Nanogels of a Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Release Behavior, Gastrointestinal Distribution, and Systemic Absorption
Abstract
1. Introduction
2. Results and Discussion
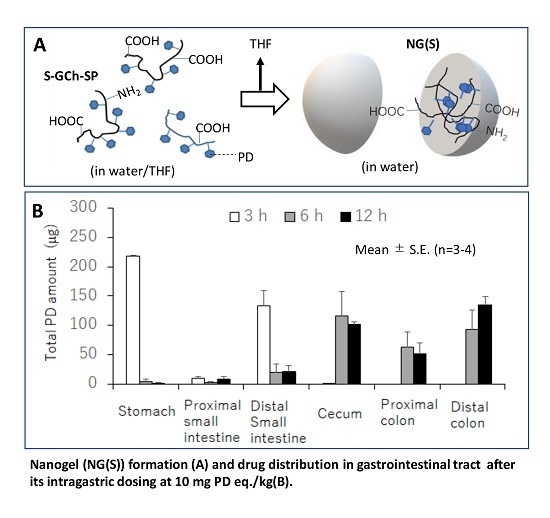
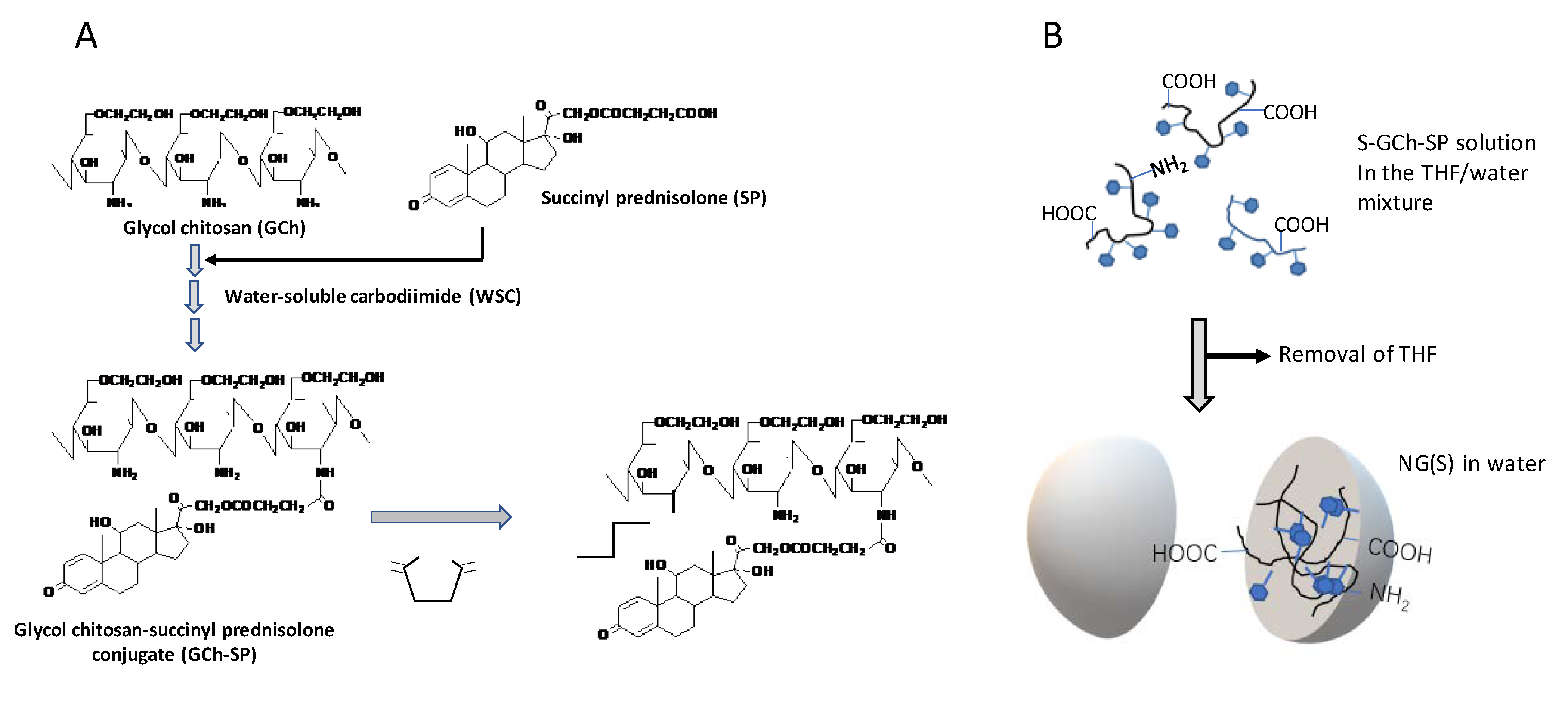
2.1. Preparation and Characterization of NG(S)
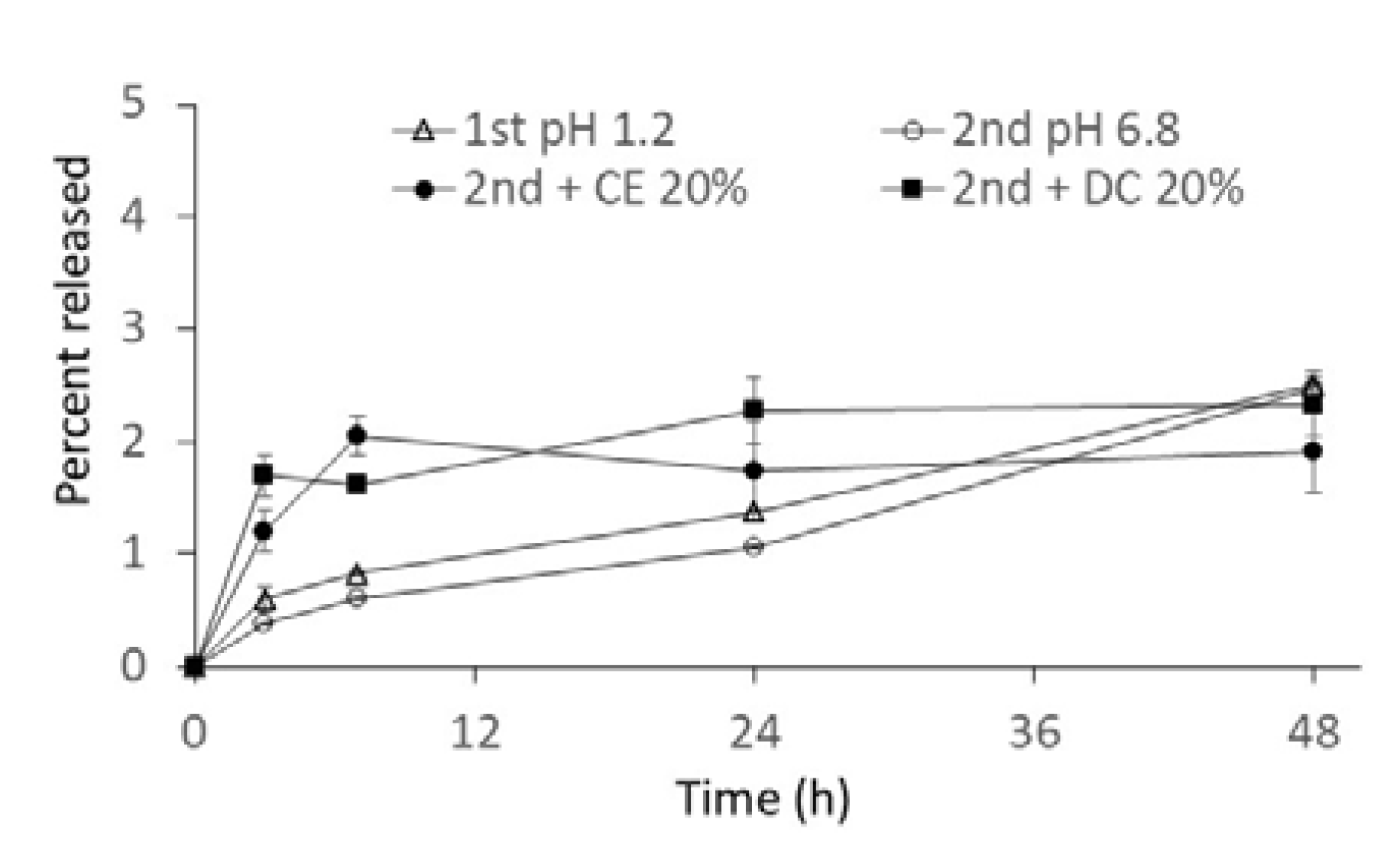
2.2. In Vitro Release from NG(S)
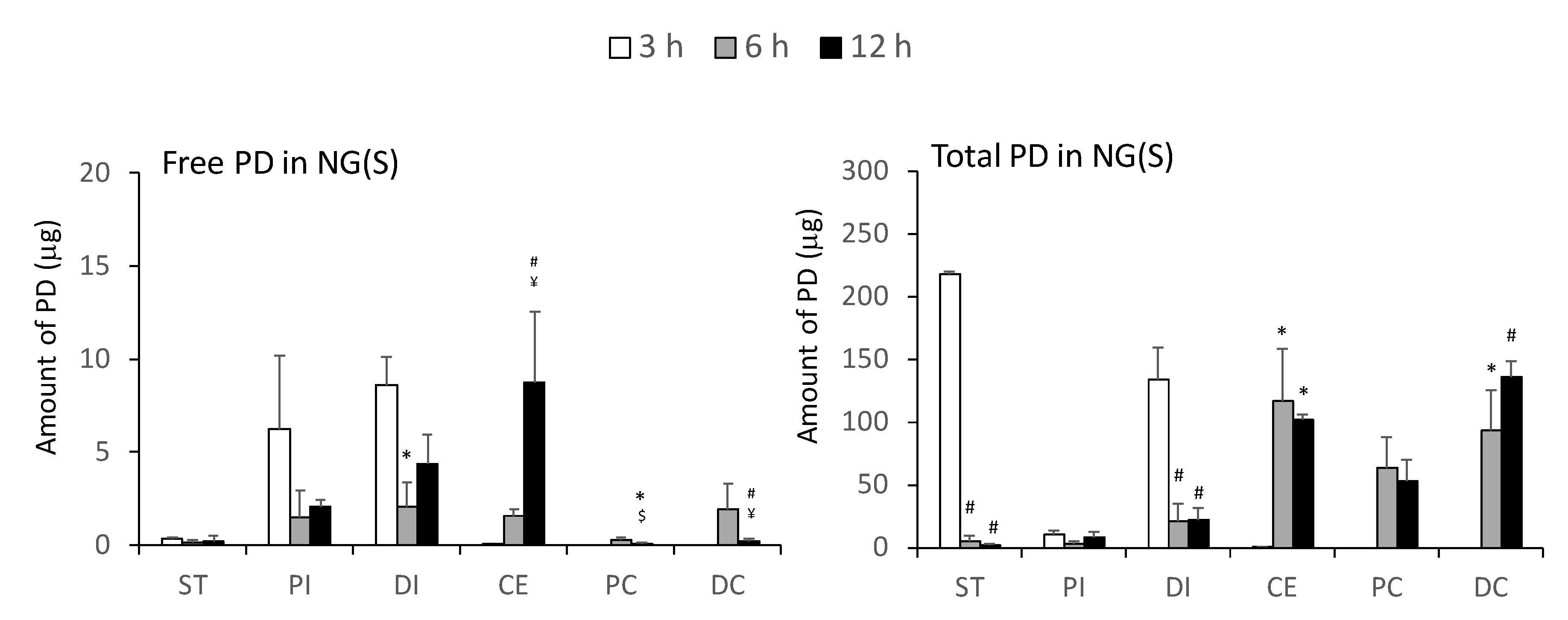
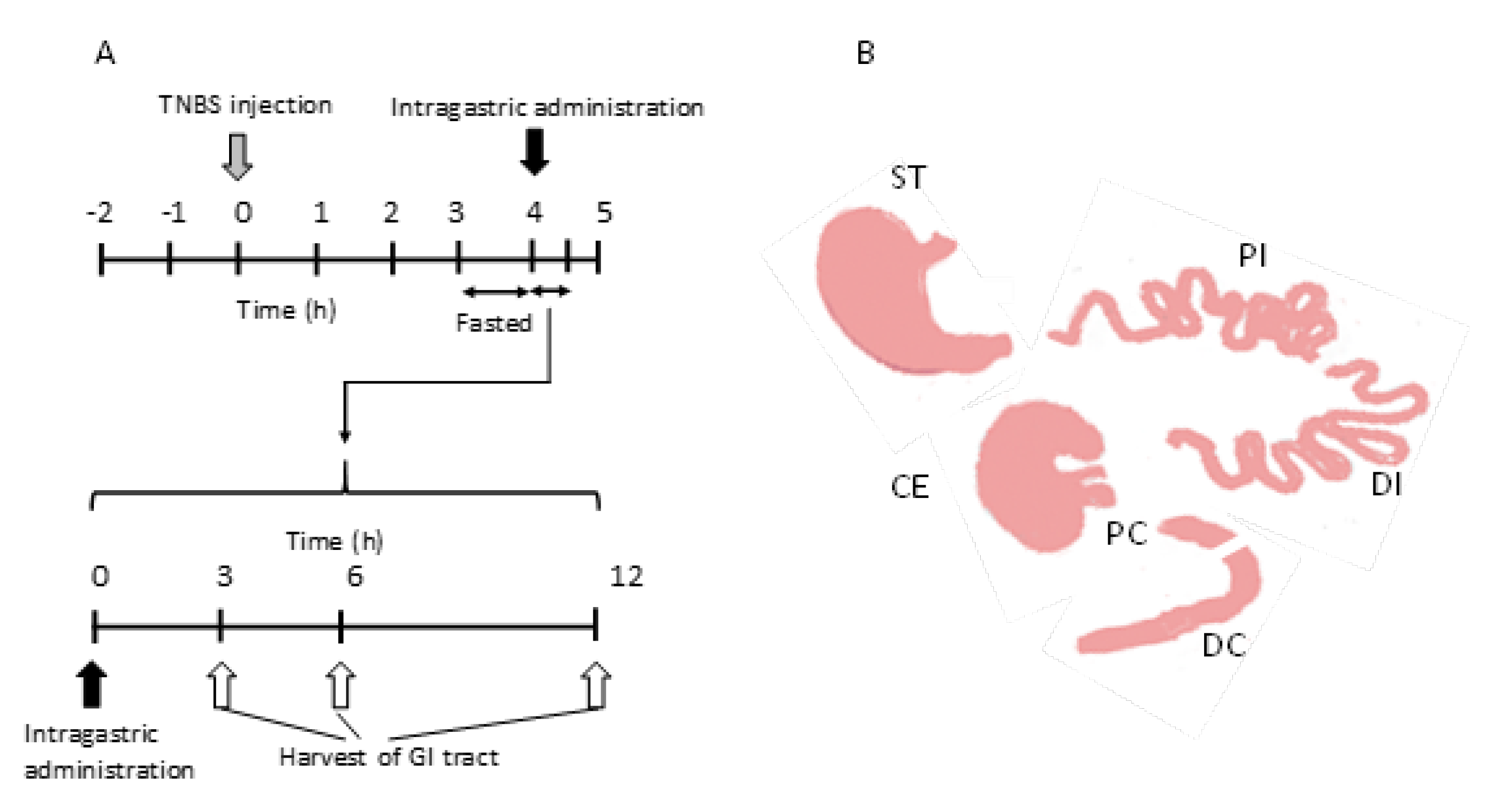
2.3. Drug Distribution in the Gastrointestinal Tract after the Intragastric Administration of NG(S)
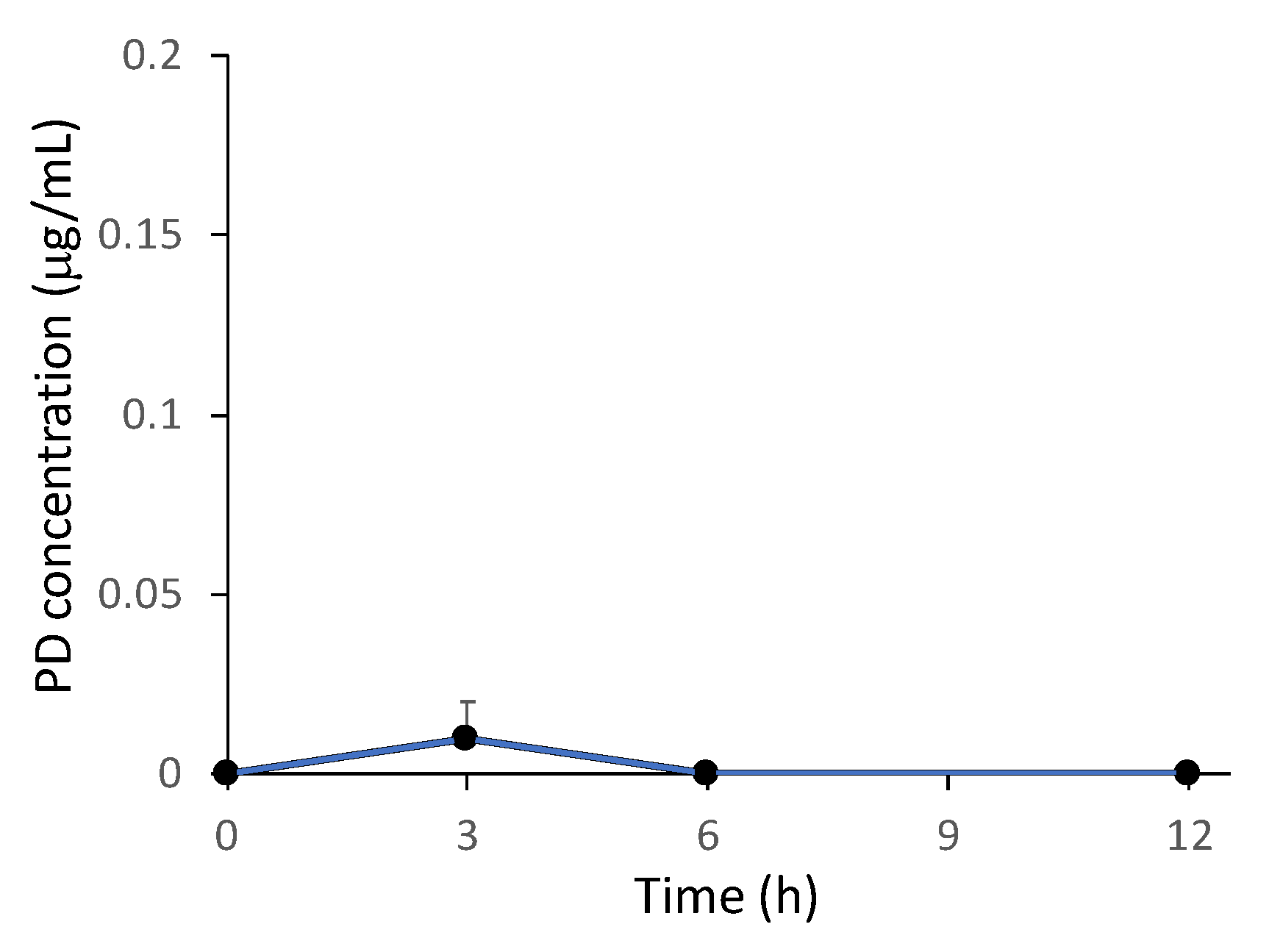
2.4. Plasma Concentration of PD after the Intragastric Administration of NG(S)
3. Materials and Methods
3.1. Materials and Animals
3.1.1. Materials
3.1.2. Animals
3.2. Equipment
3.3. Preparation of GCh-SP and S-GCh-SP
3.4. Production of the UC Model
3.5. In vitro Release Studies
3.6. Drug Distribution Studies in the Gastrointestinal Tract after Intragastric Administration
3.7. HPLC Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CE | cecum |
| DMSO | dimethyl sulfoxide |
| DC | distal colon |
| DI | distal small intestine |
| e-EPR | epithelial enhanced permeability and retention |
| GCh | glycol chitosan |
| GCh-SP | glycol chitosan-succinyl prednisolone conjugate |
| NG | Nanogel |
| NG(G) | glycol chitosan-succinyl prednisolone conjugate nanogel |
| NG(S) | succinylated glycol chitosan-succinyl prednisolone conjugate nanogel |
| NP | nanoparticle |
| PC | proximal colon |
| PD | prednisolone |
| PI | proximal small intestine |
| SA | succinic anhydride |
| S-GCh-SP | succinylated glycol chitosan-succinyl prednisolone conjugate |
| SP | succinyl prednisolone |
| ST | stomach |
| THF | tetrahyrofuran |
| TNBS | trinitrobenzene sulfonic acid |
| UC | ulcerative colitis |
| WSC | water-soluble carbodiimide |
References
- Gallo, G.; Kotze, P.G.; Spinelli, A. Surgery in ulcerative colitis: When? How? Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Medrano, L.M.; Pascual, V.; Bodas, A.; López-Palacios, N.; Salazar, I.; Espino-Paisán, L.; González-Pérez, B.; Urcelay, E.; Mendoza, J.L.; Núñez, C. Expression patterns common and unique to ulcerative colitis and celiac disease. Ann. Hum. Genet. 2019, 83, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Nakata, Y.; Nakashima, Y.; Kubota, N.; Kaiga, T.; Mamiya, T.; Mihara, Y.; Yamasaki, Y.; Jinno, D.; Kobayashi, M.; Miyata, T.; et al. A case of fulminant ulcerative colitis complicated with toxic megacolon and perforation. J. Nihon Univ. Med. Assoc. 2013, 72, 26–29. [Google Scholar]
- Kirk, A.P.; Cason, J.; Fordham, J.N.; Brown, K.A.; Goddard, D.H.; Holborow, E.J.; Lennard-Jones, J.E. Polymorphonuclear leukocyte function in ulcerative colitis and Crohn’s disease. Dig. Dis. Sci. 1983, 28, 236–248. [Google Scholar] [CrossRef]
- Nunes, R.; Neves, J.D.; Sarmento, B. Nanoparticles for the regulation of intestinal inflammation: Opportunities and challenges. Nanomedicine 2019, 14, 2631–2644. [Google Scholar] [CrossRef]
- Kane, S.V. Systematic review: Adherence issues in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 577–585. [Google Scholar] [CrossRef]
- Head, K.A.; Jurenka, J.S. Inflammatory bowel disease part I: Ulcerative colitis–pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar]
- Ishii, N.; Chiba, M.; Suzuki, T.; Iizuka, M.; Masamune, O.; Kubo, N. A successful case of intraarterial prednisolone injection therapy in severe ulcerative colitis unresponsive to an intensive intravenous regimen. Gastroenterol. Jpn. 1993, 28, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Iizuka, B.; Omori, T.; Nakamura, S.; Tokushige, K. Tacrolimus for remission induction and maintenance therapy in patients with ulcerative colitis: A retrospective evaluation study. Gastroenterol. Res. Pract. 2016, 2016, 5956316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Damião, A.O.M.C.; Azevedo, M.F.C.D.; Carlos, A.D.S.; Wada, M.Y.; Silva, T.V.M.; Feitosa, F.D.C. Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review. World J. Gastroenterol. 2019, 25, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Löfberg, R.; Thomsen, O.O.; Langholz, E.; Schiöler, R.; Danielsson, A.; Suhr, O.; Graffner, H.; Påhlman, L.; Matzen, P.; Petersen, J.F.M. Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment. Pharmacol. Ther. 1994, 8, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.I.; Jewell, D.P.; Mani, V.; Keighley, M.R.; Kingston, R.D.; Record, C.O.; Grace, R.H.; Daniels, S.; Patterson, J.; Smith, K. A randomised trial comparing mesalazine and prednisolone foam enemas in patients with acute distal ulcerative colitis. Gut 1996, 38, 229–333. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Hirayama, F.; Kamada, M.; Arima, H.; Uekama, K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: Alleviation of systemic side effect after oral administration. J. Control. Release 2002, 79, 103–112. [Google Scholar] [CrossRef]
- Wilson, J.C.; Sarsour, K.; Collinson, N.; Tuckwell, K.; Musselman, D.; Klearman, M.; Napalkov, P.; Jick, S.S.; Stone, J.H.; Meier, C.R. Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): A nested case-control analysis. Semin. Arthritis Rheum. 2017, 46, 819–827. [Google Scholar]
- Mundell, L.; Lindemann, R.; Douglas, J. Monitoring long-term oral corticosteroids. B.M.J. Open Qual. 2017, 6, e000209. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, H.; Fujita, T.; Odoriba, T.; Terabe, A.; Okabe, S.; Muranishi, S.; Yamamoto, A. Validation of a pharmacokinetic model of colon-specific drug delivery and the therapeutic effects of chitosan capsules containing 5-aminosalicylic acid on 2,4,6-trinitrobenzensulfonic acid-induced colitis in rats. J. Pharm. Pharmacol. 1999, 51, 1107–1112. [Google Scholar] [CrossRef]
- Kim, H.J.; Neophytou, C. Natural anti-inflammatory compounds for the management and adjuvant therapy of inflammatory bowel disease and its drug delivery system. Arch. Pharm. Res. 2009, 32, 997–1004. [Google Scholar] [CrossRef]
- Naeem, M.; Kim, W.; Cao, J.; Jung, Y.; Yoo, J.-W. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf. B. 2014, 123, 271–278. [Google Scholar] [CrossRef]
- Takaya, T.; Ikeda, C.; Imagawa, N.; Niwa, K.; Takada, K. Development of a colon delivery capsule and the pharmacological activity of recombinant and human granulocyte colony-stimulating factor (rhG-CSF) in beagle dogs. J. Pharm. Pharmacol. 1995, 47, 474–478. [Google Scholar] [CrossRef]
- Dianzani, C.; Cavalli, R.; Zara, G.P.; Gallicchio, M.; Lombardi, G.; Gasco, M.R.; Fantozzi, R. Cholesteryl butyrate solid lipid nanoparticles inhibit adhesion of human neutrophils to endothelial cells. Br. J. Pharmacol. 2006, 148, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Girlich, C.; Scholmerich, J. Topical delivery of steroids in inflammatory bowel disease. Curr. Drug Deliv. 2012, 9, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar] [PubMed]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef]
- Collnot, E.M.; Ali, H.; Lehr, C.M. Nano and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef]
- Tirosh, B.; Khatib, N.; Barenholz, Y.; Nissan, A.; Rubinstein, A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol. Pharm. 2009, 6, 1083–1091. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Zhou, H.; Ichikawa, A.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Nanogels of succinylated glycol. chitosan-succinyl prednisolone conjugate: Preparation, in vitro characteristics and therapeutic potential. Pharmaceutics 2019, 11, 333. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Biological fate of highly-succinylated N-succinyl-chitosan and antitumor characteristics of its water-soluble conjugate with mitomycin C at i.v. and i.p. administration into tumor-bearing mice. Biol. Pharm. Bull. 2000, 23, 1497–1503. [Google Scholar] [CrossRef][Green Version]
- Crauste-Manciet, S.; Decroix, M.; Farinotti, R.; Chaumeil, J. Cefpodoxime-proxetil hydrolysis and food effects in the intestinal lumen before absorption: In vitro comparison of rabbit and human material. Int. J. Pharm. 1997, 157, 153–161. [Google Scholar] [CrossRef]
- Hirayama, F.; Ogata, T.; Yano, H.; Arima, H.; Udo, K.; Takano, M.; Uekama, K. Release characteristics of a short-chain fatty acid, n-butyric acid, from its beta-cyclodextrin ester conjugate in rat biological media. J. Pharm. Sci. 2000, 89, 1486–1495. [Google Scholar] [CrossRef]
- Udo, K.; Hokonohara, K.; Motoyama, K.; Arima, H.; Hirayama, F.; Uekama, K. 5-Fluorouracil acetic acid/beta-cyclodextrin conjugates: Drug release behavior in enzymatic and rat cecal media. Int. J. Pharm. 2010, 388, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Ikeuchi-Takahashi, Y.; Kawano, K.; Hattori, Y. Preparation of chondroitin sulfate-glycyl-prednisolone conjugate nanogel and its efficacy in rats with ulcerative colitis. Biol. Pharm. Bull. 2019, 42, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Casabianca, L.B. Probing Amino Acid Interaction with a Polystyrene Nanoparticle Surface Using Saturation-Transfer Difference (STD)-NMR. J. Phys. Chem. Lett. 2018, 9, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Oosegi, T.; Onishi, H.; Machida, Y. Gastrointestinal distribution and absorption behavior of Eudragit-coated chitosan-prednisolone conjugate microspheres in rats with TNBS-induced colitis. Int. J. Pharm. 2008, 348, 80–88. [Google Scholar] [CrossRef]
- Onishi, H.; Kikuchi, H. Comparison of simple Eudragit microparticles loaded with prednisolone and Eudragit-coated chitosan-succinyl-prednisolone conjugate microparticles: Part II. In vivo evaluation of efficacy, toxicity, and biodisposition characteristics. Int. J. Mol. Sci. 2015, 16, 26125–26136. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef]
- Onishi, H.; Oosegi, T.; Machida, Y. Efficacy and toxicity of Eudragit-coated chitosan-succinyl-prednisolone conjugate microspheres using rats with 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis. Int. J. Pharm. 2008, 358, 296–302. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]


| Preparation | Particle Size (nm) 1 | Zeta Potential (mV) 1 | PD Content (%, w/w) 1 |
|---|---|---|---|
| NG(S) | 560 ± 41 | −34.8 ± 0.3 | 20.8 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Ikeuchi-Takahashi, Y.; Hattori, Y.; Onishi, H. Nanogels of a Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Release Behavior, Gastrointestinal Distribution, and Systemic Absorption. Int. J. Mol. Sci. 2020, 21, 2376. https://doi.org/10.3390/ijms21072376
Zhou H, Ikeuchi-Takahashi Y, Hattori Y, Onishi H. Nanogels of a Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Release Behavior, Gastrointestinal Distribution, and Systemic Absorption. International Journal of Molecular Sciences. 2020; 21(7):2376. https://doi.org/10.3390/ijms21072376
Chicago/Turabian StyleZhou, Haiyan, Yuri Ikeuchi-Takahashi, Yoshiyuki Hattori, and Hiraku Onishi. 2020. "Nanogels of a Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Release Behavior, Gastrointestinal Distribution, and Systemic Absorption" International Journal of Molecular Sciences 21, no. 7: 2376. https://doi.org/10.3390/ijms21072376
APA StyleZhou, H., Ikeuchi-Takahashi, Y., Hattori, Y., & Onishi, H. (2020). Nanogels of a Succinylated Glycol Chitosan-Succinyl Prednisolone Conjugate: Release Behavior, Gastrointestinal Distribution, and Systemic Absorption. International Journal of Molecular Sciences, 21(7), 2376. https://doi.org/10.3390/ijms21072376