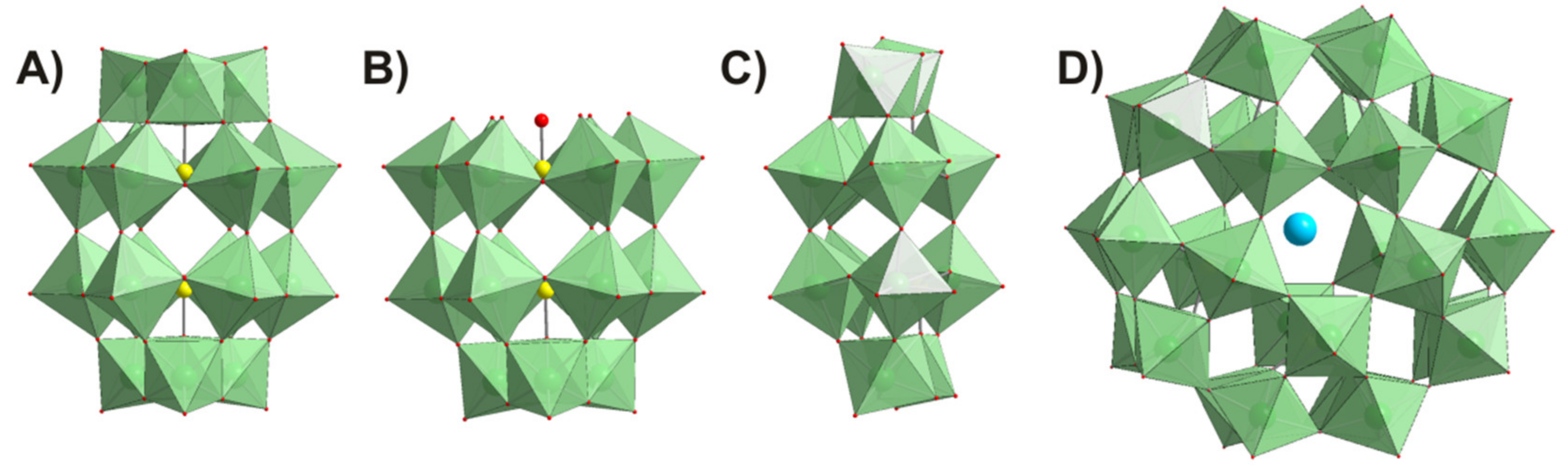
| Formula | Abbreviation | Net Charge | Charge Density (Charge/Number of Addenda Atoms Ratio) | Synthesized According to | First Structural Report in | 31P-NMR Signals Assignment According to |
|---|---|---|---|---|---|---|
| K6[α-P2W18O62]·14H2O | P2W18 | −6 | 0.33 | [54] | [56] | [54] |
| K12[α-H2P2W12O48]·16H2O | P2W12 | −12 | 1 | [54] | [57] | [54] |
| Na12[α-P2W15O56]·24H2O | P2W15 | −12 | 0.8 | [54] | [58] | [54] |
| (NH4)14[NaP5W30O110] 31H2O | P5W30 | −14 | 0.47 | [55] | [59] | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. https://doi.org/10.3390/ijms21072467
Pimpão C, da Silva IV, Mósca AF, Pinho JO, Gaspar MM, Gumerova NI, Rompel A, Aureliano M, Soveral G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. International Journal of Molecular Sciences. 2020; 21(7):2467. https://doi.org/10.3390/ijms21072467
Chicago/Turabian StylePimpão, Catarina, Inês V. da Silva, Andreia F. Mósca, Jacinta O. Pinho, Maria Manuela Gaspar, Nadiia I. Gumerova, Annette Rompel, Manuel Aureliano, and Graça Soveral. 2020. "The Aquaporin-3-Inhibiting Potential of Polyoxotungstates" International Journal of Molecular Sciences 21, no. 7: 2467. https://doi.org/10.3390/ijms21072467
APA StylePimpão, C., da Silva, I. V., Mósca, A. F., Pinho, J. O., Gaspar, M. M., Gumerova, N. I., Rompel, A., Aureliano, M., & Soveral, G. (2020). The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. International Journal of Molecular Sciences, 21(7), 2467. https://doi.org/10.3390/ijms21072467