The Current Understanding of Autophagy in Nanomaterial Toxicity and Its Implementation in Safety Assessment-Related Alternative Testing Strategies
Abstract
1. Introduction
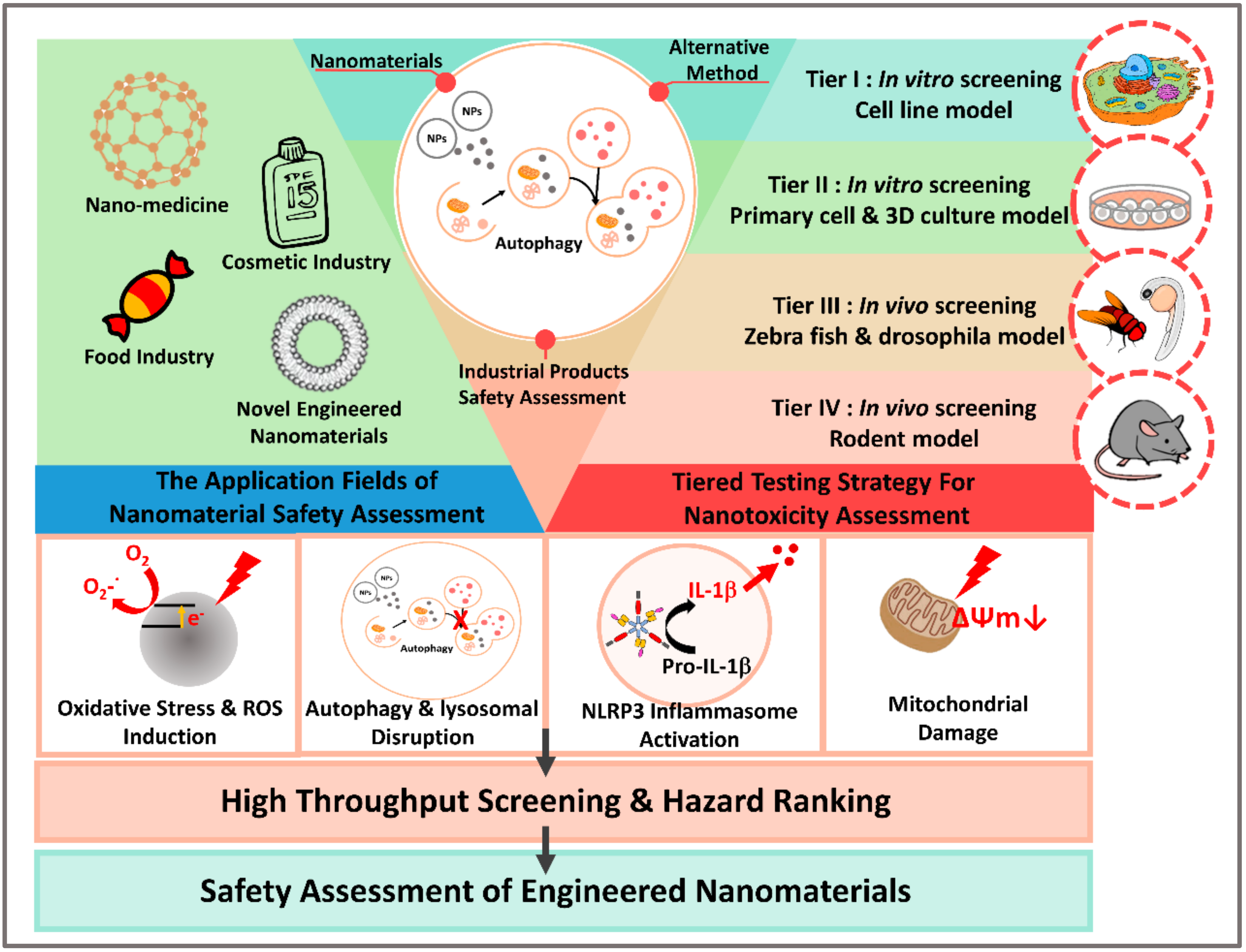
2. Alternative Testing Strategy for Nanomaterial Safety Assessments
2.1. Approach towards the Use of Alternatives to Testing on Animals
2.2. Alternative Testing Strategy for Nanomaterials
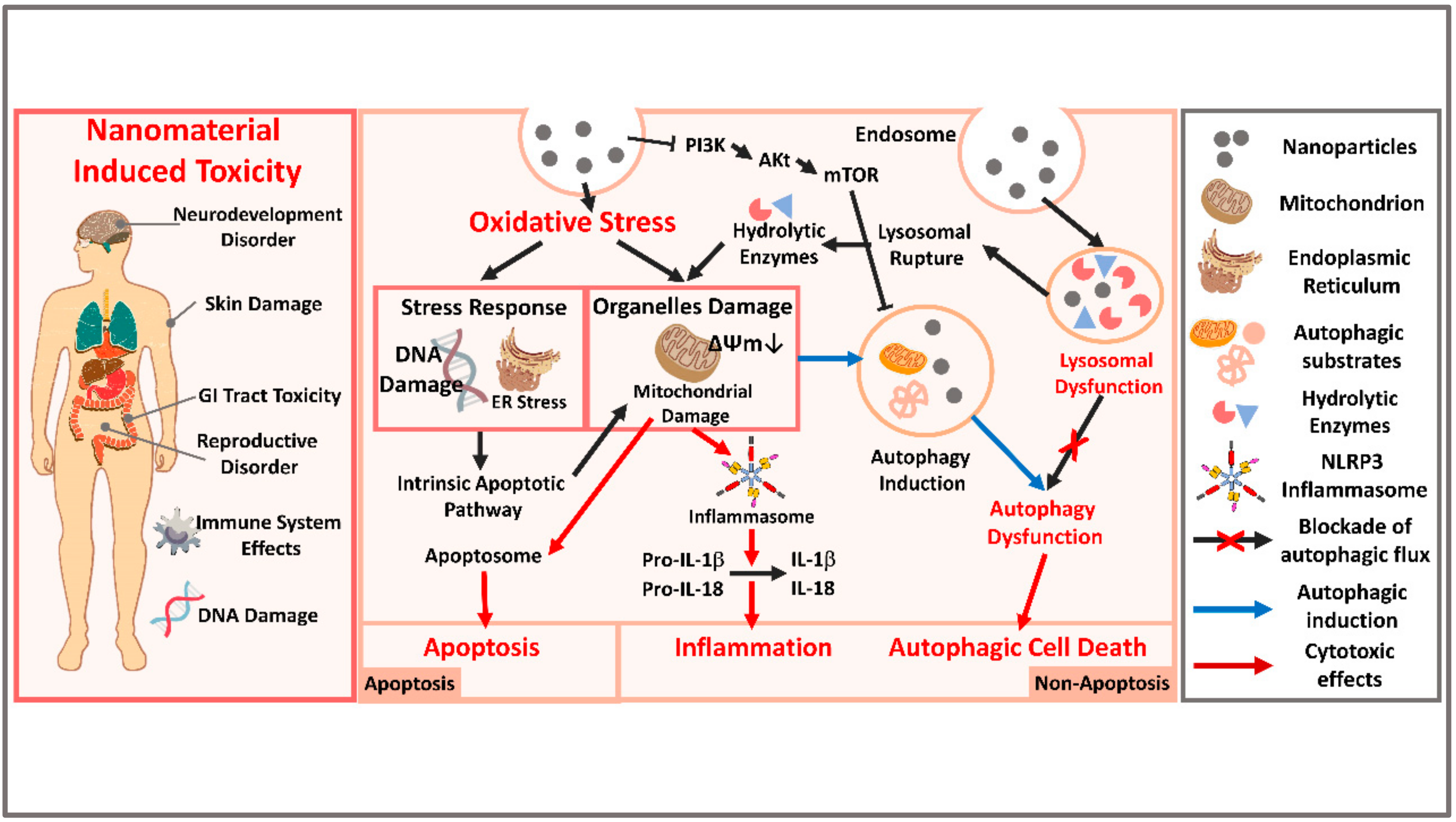
3. Updated Nanotoxicology Knowledge Regarding Autophagy Dysfunction
3.1. Autophagy and Autophagy-Induced Cell Death (ACD)
3.2. Autophagy Dysfunction as a Cell Toxicity Mechanism
3.3. Autophagy Dysfunction Induced by Nanomaterials
4. In Vivo Model Systems for the Detection of Nanomaterial Toxicity and Autophagy
4.1. C. elegans Model
4.2. Zebrafish Model
4.3. Drosophila Model
4.4. Rodent Model
5. Autophagy Detection as a Toxicity Biomarker-Like Indicator for Medical, Food, and Cosmetic NPs Safety Assessments in Future
5.1. Silver Nanoparticles-Induced Toxicity and the Possible Role of Autophagy
5.2. ZnO Nanoparticles-Induced Toxicity and the Possible Role of Autophagy
5.3. TiO2 Nanoparticles-Induced Toxicity and the Possible Role of Autophagy
6. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| 3R’s | Reduces, Refines, or Replaces |
| ACD | Autophagic Cell Death |
| AgNPs | Silver Nanoparticles |
| AMPK | AMP Kinase |
| AOP | Adverse Outcome Pathway |
| AuNPs | Gold Nanoparticles |
| BBB | Blood–Brain Barrier |
| BPR | Biocidal Products Regulations |
| CLP | Classification, Labelling and Packaging |
| CuONPs | Copper Oxide Nanoparticles |
| DMH | Dimethylhydrazine |
| ECVAM | European Center for the Validation of Alternative Methods |
| ER | Endoplasmic Reticulum |
| FADD | Fas-Associated Protein with Death Domain |
| GIT | Gastrointestinal Tract |
| INRA | French National Institute for Agricultural Research |
| ISO | International Organization for Standardization |
| LMP | Lysosomal Membrane Permeabilization |
| NCI | National Cancer Institute |
| NCL | Nanotechnology Characterization Lab |
| NMs | Nanomaterials |
| NRC | National Research Council |
| OECD | Organization for Economic Co-operation and Development |
| PDT | Photodynamic Therapy |
| REACH | Registration, Evaluation, Authorization and Restriction of Chemicals |
| ROS | Reactive Oxygen Species |
| SC | Stratum Corneum |
| SCCS | Scientific Committee on Consumer Safety |
| SEURAT | Safety Evaluation Ultimately Replacing Animal Testing |
| TEM | Transmission Electron Microscopy |
| TiO2NPs | Titanium Dioxide Nanoparticles |
| TT21C | Toxicity Testing in the 21st Century |
| UV | Ultraviolet |
| UV-light | Ultraviolet Light |
| ZnONPs | Zinc Oxide Nanoparticles |
References
- European Commission. Commission Recommendation of 18 October 2011 on the definition of nanomaterial. J. Off. Eur. Union 2011, 275, 38–40. [Google Scholar]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.G.; Proykova, A.; Santos, G.M.L. Dealing with nanosafety around the globe—Regulation vs. innovation. Int. J. Pharm. 2016, 509, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Ramachandran, G.; Kandlikar, M. The impact of toxicity testing costs on nanomaterial regulation. Environ. Sci. Technol. 2009, 43, 3030–3034. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Verdon, R.; Gillies, S.; Brown, D.M.; Fernandes, T.F.; Henry, T.B.; Rossi, A.G.; Tran, L.; Tucker, C.; Tyler, C.R.; et al. Adoption of in vitro systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanomaterials. Crit. Rev. Toxicol. 2018, 48, 252–271. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Acosta, D., Jr.; Andersen, M.; Anderson, H.; Bailar, J.C., III; Boekelheide, K.; Brent, R.; Charnley, G.; Cheung, V.G.; Green, S., Jr.; et al. Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health Part B 2010, 13, 51–138. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy; National Academies Press: Washington, DC, USA, 2007; ISBN 978-030-910-992-5. [Google Scholar]
- Shatkin, J.; Ong, K. Alternative testing strategies for nanomaterials: State of the science and considerations for risk analysis. Risk Anal. 2016, 36, 1564–1580. [Google Scholar] [CrossRef]
- Dusinska, M.; Boland, S.; Saunders, M.; Juillerat-Jeanneret, L.; Tran, L.; Pojana, G.; Marcomini, A.; Volkovova, K.; Tulinska, J.; Knudsen, L.E.; et al. Towards an alternative testing strategy for nanomaterials used in nanomedicine: Lessons from NanoTEST. Nanotoxicology 2015, 9 (Suppl. 1), 118–132. [Google Scholar] [CrossRef]
- Collins, F.S.; Gray, G.M.; Bucher, J.R. Transforming environmental health protection. Science 2008, 319, 906–907. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. The lysosome. Sci. Am. 1963, 208, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy in protein and organelle turnover. Cold Spring Harbor Symp. Quant. Biol. 2011, 76, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Levine, B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 2014, 157, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ju, D. The role of autophagy in nanoparticles-induced toxicity and its related cellular and molecular mechanisms. Adv. Exp. Med. Biol. 2018, 1048, 71–84. [Google Scholar]
- Lee, Y.-H.; Cheng, F.-Y.; Chiu, H.-W.; Tsai, J.-C.; Fang, C.-Y.; Chen, C.-W.; Wang, Y.-J. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 2014, 35, 4706–4715. [Google Scholar] [CrossRef]
- Mao, B.H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Fang, C.-Y.; Chiu, H.-W.; Cheng, F.-Y.; Tsai, J.-C.; Chen, C.-W.; Wang, Y.-J. Endoplasmic reticulum stress-triggered autophagy and lysosomal dysfunction contribute to the cytotoxicity of amine-modified silver nanoparticles in NIH 3T3 cells. J. Biomed. Nanotechnol. 2017, 13, 778–794. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Z.; Okweesi Aggrey, M.; Li, C.; Chen, J.; Tong, L. Nanotoxicity: The toxicity research progress of metal and metal-containing nanoparticles. Mini Rev. Med. Chem. 2015, 15, 529–542. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Andersen, M.; Tyshenko, M.; Krishnan, K.; Hartung, T.; Boekelheide, K.; Wambaugh, J.; Jones, D.; Whelan, M.; Thomas, R.; et al. Toxicity testing in the 21st century: Progress in the past decade and future perspectives. Arch. Toxicol. 2019, 94, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Gocht, T.; Berggren, E.; Ahr, H.J.; Cotgreave, I.; Cronin, M.T.; Daston, G.; Hardy, B.; Heinzle, E.; Hescheler, J.; Knight, D.J. The SEURAT-1 approach towards animal free human safety assessment. ALTEX Altern. Anim. Exp. 2015, 32, 9–24. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Non-Animal Approaches-Current Status of Regulatory Applicability under the REACH, CLP and Biocidal Products Regulations; European Chemicals Agency: Helsinki, Finland, 2017; ISBN 978-92-9020-208-0. [Google Scholar]
- Rusyn, I.; Greene, N. The impact of novel assessment methodologies in toxicology on green chemistry and chemical alternatives. Toxicol. Sci. 2018, 161, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Van Den Berghe, C.; Casati, S.; Castell, J.; Clemedson, C.; Coecke, S.; Colombo, A.; Curren, R.; Negro, G.D.; Goldberg, A.; et al. Strategies to replace in vivo acute systemic toxicity testing: The report and recommendations of ECVAM workshop 50. ATLA Altern. Lab. Anim. 2004, 32, 437–459. [Google Scholar] [CrossRef]
- Andrew, D.J. Acute systemic toxicity: Oral, dermal and inhalation exposures. In Reducing, Refining and Replacing the Use of Animals in Toxicity Testing; Allen, D., Waters, M.D., Eds.; Royal Society of Chemistry: London, UK, 2013; pp. 183–214. [Google Scholar]
- Prieto, P.; Graepel, R.; Gerloff, K.; Lamon, L.; Sachana, M.; Pistollato, F.; Gribaldo, L.; Bal-Price, A.; Worth, A. Investigating cell type specific mechanisms contributing to acute oral toxicity. ALTEX Altern. Anim. Exp. 2019, 36, 39–64. [Google Scholar] [CrossRef]
- National Institutes of Health. Report on the ICCVAM-NICEATM/ECVAM/JaCVAM Scientific Workshop on Acute Chemical Safety Testing: Advancing In Vitro Approaches and Humane Endpoints for Systemic Toxicity Evaluations; National Toxicology Program; National Institutes of Health: Bethesda, MD, USA, 2009.
- Saifi, M.A.; Khan, W.; Godugu, C. Cytotoxicity of nanomaterials: Using nanotoxicology to address the safety concerns of nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef]
- Bai, X.; Liu, F.; Liu, Y.; Li, C.; Wang, S.; Zhou, H.; Wang, W.; Zhu, H.; Winkler, D.A.; Yan, B. Toward a systematic exploration of nano-bio interactions. Toxicol. Appl. Pharmacol. 2017, 323, 66–73. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Zhang, C.; Cui, X.; Zhai, S.; Liu, Y.; Li, C.; Zhu, H.; Qu, G.; Jiang, G.; et al. Tuning cell autophagy by diversifying carbon nanotube surface chemistry. ACS Nano 2014, 8, 2087–2099. [Google Scholar] [CrossRef]
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359. [Google Scholar] [CrossRef] [PubMed]
- Drasler, B.; Sayre, P.; Steinhauser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. Nanoimpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Gomes, T.; Machado, M.R.F.; Rocha, T.L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: From morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.; Baehrecke, E.H. Life, death and autophagy. Nat. Cell Biol. 2018, 20, 1110–1117. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Lam, H.C.; Jin, Y.; Kim, H.-P.; Cao, J.; Lee, S.-J.; Ifedigbo, E.; Parameswaran, H.; Ryter, S.W.; Choi, A.M. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. USA 2010, 107, 18880–18885. [Google Scholar] [CrossRef]
- White, E.; DiPaola, R.S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef]
- Monick, M.M.; Powers, L.S.; Walters, K.; Lovan, N.; Zhang, M.; Gerke, A.; Hansdottir, S.; Hunninghake, G.W. Identification of an autophagy defect in smokers’ alveolar macrophages. J. Immunol. 2010, 185, 5425–5435. [Google Scholar] [CrossRef]
- Mao, B.H.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Belanger, E.C.; Veinot, J.P. Lysosomal storage disorders affecting the heart: A review. Cardiovasc. Pathol. 2019, 39, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257–5276. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Lu, K.; Yang, M.; Li, Y.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int. J. Nanomed. 2017, 12, 809–825. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Shahnazari, S.; Brech, A.; Lamark, T.; Johansen, T.; Brumell, J.H. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009, 183, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver nanoparticle-induced autophagic-lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.-R.; Dong, L.; Xu, M.-R.; Zhang, L.; Ding, W.-P.; Zhang, J.-Q.; Lin, J.; Zhang, Y.-J.; Qiu, B.-S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef]
- Fan, J.; Wang, S.; Zhang, X.; Chen, W.; Li, Y.; Yang, P.; Cao, Z.; Wang, Y.; Lu, W.; Ju, D. Quantum dots elicit hepatotoxicity through lysosome-dependent autophagy activation and reactive oxygen species production. ACS Biomater. Sci. Eng. 2018, 4, 1418–1427. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Horibe, A.; Otsuki, Y.; Kondo, Y. Ethanol-induced mitochondrial damage in sertoli cells is associated with parkin overexpression and activation of mitophagy. Cells 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, A.J.; Buss, F. Actin cages isolate damaged mitochondria during mitophagy. Autophagy 2018, 14, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tu, B.; Jiang, X.; Xu, G.; Bai, L.; Zhang, L.; Meng, P.; Qin, X.; Chen, C.; Zou, Z. Lysosomal dysfunction is associated with persistent lung injury in dams caused by pregnancy exposure to carbon black nanoparticles. Life Sci. 2019, 233, 116741. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, X.; Lin, H.; Chen, H.; Huang, D.; Li, D.; Shan, H.; Gao, J. Gold nanoparticles impair autophagy flux through shape-dependent endocytosis and lysosomal dysfunction. J. Mater. Chem. B 2018, 6, 8127–8136. [Google Scholar] [CrossRef]
- Vermeulen, L.M.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef]
- Anozie, U.C.; Dalhaimer, P. Molecular links among non-biodegradable nanoparticles, reactive oxygen species, and autophagy. Adv. Drug Deliv. Rev. 2017, 122, 65–73. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Wang, Q.; Zhou, Y.; Fu, R.; Zhu, Y.; Song, B.; Zhong, Y.; Wu, S.; Shi, Y.; Wu, Y.; Su, Y.; et al. Distinct autophagy-inducing abilities of similar-sized nanoparticles in cell culture and live C. elegans. Nanoscale 2018, 10, 23059–23069. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A.C. C. elegans as a tool for in vivo nanoparticle assessment. Adv. Colloid Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, X.; Zhang, P.; Qi, L.; Liang, Q.; Zhang, X.; Huang, J.; Fang, B.; Hou, W.; Han, J.; et al. Genome-wide screen identifies signaling pathways that regulate autophagy during Caenorhabditis elegans development. EMBO Rep. 2014, 15, 705–713. [Google Scholar]
- Kim, J.H.; Lee, S.H.; Cha, Y.J.; Hong, S.J.; Chung, S.K.; Park, T.H.; Choi, S.S. C. elegans-on-a-chip for in situ and in vivo Ag nanoparticles’ uptake and toxicity assay. Sci. Rep. 2017, 7, 40225. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In vitro methods for assessing nanoparticle toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar]
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials (Basel) 2018, 8, 561. [Google Scholar] [CrossRef]
- Pappus, S.A.; Mishra, M. A drosophila model to decipher the toxicity of nanoparticles taken through oral routes. Adv. Exp. Med. Biol. 2018, 1048, 311–322. [Google Scholar]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed. J. 2016, 20, 1–11. [Google Scholar] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, R.L. The rise of zebrafish as a model for toxicology. Toxicol. Sci. 2018, 163, 3–4. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; Van Eeden, F.J.; Jiang, Y.J.; Heisenberg, C.P.; et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar] [PubMed]
- Stainier, D.Y.; Fishman, M.C. The zebrafish as a model system to study cardiovascular development. Trends Cardiovasc. Med. 1994, 4, 207–212. [Google Scholar] [CrossRef]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; De Bruijn, E.; Van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Chakraborty, C. The zebrafish model: Use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr. Neurovasc. Res. 2007, 4, 111–120. [Google Scholar] [CrossRef]
- Liu, H.; Gooneratne, R.; Huang, X.; Lai, R.; Wei, J.; Wang, W. A rapid in vivo zebrafish model to elucidate oxidative stress-mediated PCB126-induced apoptosis and developmental toxicity. Free Radic. Biol. Med. 2015, 84, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.M.; Nonarath, H.J.T.; Bostrom, J.R.; Link, B.A. Establishment and validation of an endoplasmic reticulum stress reporter to monitor zebrafish ATF6 activity in development and disease. Dis. Model Mech. 2020, 13. [Google Scholar] [CrossRef]
- Mathai, B.J.; Meijer, A.H.; Simonsen, A. Studying autophagy in zebrafish. Cells 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Capelle, M.; Fent, K. Silver nanoparticles induce endoplasmatic reticulum stress response in zebrafish. Toxicol. Appl. Pharmacol. 2013, 272, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Tilton, S.C.; Zaikova, T.; Richman, E.; Waters, K.M.; Hutchison, J.E.; Tanguay, R.L. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology 2013, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Santonastaso, M.; Mottola, F.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015, 113, 223–230. [Google Scholar] [CrossRef]
- Villacis, R.A.R.; Filho, J.S.; Pina, B.; Azevedo, R.B.; Pic-Taylor, A.; Mazzeu, J.F.; Grisolia, C.K. Integrated assessment of toxic effects of maghemite (gamma-Fe2O3) nanoparticles in zebrafish. Aquat. Toxicol. 2017, 191, 219–225. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, R.-O.; Yoon, S.; Kim, W.-K. Developmental toxicity of zinc oxide nanoparticles to zebrafish (Danio rerio): A transcriptomic analysis. PLoS ONE 2016, 11, e0160763. [Google Scholar] [CrossRef]
- Duan, J.; Liang, S.; Yu, Y.; Li, Y.; Wang, L.; Wu, Z.; Chen, Y.; Miller, M.R.; Sun, Z. Inflammation-coagulation response and thrombotic effects induced by silica nanoparticles in zebrafish embryos. Nanotoxicology 2018, 12, 470–484. [Google Scholar] [CrossRef]
- Varga, M.; Fodor, E.; Vellai, T. Autophagy in zebrafish. Methods 2015, 75, 172–180. [Google Scholar] [CrossRef]
- Simonsen, A.; Tooze, S.A. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J. Cell Biol. 2009, 186, 773–782. [Google Scholar] [CrossRef]
- George, A.A.; Hayden, S.; Stanton, G.R.; Brockerhoff, S.E. Arf6 and the 5′phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors. BioEssays 2016, 38 (Suppl. 1), S119–S135. [Google Scholar] [CrossRef]
- Kotil, T.; Akbulut, C.; Yon, N.D. The effects of titanium dioxide nanoparticles on ultrastructure of zebrafish testis (Danio rerio). Micron 2017, 100, 38–44. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, H. Drosophila as a model organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar]
- Lorincz, P.; Mauvezin, C.; Juhasz, G. Exploring autophagy in drosophila. Cells 2017, 6, 22. [Google Scholar] [CrossRef]
- Ong, C.; Yung, L.Y.; Cai, Y.; Bay, B.H.; Baeg, G.H. Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 2015, 9, 396–403. [Google Scholar] [CrossRef]
- Baeg, E.; Sooklert, K.; Sereemaspun, A. Copper oxide nanoparticles cause a dose-dependent toxicity via inducing reactive oxygen species in drosophila. Nanomaterials (Basel) 2018, 8, 824. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- He, X. In vivo nanotoxicity assays in animal models. In Toxicology of Nanomaterials; Zhao, Y., Zhang, Z., Eds.; Wiley-VCH: Weinheim, Germany, 2016; pp. 151–197. [Google Scholar]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials (Basel) 2018, 8, 634. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef]
- Li, Y.P.; Pei, Y.Y.; Zhang, X.Y.; Gu, Z.H.; Zhou, Z.H.; Yuan, W.F.; Zhou, J.J.; Zhu, J.H.; Gao, X.J. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Baker, G.L.; Gupta, A.; Clark, M.L.; Valenzuela, B.R.; Staska, L.M.; Harbo, S.J.; Pierce, J.T.; Dill, J.A. Inhalation toxicity and lung toxicokinetics of C-60 fullerene nanoparticles and microparticles. Toxicol. Sci. 2008, 101, 122–131. [Google Scholar] [CrossRef]
- Lei, R.; Wu, C.; Yang, B.; Ma, H.; Shi, C.; Wang, Q.; Wang, Q.; Yuan, Y.; Liao, M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008, 232, 292–301. [Google Scholar] [CrossRef]
- Carraro, T.C.M.M.; Altmeyer, C.; Khalil, N.M.; Mainardes, R.M. Assessment of in vitro antifungal efficacy and in vivo toxicity of Amphotericin B-loaded PLGA and PLGA-PEG blend nanoparticles. J. Mycol. Med. 2017, 27, 519–529. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Korani, M.; Rezayat, S.M.; Arbabi Bidgoli, S. Sub-chronic dermal toxicity of silver nanoparticles in guinea pig: Special emphasis to heart, bone and kidney toxicities. Iran. J. Pharm. Res. 2013, 12, 511–519. [Google Scholar]
- Chuang, H.C.; Hsiao, T.C.; Wu, C.K.; Chang, H.H.; Lee, C.H.; Chang, C.C.; Cheng, T.J.; Taiwan CardioPulmonary Research Group. Allergenicity and toxicology of inhaled silver nanoparticles in allergen-provocation mice models. Int. J. Nanomed. 2013, 8, 4495–4506. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Gurunathan, S. Combination of graphene oxide-silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int. J. Nanomed. 2017, 12, 6537–6558. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Guo, D.W.; Sun, L.L.; Huang, Z.H.; Zhang, X.Y.; Ma, W.J.; Wu, J.; Xiao, L.; Zhao, Y.; Gu, N. Activation of autophagy by elevated reactive oxygen species rather than released silver ions promotes cytotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in hematopoietic cells. Nanoscale 2017, 9, 5489–5498. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Yuan, X.; Xie, J.P.; Leong, D.T. The influence of lysosomal stability of silver nanomaterials on their toxicity to human cells. Biomaterials 2014, 35, 6707–6715. [Google Scholar] [CrossRef]
- Kroemer, G.; Jaattela, M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer 2005, 5, 886–897. [Google Scholar] [CrossRef]
- Lee, T.Y.; Liu, M.S.; Huang, L.J.; Lue, S.I.; Lin, L.C.; Kwan, A.L.; Yang, R.C. Bioenergetic failure correlates with autophagy and apoptosis in rat liver following silver nanoparticle intraperitoneal administration. Part. Fibre Toxicol. 2013, 10, 40. [Google Scholar] [CrossRef]
- Comfort, K.K.; Braydich-Stolle, L.K.; Maurer, E.I.; Hussain, S.M. Less is more: Long-term in vitro exposure to low levels of silver nanoparticles provides new insights for nanomaterial evaluation. ACS Nano 2014, 8, 3260–3271. [Google Scholar] [CrossRef]
- Zielinska, E.; Zauszkiewicz-Pawlak, A.; Wojcik, M.; Inkielewicz-Stepniak, I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget 2018, 9, 4675–4697. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wang, L.M.; Bai, R.; Zhang, T.L.; Chen, C.Y. Silver nanoparticles impede phorbol myristate acetate-induced monocyte-macrophage differentiation and autophagy. Nanoscale 2015, 7, 16100–16109. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Taccola, L.; Raffa, V.; Riggio, C.; Vittorio, O.; Iorio, M.C.; Vanacore, R.; Pietrabissa, A.; Cuschieri, A. Zinc oxide nanoparticles as selective killers of proliferating cells. Int. J. Nanomed. 2011, 6, 1129–1140. [Google Scholar]
- Mu, L.; Sprando, R.L. Application of nanotechnology in cosmetics. Pharm. Res. 2010, 27, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Fakhravar, Z.; Ebrahimnejad, P.; Daraee, H.; Akbarzadeh, A. Nanoliposomes: Synthesis methods and applications in cosmetics. J. Cosmet. Laser Ther. 2016, 18, 174–181. [Google Scholar] [CrossRef]
- Subramaniam, V.D.; Prasad, S.V.; Banerjee, A.; Gopinath, M.; Murugesan, R.; Marotta, F.; Sun, X.-F.; Pathak, S. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem. Toxicol. 2019, 42, 84–93. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: An in vitro and in vivo study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef]
- Nikolic, S.; Keck, C.M.; Anselmi, C.; Müller, R.H. Skin photoprotection improvement: Synergistic interaction between lipid nanoparticles and organic UV filters. Int. J. Pharm. 2011, 414, 276–284. [Google Scholar] [CrossRef]
- Gupta, R.; Rai, B. Effect of size and surface charge of gold nanoparticles on their skin permeability: A molecular dynamics study. Sci. Rep. 2017, 7, 45292. [Google Scholar] [CrossRef]
- Dorier, M.; Brun, E.; Veronesi, G.; Barreau, F.; Pernet-Gallay, K.; Desvergne, C.; Rabilloud, T.; Carapito, C.; Herlin-Boime, N.; Carriere, M. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale 2015, 7, 7352–7360. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Hathaway, Q.A.; Nichols, C.E.; Shepherd, D.L.; Stapleton, P.A.; McLaughlin, S.L.; Stricker, J.C.; Rellick, S.L.; Pinti, M.V.; Abukabda, A.B.; McBride, C.R.; et al. Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H446–H458. [Google Scholar] [CrossRef] [PubMed]
- Zvyagin, A.V.; Zhao, X.; Gierden, A.; Sanchez, W.; Ross, J.A.; Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J. Biomed. Opt. 2008, 13, 064031. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Rakshit, M.; Nguyen, K.T.; Kathawala, M.H.; Nguyen, L.T.H.; Tay, C.Y.; Wong, E.; Ng, K.W. Understanding the implications of engineered nanoparticle induced autophagy in human epidermal keratinocytes in vitro. NanoImpact 2019, 15, 100177. [Google Scholar] [CrossRef]
- Johnson, B.M.; Fraietta, J.A.; Gracias, D.T.; Hope, J.L.; Stairiker, C.J.; Patel, P.R.; Mueller, Y.M.; McHugh, M.D.; Jablonowski, L.J.; Wheatley, M.A.; et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology 2015, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef]
- Zhao, Y.; Howe, J.L.; Yu, Z.; Leong, D.T.; Chu, J.J.; Loo, J.S.; Ng, K.W. Exposure to titanium dioxide nanoparticles induces autophagy in primary human keratinocytes. Small 2013, 9, 387–392. [Google Scholar] [CrossRef]
- Lopes, V.R.; Loitto, V.; Audinot, J.N.; Bayat, N.; Gutleb, A.C.; Cristobal, S. Dose-dependent autophagic effect of titanium dioxide nanoparticles in human HaCaT cells at non-cytotoxic levels. J. Nanobiotechnol. 2016, 14, 22. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Antitumor activities of metal oxide nanoparticles. Nanomaterials (Basel). 2015, 5, 1004–1021. [Google Scholar] [CrossRef]
- Cai, R.; Kubota, Y.; Shuin, T.; Sakai, H.; Hashimoto, K.; Fujishima, A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992, 52, 2346–2348. [Google Scholar]
- Moosavi, M.A.; Sharifi, M.; Ghafary, S.M.; Mohammadalipour, Z.; Khataee, A.; Rahmati, M.; Hajjaran, S.; Los, M.J.; Klonisch, T.; Ghavami, S. Photodynamic N-TiO2 nanoparticle treatment induces controlled ROS-mediated autophagy and terminal differentiation of leukemia cells. Sci. Rep. 2016, 6, 34413. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wojcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles exposure on human health—A review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Nguyen, T.H.; Lin, M.; Mustapha, A. Engineered nanoparticles as potential food contaminants and their toxicity to Caco-2 cells. J. Food Sci. 2016, 81, T2107–T2113. [Google Scholar] [CrossRef]
- Yu, S.; Mu, Y.; Zhang, X.; Li, J.; Lee, C.; Wang, H. Molecular mechanisms underlying titanium dioxide nanoparticles (TiO2NP) induced autophagy in mesenchymal stem cells (MSC). J. Toxicol. Environ. Health 2019, 82, 997–1008. [Google Scholar] [CrossRef]
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology 2018, 12, 357–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Yao, M.; Dong, T.; Mao, Z.; Hang, B.; Han, X.; Lin, Z.; Bian, Q.; Li, M.; et al. Titanium dioxide nanoparticles induce proteostasis disruption and autophagy in human trophoblast cells. Chem. Biol. Interact. 2018, 296, 124–133. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Comera, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Tache, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef]
- Hong, F.; Si, W.; Zhao, X.; Wang, L.; Zhou, Y.; Chen, M.; Ge, Y.; Zhang, Q.; Wang, Y.; Zhang, J. TiO2 nanoparticle exposure decreases spermatogenesis via biochemical dysfunctions in the testis of male mice. J. Agric. Food Chem. 2015, 63, 7084–7092. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-J.; Chen, Y.-Y.; Liao, M.-Y.; Lee, Y.-H.; Chen, Z.-Y.; Yan, S.-J.; Yeh, Y.-L.; Yang, L.-X.; Lee, Y.-L.; Wu, Y.-H.; et al. The Current Understanding of Autophagy in Nanomaterial Toxicity and Its Implementation in Safety Assessment-Related Alternative Testing Strategies. Int. J. Mol. Sci. 2020, 21, 2387. https://doi.org/10.3390/ijms21072387
Chen R-J, Chen Y-Y, Liao M-Y, Lee Y-H, Chen Z-Y, Yan S-J, Yeh Y-L, Yang L-X, Lee Y-L, Wu Y-H, et al. The Current Understanding of Autophagy in Nanomaterial Toxicity and Its Implementation in Safety Assessment-Related Alternative Testing Strategies. International Journal of Molecular Sciences. 2020; 21(7):2387. https://doi.org/10.3390/ijms21072387
Chicago/Turabian StyleChen, Rong-Jane, Yu-Ying Chen, Mei-Yi Liao, Yu-Hsuan Lee, Zi-Yu Chen, Shian-Jang Yan, Ya-Ling Yeh, Li-Xing Yang, Yen-Ling Lee, Yuan-Hua Wu, and et al. 2020. "The Current Understanding of Autophagy in Nanomaterial Toxicity and Its Implementation in Safety Assessment-Related Alternative Testing Strategies" International Journal of Molecular Sciences 21, no. 7: 2387. https://doi.org/10.3390/ijms21072387
APA StyleChen, R.-J., Chen, Y.-Y., Liao, M.-Y., Lee, Y.-H., Chen, Z.-Y., Yan, S.-J., Yeh, Y.-L., Yang, L.-X., Lee, Y.-L., Wu, Y.-H., & Wang, Y.-J. (2020). The Current Understanding of Autophagy in Nanomaterial Toxicity and Its Implementation in Safety Assessment-Related Alternative Testing Strategies. International Journal of Molecular Sciences, 21(7), 2387. https://doi.org/10.3390/ijms21072387




