Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM
Abstract
1. Introduction
2. Results
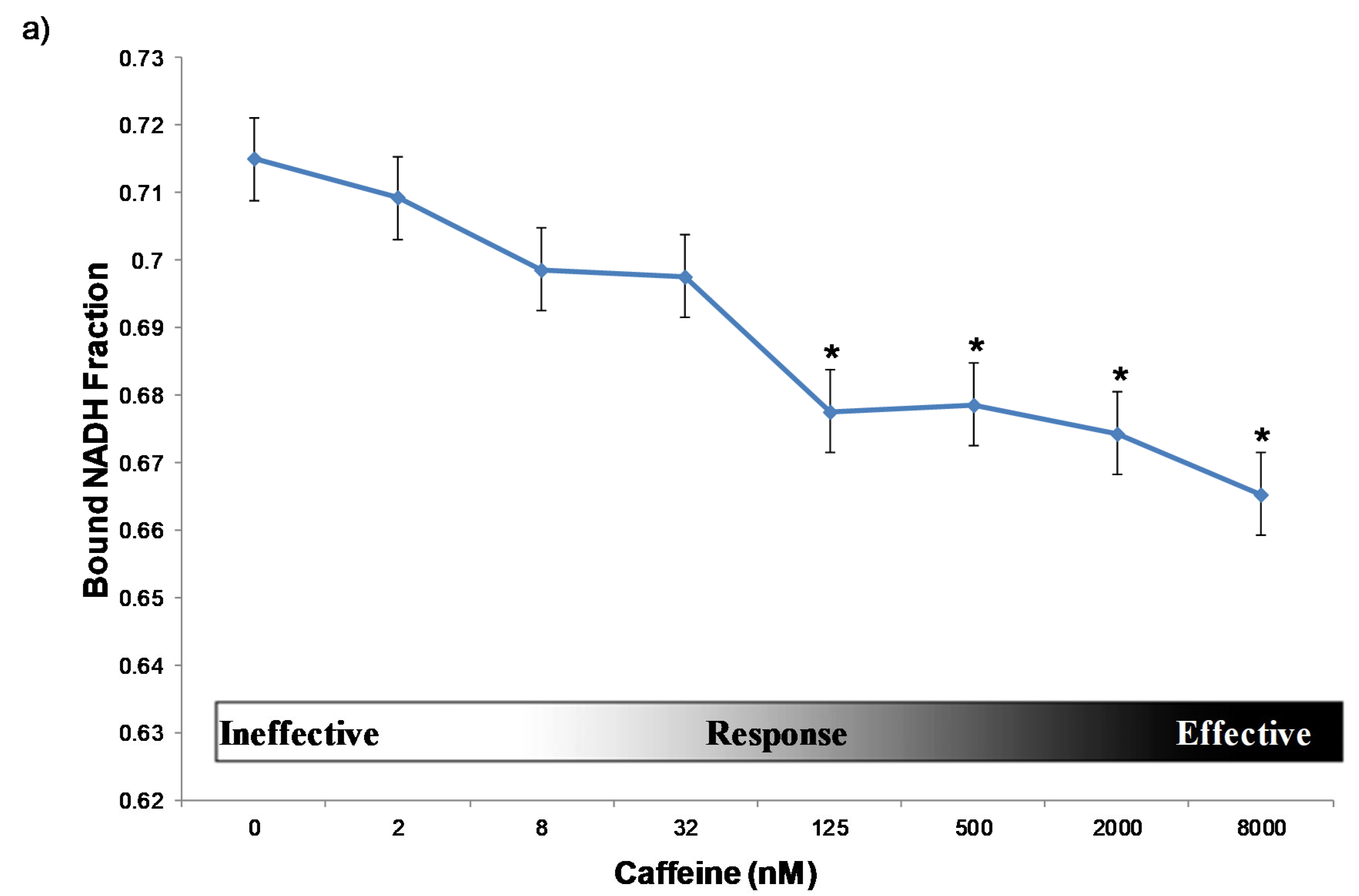
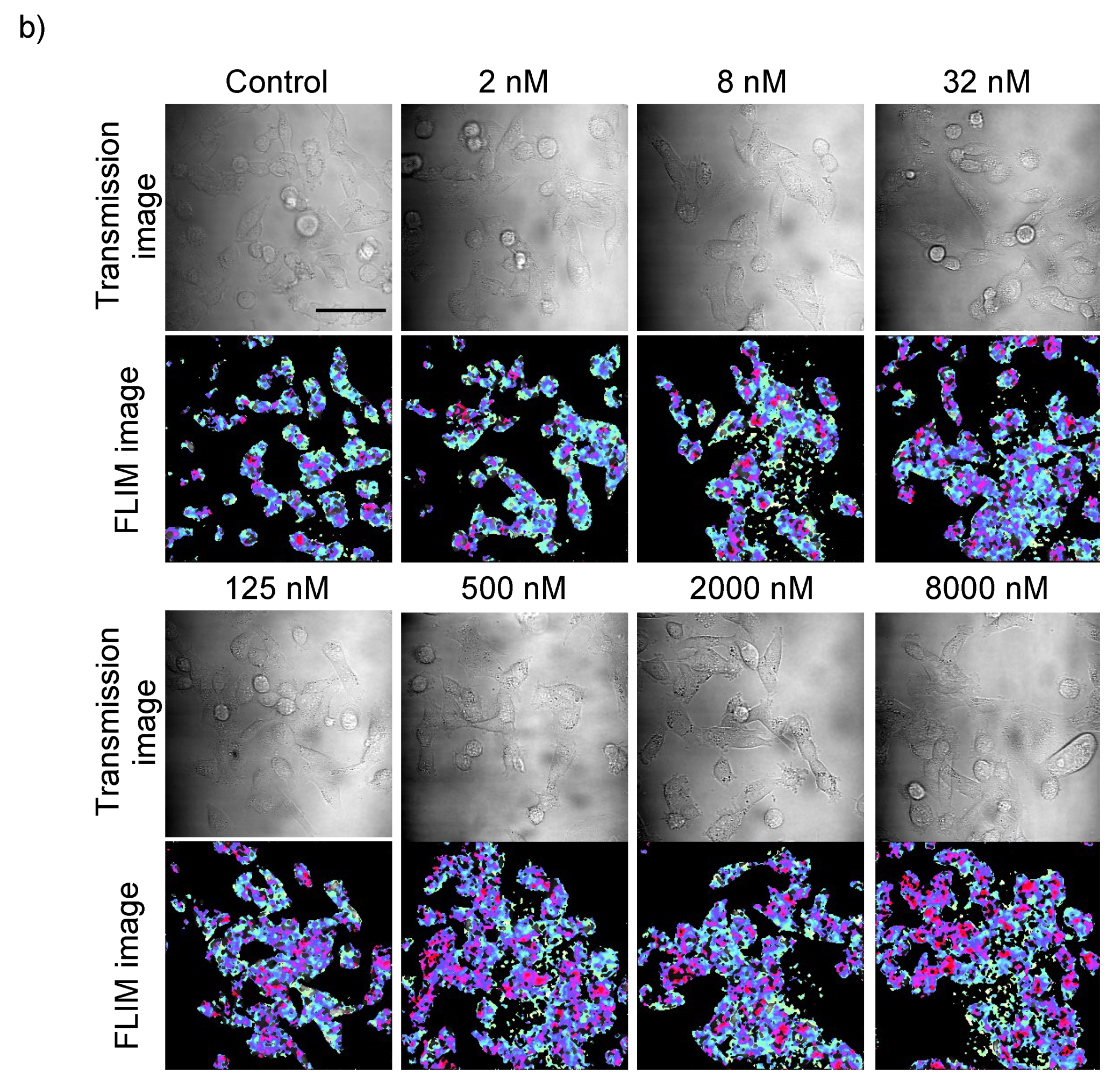
2.1. Dosage Dependency of Caffeine
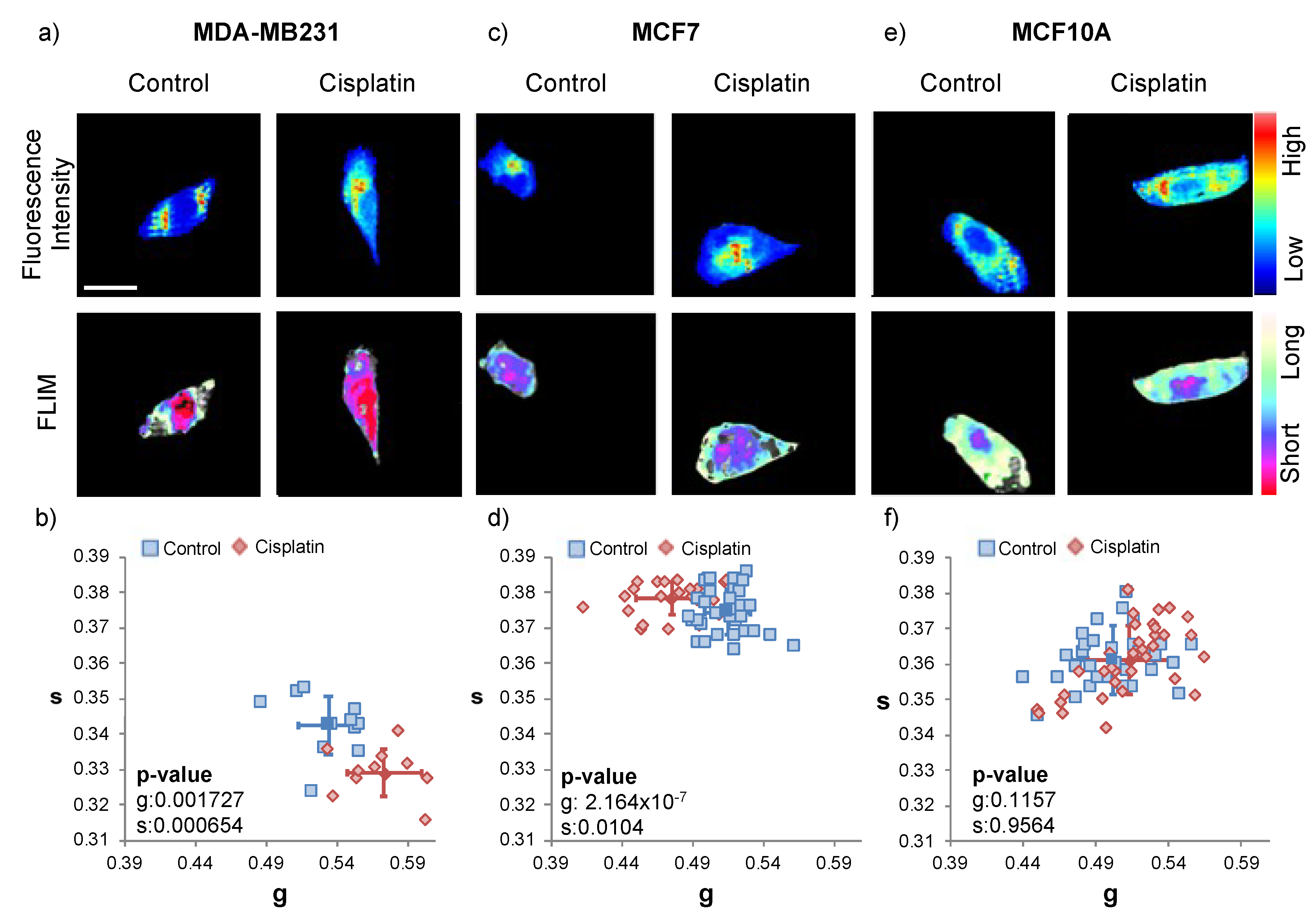
2.2. Cisplatin Treatment
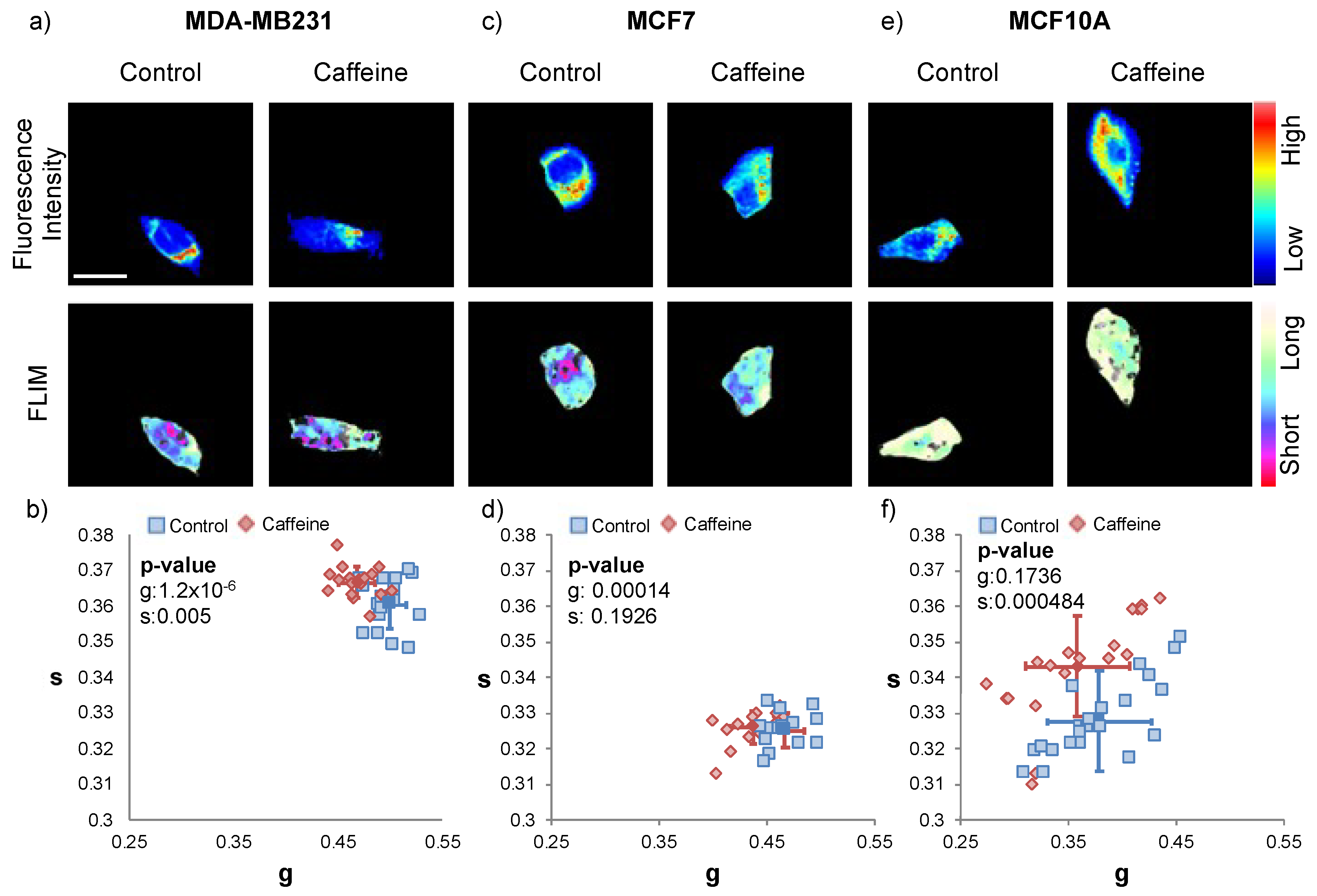
2.3. Caffeine Treatment
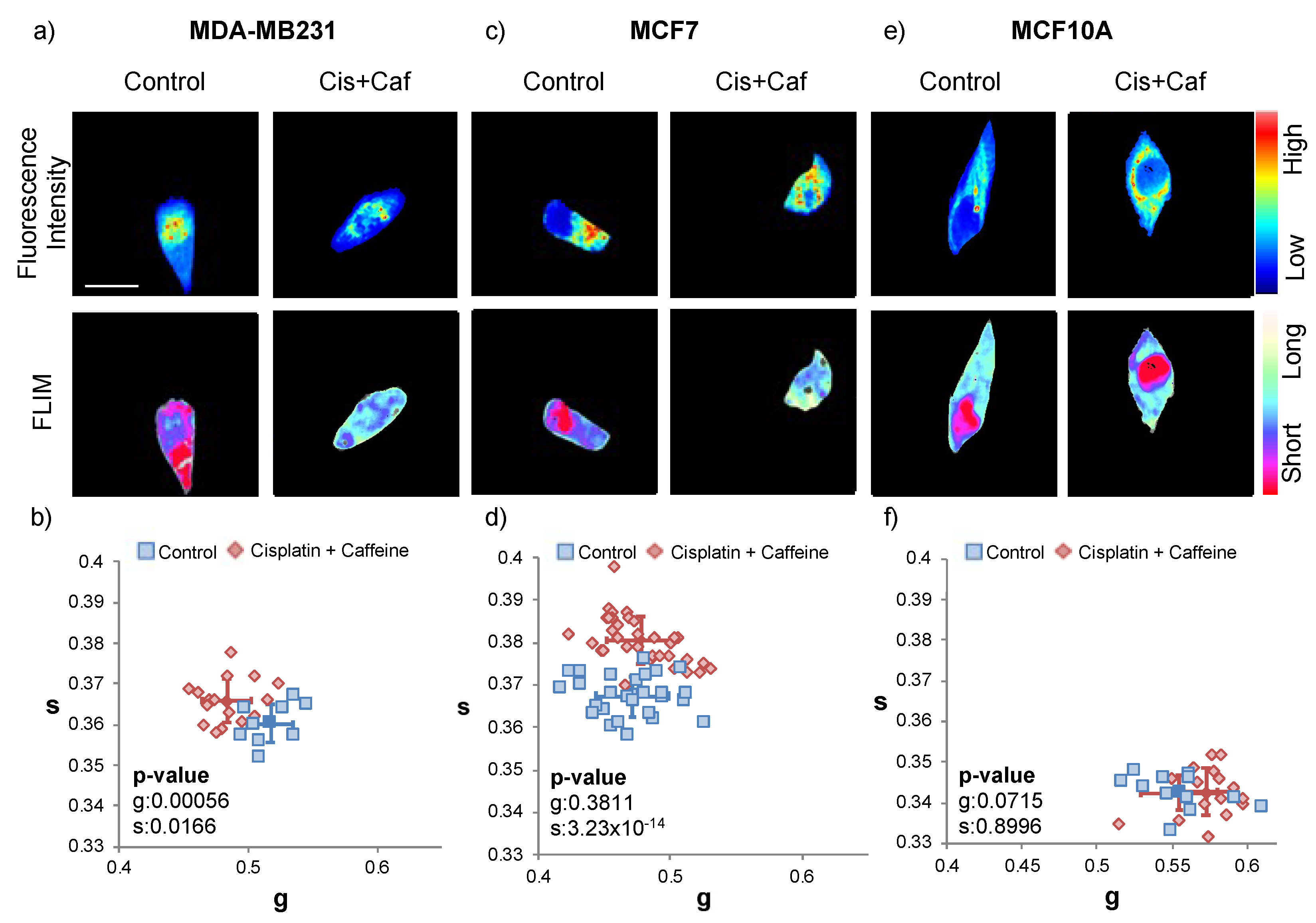
2.4. Cisplatin and Caffeine Treatment
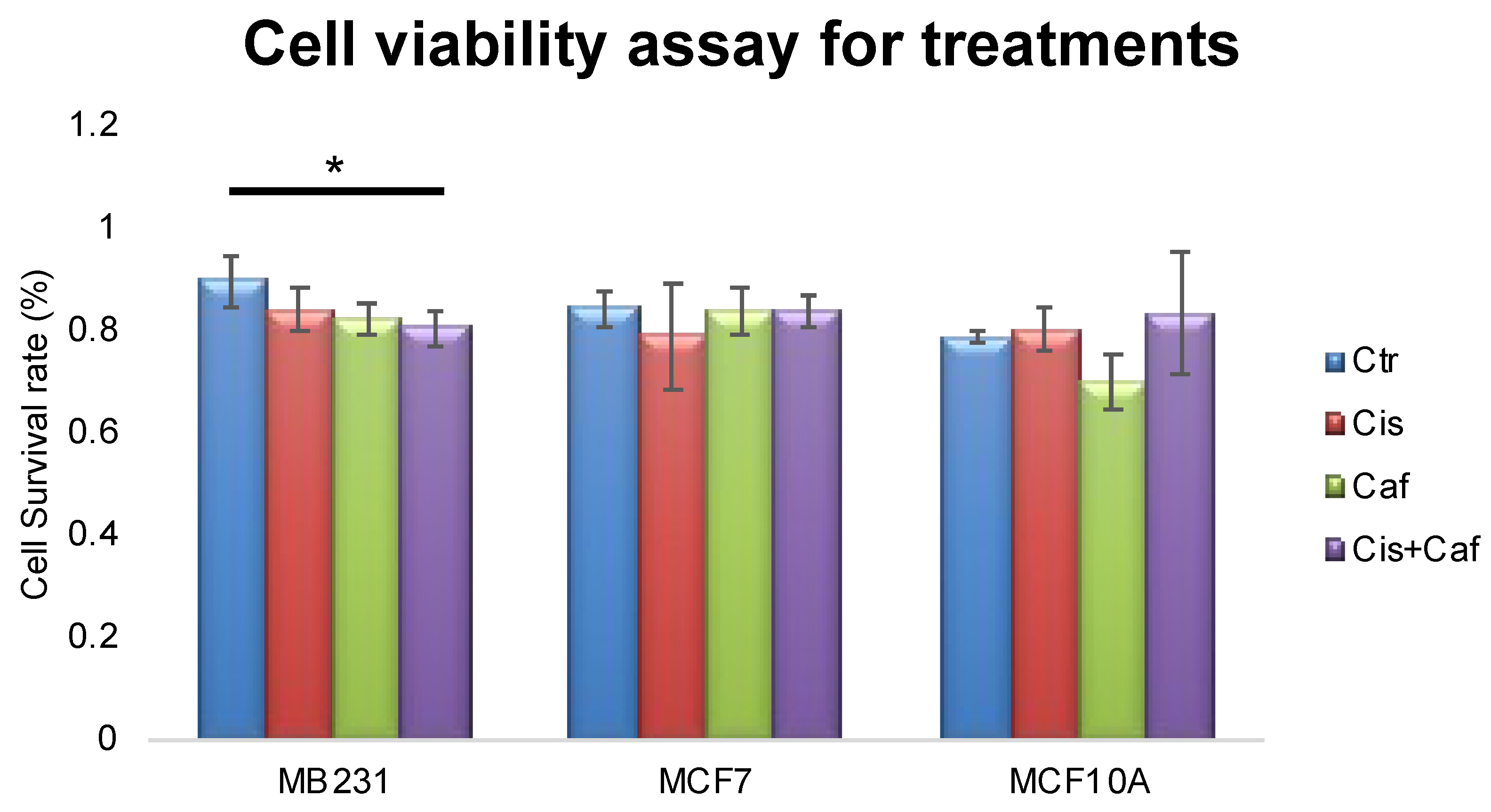
2.5. Cell Viability with Different Treatments
3. Discussion
4. Materials and Methods
4.1. Fluorescence Lifetime Imaging Microscopy (FLIM)
4.2. Phasor Approach
4.3. Cell Preparation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NBCF. Breast Cancer Facts: The National Breast Cancer Foundation. 2019. Available online: https://www.nationalbreastcancer.org/breast-cancer-facts (accessed on 25 February 2020).
- Institute, N.C. Biological Therapies for Cancer—National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet (accessed on 31 May 2019).
- Zhong, Z.; Pannu, V.; Rosenow, M.; Stark, A.; Spetzler, D. KIAA0100 Modulates Cancer Cell Aggression Behavior of MDA-MB-231 through Microtubule and Heat Shock Proteins. Cancers 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Irvin, W.J., Jr.; Carey, L.A. What is triple-negative breast cancer? Eur. J. Cancer 2008, 44, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35. [Google Scholar] [CrossRef] [PubMed]
- Digman, M.A.; Caiolfa, V.R.; Zamai, M.; Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 2008, 94, L14–L16. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug. Discov. 2005, 4, 307. [Google Scholar] [CrossRef]
- Wu, K.; Tan, M.-Y.; Jiang, J.-T.; Mu, X.-Y.; Wang, J.-R.; Zhou, W.-J.; Wang, X.; Li, M.-q.; He, Y.-Y.; Liu, Z.-H. Cisplatin inhibits the progression of bladder cancer by selectively depleting G-MDSCs: A novel chemoimmunomodulating strategy. Clin. Immunol. 2018, 193, 60–69. [Google Scholar] [CrossRef]
- Högberg, T.; Glimelius, B.; Nygren, P. A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol. 2001, 40, 340–360. [Google Scholar] [CrossRef]
- Society, A.C. Chemotherapy Side Effects. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html (accessed on 31 May 2019).
- Wang, G.; Bhoopalan, V.; Wang, D.; Wang, L.; Xu, X. The effect of caffeine on cisplatin-induced apoptosis of lung cancer cells. Exp. Hematol. Oncol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.-L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Busby, E.C.; Tibbetts, R.S.; Roos, P.; Taya, Y.; Karnitz, L.M.; Abraham, R.T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999, 59, 4375–4382. [Google Scholar]
- Selby, C.P.; Sancar, A. Molecular mechanisms of DNA repair inhibition by caffeine. Proc. Natl. Acad. Sci. USA 1990, 87, 3522–3525. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Nagata, M.; Kohno, T.; Ichimiya, A.; Iwakiri, T.; Okumura, M.; Arimori, K. Caffeine increases the antitumor effect of Cisplatin in human hepatocellular carcinoma cells. Biol. Pharm. Bull. 2012, 35, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Takahashi, Y.; Takazawa, K.; Tsuchiya, H.; Tomita, K.; Yokogawa, K.; Miyamoto, K.-i. Caffeine dose-dependently potentiates the antitumor effect of cisplatin on osteosarcomas. Anticancer Res. 2008, 28, 1681–1685. [Google Scholar] [PubMed]
- Xu, Y.; Ho, C.-T.; Amin, S.G.; Han, C.; Chung, F.-L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992, 52, 3875–3879. [Google Scholar]
- Lu, G.; Liao, J.; Yang, G.; Reuhl, K.R.; Hao, X.; Yang, C.S. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone–induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006, 66, 11494–11501. [Google Scholar] [CrossRef]
- Conney, A.H.; Zhou, S.; Lee, M.-J.; Xie, J.G.; Yang, C.S.; Lou, Y.R.; Lu, Y. Stimulatory effect of oral administration of tea, coffee or caffeine on UVB-induced apoptosis in the epidermis of SKH-1 mice. Toxicol. Appl. Pharmacol. 2007, 224, 209–213. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Gertig, D.M.; Gates, M.A.; Hecht, J.L.; Hankinson, S.E. Caffeine, alcohol, smoking, and the risk of incident epithelial ovarian cancer. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 1169–1177. [Google Scholar] [CrossRef]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef]
- Network, C.G.A. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61. [Google Scholar] [CrossRef]
- Aminzadeh, S.; Vidali, S.; Sperl, W.; Kofler, B.; Feichtinger, R.G. Energy metabolism in neuroblastoma and Wilms tumor. Transl. Pediatrics 2015, 4, 20. [Google Scholar]
- Dirks, A.J.; Hofer, T.; Marzetti, E.; Pahor, M.; Leeuwenburgh, C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006, 5, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tan, M.; Cai, Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015, 356, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.A.; Hossain, S.; Chowdhury, E.H. Potential therapeutic targets in energy metabolism pathways of breast cancer. Curr. Cancer Drug Targets 2017, 17, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Cinquin, A.; Cinquin, O.; Digman, M.A.; Donovan, P.J.; Gratton, E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.K.; Yan, L.; Vrotsos, K.M.; Eliceiri, K.W.; Vaughan, E.M.; Keely, P.J.; White, J.G.; Ramanujam, N. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res. 2005, 65, 8766–8773. [Google Scholar] [CrossRef]
- Ma, N.; Digman, M.A.; Malacrida, L.; Gratton, E. Measurements of absolute concentrations of NADH in cells using the phasor FLIM method. Biomed. Opt. Express 2016, 7, 2441–2452. [Google Scholar] [CrossRef]
- Skala, M.C.; Riching, K.M.; Bird, D.K.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; Keely, P.J.; Ramanujam, N. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J. Biomed. Opt. 2007, 12, 024014. [Google Scholar] [CrossRef]
- Ma, N.; de Mochel, N.R.; Pham, P.D.; Yoo, T.Y.; Cho, K.W.; Digman, M.A. Label-free assessment of pre-implantation embryo quality by the Fluorescence Lifetime Imaging Microscopy (FLIM)-phasor approach. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Cinco, R.; Digman, M.A.; Gratton, E.; Luderer, U. Spatial characterization of bioenergetics and metabolism of primordial to preovulatory follicles in whole ex vivo murine ovary. Biol. Reprod. 2016, 95, 129. [Google Scholar] [CrossRef]
- Stringari, C.; Edwards, R.A.; Pate, K.T.; Waterman, M.L.; Donovan, P.J.; Gratton, E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci. Rep. 2012, 2, 568. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999, 59, 1903–1910. [Google Scholar] [PubMed]
- Lin, W.; Lin, F.; Nevins, J. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 2001, 15, 1833–1844. [Google Scholar]
- Amoedo, N.; Obre, E.; Rossignol, R. Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta (Bba)-Bioenerg. 2017, 1858, 674–685. [Google Scholar] [CrossRef] [PubMed]
- König, K.; So, P.C.; Mantulin, W.; Tromberg, B.; Gratton, E. Two-photon excited lifetime imaging of autofluorescence in cells during UV A and NIR photostress. J. Microsc. 1996, 183, 197–204. [Google Scholar] [PubMed]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascua, S.M.; McGahey, G.E.; Ma, N.; Wang, J.J.; Digman, M.A. Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM. Int. J. Mol. Sci. 2020, 21, 2443. https://doi.org/10.3390/ijms21072443
Pascua SM, McGahey GE, Ma N, Wang JJ, Digman MA. Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM. International Journal of Molecular Sciences. 2020; 21(7):2443. https://doi.org/10.3390/ijms21072443
Chicago/Turabian StylePascua, Stephanie M., Gabrielle E. McGahey, Ning Ma, Justin J. Wang, and Michelle A. Digman. 2020. "Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM" International Journal of Molecular Sciences 21, no. 7: 2443. https://doi.org/10.3390/ijms21072443
APA StylePascua, S. M., McGahey, G. E., Ma, N., Wang, J. J., & Digman, M. A. (2020). Caffeine and Cisplatin Effectively Targets the Metabolism of a Triple-Negative Breast Cancer Cell Line Assessed via Phasor-FLIM. International Journal of Molecular Sciences, 21(7), 2443. https://doi.org/10.3390/ijms21072443



